Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.1 Lima Jan./Mar 2020
http://dx.doi.org/10.17843/rpmesp.2020.371.4468
Original article
Differential expression of circulating micro-RNAs in patients with active and latent tuberculosis
1 Laboratorio de Referencia Nacional de Biotecnología y Biología Molecular, Instituto Nacional de Salud, Lima, Perú.
2 Departamento Académico de Biología, Universidad Nacional de San Agustín de Arequipa, Arequipa, Perú.
INTRODUCCIÓN
Tuberculosis (TB), one of the top ten killer diseases in the world, is caused by the Mycobacterium tuberculosis bacillus (Mtb). In 2017, the World Health Organization (WHO) reported 31,120 TB cases in Peru 1,2. In addition, 1.7 thousand million worldwide people have latent TB, and according to Houben and Dodd 20% to 30% of the Peruvian population have latent TB 3.
The search for new biomarkers, derived from the pathogen or the host, is focusing on concepts of cellular communication, represented by the extracellular vesicles called exosomes. The study of circulating microRNAs contained in these exosomes proposes new diagnostic methods in different diseases, including infectious diseases 4, so they can be easily detected in the different types of biofluids in the human body.
KEY MESSAGES
Motivation for the study: The search for a biomarker that will allow the rapid and reliable diagnosis of latent TB in Peru.
Main findings: Micro-RNA (miR-155) that would be overexpressed in subjects with a latent TB diag nosis.
Implications: Since a high percentage of the Peruvian population is infected with latent TB, an ad equate biomarker will help us to diagnose TB infection in a timely manner before the active disease develops and to direct treatment or prophylaxis to those who really need it.
Particularly, at the level of microRNAs (miRNAs) and proteins, the analysis of dynamic changes in exosome cargo offers the possibility to study the activation of cellular and immunological signaling pathways in real time, in order to validate bio markers that correspond to symptomatic and asymptomatic stages (5.
Currently, TB diagnostic methods only detect an active disease, so there are no biomarkers that differentiate latent and active TB for a more specific diagnosis. For this reason, for some years now, research has been conducted on the difference in molecules produced by the host (microRNA) in the context of an infectious disease versus healthy people as a control, this process is called “differential expression” and is evaluated by real-time PCR (Polymerase Chain Reaction), considering a normalizing or reference gene (6,7. These molecules are microRNAs with 18 24 non-coding, endogenously expressed and highly conserved nucleotides, which regulate the post-transcriptional expression of genes through sub/over expression of RNAs in a wide range of organisms in both normal physiological and pathological circumstances.
The deregulation of microRNAs is variable in infectious diseases, and it depends on the population studied. In this sense, the study of these microRNAs in TB has been carried out mainly in European and Asian populations, where diverse microRNAs expressed differentially among pathologies 8,9. Technological advances have generated a multitude of platforms for the creation of microRNA profiles, and an understanding of the strengths and difficulties of the different approaches can help their effective use 10.
This study evaluates the differential expression of miR 21, miR-29a, miR-99b and miR-155 microRNAs taken from patient serum samples in order to find a different miRNA profile in latent TB in comparison with active TB and healthy controls.
MATERIALS AND METHODS
Clinical samples and ARN extraction
The clinical samples used were 28 serum samples taken from patients with active TB diagnosis (n=9), patients with latent TB (n=10) and healthy controls (n=9) (Table 1). The samples were stored in 2 ml cryovials in the freezer at -80 °C (Thermo Fisher Scientific Inc.) of the Immunology area of the National Reference Laboratory of Biotechnology and Molecular Biology of the National Center of Public Health of the National Institute of Health.
Table 1 Clinical and auxiliary examination characteristics in patients with latent TB, active TB and healthy controls.
