Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.2 Lima Apr-Jun 2020
http://dx.doi.org/10.17843/rpmesp.2020.372.4772
Original article
Neonatal meningitis: a multicenter study in Lima, Peru
1 Universidad Peruana Cayetano Heredia, Lima, Perú.
2 Hospital Cayetano Heredia, Lima, Perú.
3 Instituto Nacional Materno Perinatal, Lima, Perú.
4 Hospital Nacional Docente Madre Niño San Bartolomé, Lima, Perú.
5 Hospital Nacional Arzobispo Loayza, Lima, Perú.
6 Hospital Nacional Daniel Alcides Carrión, Lima, Perú.
7 Hospital Nacional Guillermo Almenara Irigoyen, Lima, Perú.
INTRODUCTION
Neonatal meningitis (NM) is a devastating disease, known to exist for over a century. Early publications emphasized its clinical rarity and cumbersome diagnostic process 1 2. However, over time it has been reported on every continent, and despite scientific and technological advances, it remains a public health problem 3.
Incidence of NM varies considerably. In developed countries, it is estimated to be around 0.3 cases per 1,000 live births, while in developing countries this incidence can be as high as 6.1 cases per 1,000 live births 3. With the new methods, detection has improved and lethality has decreased; however, morbidity remains high (20-60%) 4