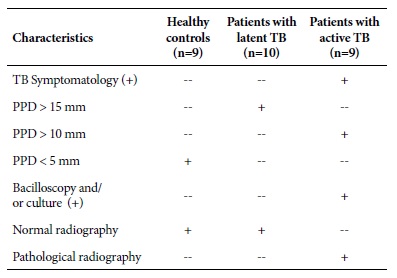
PPD: Mycobacterium tuberculosis Purified Protein Derivative; TB: Tuberculosis; mm: millimeters; (+) positive test result.
The samples were collected between 2012 and 2016, and they are part of a study approved by the Research Ethics Commit tee of the National Institute of Health, “Study of the immunological characteristics of latent tuberculosis in the Peruvian pop ulation, year 2012-2014” (according to OGIIT code OI 01-072- 10). Each participant in the study signed an informed consent form previously approved by the Ethics Committee of the Peruvian National Institute of Health.
The miRNeasy (Qiagen) kit was used for RNA extraction from selected sera, and was used according the manufacturer’s recommendations. Total RNA concentration was quantified using a Nanodrop 8000 (Thermo Fisher) kit. The extracted RNA was stored at - 20 °C in ultra-pure water free of RNases 11 and processed immediately by retrotranscription to complementary DNA within 72 hours.
Selection of microRNAs for real-time PCR validation
A search was made for the differentially expressed microRNAs in previous publications that included different populations, finding mainly works in European and Asian populations 8,9,12-14. The selection criteria for microRNAs included: a) mi croRNAs expressed in TB patients, b) microRNAs differentially expressed in serum by real time PCR and c) overexpressed microRNAs in TB patients by the Microarray and Next generation sequencing platforms. Taking into account these criteria, four microRNAs were selected from a group of TB-related microRNAs: miR-21, miR-29a, miR-99b and miR-155 as possible biomarkers for a differential diagnosis between latent and active TB 9,10,13-16 in samples from the Peruvian population.
Real-time PCR and differential expression analysis of microRNA
The previously designed primers were hsa-miR-21 (MI0000077: UAGCUUAUCAGACUGAUGUGA), hsa-miR-29a (MI0000087: UAGCACCAUCUGAAUC- GGUUA), hsa-miR-99b (MI0000746: UGAGGUAGGUUGUAUAGUU) and hsa-miR-155 (MI0000681: UUAAUGCUAAUCGUGAUUA-GGGGU) (Applied Biosystem) obtained from the miRbase database (http://www.mirbase.org/) 17. The amplification of the microRNAs was performed in two steps: an initial retrotranscription was performed to obtain the complementary DNA, using the TaqMan MicroRNA Reverse transcription kit (Applied Biosystem) followed by a real-time PCR amplification using the (Applied Biosystem) TaqMan Universal PCR Master Mix II kit. Sample volumes and con centrations followed manufacturer’s recommendations (18. Retrotranscription was performed using a (Applied Biosystem) Veriti thermal cycler under the following conditions: 16 °C for 30 minutes, 42 °C for 30 minutes and 85 °C for 5 minutes. The subsequent amplification was performed in the Rotor Gene Q (Qiagen) thermal cycler under the following conditions: 95 °C for 10 minutes, 45 cycles of 95 °C for 15 seconds and 60 °C for 60 seconds. Each sample was run twice and the thermocycler considered an average Ct of both runs per sample and gene evaluated. RNA RNU48 was used as the reference gene (11,19 to normalize the expression of the genes under study.
The differential expression analysis was calculated using the Livak method, which uses the Ct values (threshold value to discriminate between negative and positive, maximum Ct considered was 35) (19 of the diseased and healthy samples to finally be normalized with the reference gene (RNU48). The median Ct values were used to calculate the relative amount by the ddCt comparative method. The ddCt values were used to find the differential expression level or relative quantification normalized by the Livak method or 2 (-ddCt) method, where dCt diseased samples = (Ct microRNA of interest - Ct RNU48), dCt healthy samples = (Ct microRNA of interest - Ct RNU48) and the ddCt = (dCt diseased samples - dCt healthy samples) and the standardized relative quantification or differential expression is equal to 2-ddCt or Fold Change (FC, change in the expression from the control group or healthy samples) 7.
Statistical analysis
MicroRNA expression levels were analyzed using the non-parametric Kruskal-Wallis test. It was used to make multiple com parisons between latent TB, active TB and healthy controls before performing the Mann-Whitney test. The Prism 6 statistical package (GraphPad Software, Inc, CA, USA) was the one used.
RESULTS
Relative quantification of microRNAs using real-time PCR
The results of relative quantification using real time PCR show different Ct values in each fluorescence curve for miR-21, miR 29a, miR-99b and miR-155 regarding the RNU48 normalizer. Which gave us Ct values lower than 35, these Ct values were used to find the ddCt and the normalized relative expression level.
When analyzing the Ct data obtained by real-time PCR, it was found that the four selected microRNAs were differentially expressed (without reaching a statistically significant difference when comparing the groups using the Kruskal-Wallis and Mann-Whitney non-parametric test in patients with active TB and patients with latent TB compared to healthy controls).
Data standardization and analysis
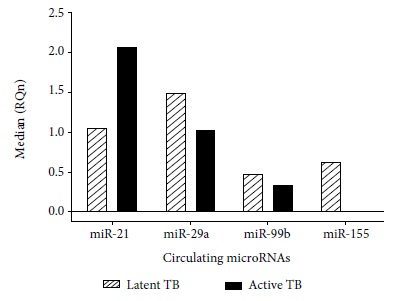
According to the Livak method, the standardized relative quantification or gene expression for miR-21, miR-29a, miR-99b and miR-155 in serum samples is represented by the number of times more, expressed in active TB and latent TB samples versus the control group. The standardized relative quantification (RQn) of these microRNAs between latent TB and active TB is represented by the relative median between both pathologies (Table 2).
Table 2 Standardized differential expression analysis of miR-21, miR-29a, miR-99b and miR-155 in patients with latent TB and patients with active TB versus healthy controls.
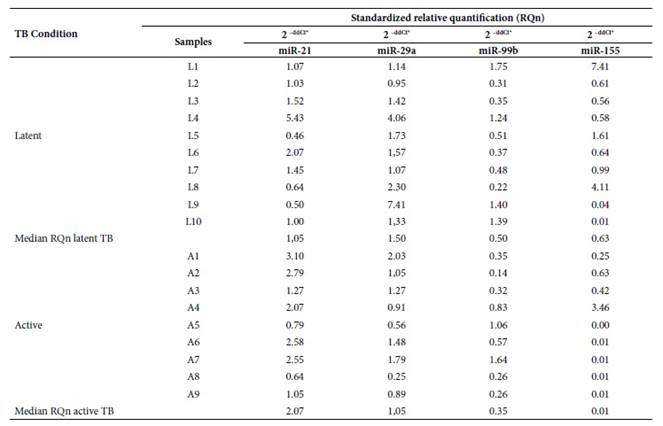
L1-L10: Patients with clinical diagnosis of latent tuberculosis; A1- A9: Patients with clinical diagnosis of active tuberculosis; ddCt*: Livak method or expression (fold change); TB: Tuberculosis.
No statistical differences were found in the miR-99b gene expression in patients with active TB and latent TB (Figures 1 and 2).
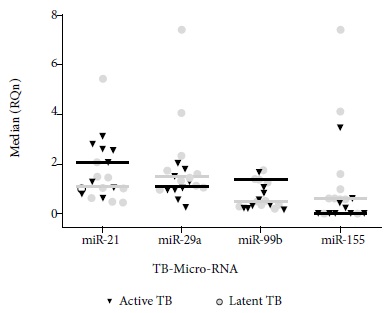
Figure 1 Standardized relative quantification in ten patients with latent TB and nine patients with active TB versus healthy controls. Analysis performed by the Livak method.
DISCUSSION
For some years now, transcriptomic analyses have been used to obtain information on how genes are being expressed and how small RNAs regulate them, among these, the microRNAs. Currently, these small molecules (miRNAs) are being used as potential components of vaccines, biomarkers for early cancer diagnosis and predictors of metabolic diseases 20,21. Likewise, the expression of other groups of small RNAs, whose function was not very clearly known, are now being considered as disease biomarkers 22.
The findings related to the deregulation of these microRNAs are varied. Wang et al. showed that miR-21 is expressed in a differential manner between patients with active TB and patients with latent TB, thus showing that this microRNA could be used as a biomarker to differentiate people with latent TB from those with active TB (2.99 FC) 23. In our research we found an overexpression of miR-21 in active TB samples (2.07 FC), congruent with the work of Wu et al. who pointed out that miR-21 could be involved in the regulation of the antimicobacterial response. Wu et al. described that miR-21 may be induced after BCG vaccination by NF-kB activation. They also reported that miR-21 may suppress IL-12 production 24 and this may indicate that in patients with latent TB, miR-21 is also over expressed. Similar findings were reported by Kleinsteuber et al. who compared miR-21 expression in healthy donors (negative PPD), individuals with latent TB and patients with active TB; finding higher expression of miR-21 in patients with latent TB (0.0438 FC) than in the presence of PPD (0.0393 FC) and patients with active TB (0.0121 FC) 9. However, according to our results, the miR-21 expression profile in latent TB samples is higher than the one from control samples. On the other hand, the miR-21 expression profile in latent TB samples is lesser in the presence of active TB. Therefore, miR-21 could be an effective strategy that uses Mtb against the host immune response to evade the defense cells and develop active TB 14, but when comparing the expression between latent and active TB, there are no significant differences, so it would not be used as a biomarker to differentiate both pathologies.
On the other hand, Wu et al. reported that the expression profile of miR-155 (3.7 FC) in the presence of PPD (Mtb-derived protein to detect latent TB by an antibody-antigen immune response) could be a potential marker for the diagnosis of TB in the presence of Mtb-specific antigens 25. However, our results show higher expression of miR-155 in latent TB (0.63 FC) than in active TB (0.01 FC) with respect to control samples, thus miR-155 could be a possible biomarker of latent TB. This data is not totally opposite to that of J. Wu et al. since they also found an overexpression of miR-155 in the presence of PPD. This Mtb protein activates the early immune system, expressing immune effectors against latent TB as shown by Fu et al. in a recent study, where they state that miR-155 regulates the adaptive immune response 13.
The immune system, when regulating the Mtb invasion, does not cause clinical symptoms. Therefore, the infection becomes latent in the host, thus an overexpression of miR-155 is noted in serum samples. This was predicted by Kumar et al, where miR-155 is overexpressed in Mtb infected macrophages as an early immune response against TB infection 26.This ratifies the results obtained in this study regarding overexpression of miR-155 in serum samples with latent TB (0.63 FC) when compared to active TB (0.01 FC). Likewise, Wang et al. reported differential expression of miR-155 between active and latent TB, since miR-155 is underexpressed in active TB samples (0.51 FC) and overexpressed in latent TB samples (1.97 FC) 23. On the other hand, Zhou et al. describe that miR155 is underexpressed in active TB samples taken from children 27; this finding would allow the use of miR-155 as a biomarker of latent TB in different life stages.
In another study, Wagh et al. highlighted the underexpression of miR-155 in multidrug resistant TB (MDR-TB) samples, with regard to the control group and patients with TB under treatment, which shows that TB virulence can be diagnosed by the underexpression of miR 155 28. Therefore, the results of our research suggest that miR-155 could be an effective biomarker for the diagnosis of latent TB and mark the progression of the disease in its different stages.
The miR-29a expression level found in our study is high in active and latent TB samples compared to the control group, but it was more overexpressed in the latent TB samples than in active TB samples. This result is consistent with the studies by Fu et al. who found overexpression of miR-29a (11.9 FC), which could differentiate TB patients from healthy controls 13,29. However, we also observed an overexpression of miR-29a (1.50 FC) in latent TB with respect to the expression in active TB samples (1.05 FC), but without finding significant differences in the expression between latent TB and active TB.
It should be mentioned that one of the limitations for the present study was the total number of participants, because it was planned as an exploratory study, so the results obtained should be validated with a larger number of participants.
On the other hand, studies are currently being carried out on exosomal proteins that can be used in the routine diag nosis of some cancers 30) and we consider that the next step for the diagnosis of infectious diseases will be with the use of microRNAs.
In conclusion, the analysis of the differential expression levels of microRNA in the serum of patients with active TB and latent TB is interesting for the diagnosis of this pathology. Based on the results of this study, we can conclude that significant overexpression of miR-155, in samples with latent TB, could be a possible biomarker for the differentiation between latent and active TB. However, studies with larger numbers of participants are required to corroborate our findings.
REFERENCES
1. GBD Tuberculosis Collaborators. Global, regional, and national burden of tuberculosis, 1990-2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect Dis. 2018;18(12):1329-1349. doi:10.1016/S1473-3099(18)30625-X [ Links ]
2. World Health Organization. Global tuberculosis report 2018 [Internet]. Geneva: WHO; 2018 [citado el 14 de septiembre de 2019]. Disponible en: https://www.who.int/tb/publications/global_report/gtbr2018_main_text_28Feb2019.pdf?ua=1. [ Links ]
3. Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [ Links ]
4. Zhang W, Jiang X, Bao J, Wang Y, Liu H, Tang L. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front Immunol. 2018;9:90. doi: 10.3389/fimmu.2018.00090. [ Links ]
5. Smith VL, Cheng Y, Bryant BR, Schorey JS. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci Rep. 2017;7:43578. doi: 10.1038/srep43578. [ Links ]
6. Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of micro-RNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33(20):e179. doi: 10.1093/nar/gni178. [ Links ]
7. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101-8. doi: 10.1038/nprot.2008.73. [ Links ]
8. Correia CN, Nalpas NC, McLoughlin KE, Browne JA, Gordon SV, MacHugh DE, et al. Circulating microRNAs as Potential Biomarkers of Infectious Disease. Front Immunol. 2017;8:118. doi: 10.3389/fimmu.2017.00118. [ Links ]
9. Kleinsteuber K, Heesch K, Schattling S, Kohns M, Sander-Julch C, Walzl G, et al. Decreased expression of miR-21, miR-26a, miR-29a, and miR-142-3p in CD4(+) T cells and peripheral blood from tuberculosis patients. PloS One. 2013;8(4):e61609. doi: 10.1371/journal.pone.0061609. [ Links ]
10. Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358-69. doi: 10.1038/nrg3198. [ Links ]
11. Yareta Yareta JL. Validación de un perfil de microARNs para el diagnóstico diferencial de tuberculosis latente y tuberculosis activa 2018 [Tesis]. Arequipa: Universidad Nacional San Agustín de Arequipa; 2018. Disponible en: http://repositorio.unsa.edu.pe/bitstream/handle/UNSA/4795/BIyayajl.pdf?sequence=1&isAllowed=y. [ Links ]
12. Abd-El-Fattah AA, Sadik NA, Shaker OG, Aboulftouh ML. Differential microRNAs expression in serum of patients with lung cancer, pulmonary tuberculosis, and pneumonia. Cell Biochem Biophys. 2013;67(3):875-84. doi: 10.1007/s12013-013-9575-y. [ Links ]
13. Fu Y, Yi Z, Wu X, Li J, Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J Clin Microbiol. 2011;49(12):4246-51. doi: 10.1128/ JCM.05459-11. [ Links ]
14. Harapan H, Fitra F, Ichsan I, Mulyadi M, Miotto P, Hasan NA, et al. The roles of microRNAs on tuberculosis infection: meaning or myth?. Tuberculosis (Edinb). 2013;93(6):596-605. doi: 10.1016/j.tube.2013.08.004. [ Links ]
15. Huang J, Jiao J, Xu W, Zhao H, Zhang C, Shi Y, et al. MiR-155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol Med Rep. 2015;12(5):7102-8. doi: 10.3892/mmr.2015.4250. [ Links ]
16. Mashima R. Physiological roles of miR-155. Immunology. 2015;145(3):323-33. doi: 10.1111/imm.12468. [ Links ]
17. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68-73. doi: 10.1093/nar/gkt1181. [ Links ]
18. Biosystems A. TaqMan(r) Universal Master Mix II. Protocol: Life Technologies Corporation [Internet] thermoFisher scientific; 2010 [citado el 20 de septiembre del 2019]. Disponible en: https://assets.thermofisher.com/TFSAssets/LSG/manuals/cms_069368.pdf. [ Links ]
19. Miotto P, Mwangoka G, Valente IC, Norbis L, Sotgiu G, Bosu R, et al. miRNA signatures in sera of patients with active pulmonary tuberculosis. PloS One. 2013;8(11):e80149. doi: 10.1371/journal.pone.0080149. [ Links ]
20. Chakraborty C, George Priya Doss C, Bandyopadhyay S. miRNAs in insulin resistance and diabetes-associated pancreatic cancer: the 'minute and miracle' molecule moving as a monitor in the 'genomic galaxy'. Curr Drug Targets. 2013;14(10):1110-7. doi: 10.2174/13894501113149990182. [ Links ]
21. Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumour biol. 2016;37(8):10703-14. doi: 10.1007/s13277-016-4939-8. [ Links ]
22. Qian Z, Liu H, Li M, Shi J, Li N, Zhang Y, et al. Potential Diagnostic Power of Blood Circular RNA Expression in Active Pulmonary Tuberculosis. EBioMedicine. 2018;27:18-26. Doi: 10.1016/j.ebiom.2017.12.007. [ Links ]
23. Wang C, Yang S, Sun G, Tang X, Lu S, Neyrolles O, et al. Comparative miRNA expression profiles in individuals with latent and active tuberculosis. PloS One. 2011;6(10):e25832. Doi: 10.1371/journal.pone.0025832. [ Links ]
24. Wu Z, Lu H, Sheng J, Li L. Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS letters. 2012;586(16):2459-67. doi: 10.1016/j.febslet.2012.06.004. [ Links ]
25. Wu J, Lu C, Diao N, Zhang S, Wang S, Wang F, et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155* as potential diagnostic markers for active tuberculosis: a preliminary study. Hum immunol. 2012;73(1):31-7. doi: 10.1016/j.humimm.2011.10.003. [ Links ]
26. Kumar R, Halder P, Sahu SK, Kumar M, Kumari M, Jana K, et al. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol. 2012;14(10):1620-31. doi: 10.1111/j.1462-5822.2012.01827.x. [ Links ]
27. Zhou M, Yu G, Yang X, Zhu C, Zhang Z, Zhan X. Circulating microRNAs as biomarkers for the early diagnosis of childhood tuberculosis infection. Mol Med Rep. 2016;13(6):4620-6. doi: 10.3892/mmr.2016.5097. [ Links ]
28. Wagh V, Urhekar A, Modi D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis (Edinb). 2017;102:24-30. doi: 10.1016/j.tube.2016.10.007. [ Links ]
29. Fu Y, Yi Z, Li J, Li R. Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J Cell Mol Med. 2014;18(3):503-13. doi: 10.1111/jcmm.12205. [ Links ]
30. Zhang W, Ou X, Wu X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: A potential role in the coagulation cascade, diagnosis and prognosis. Int J Oncol. 2019;54(5):1719-33. doi: 10.3892/ijo.2019.4742. [ Links ]
Received: April 16, 2019; Accepted: January 29, 2020











 text in
text in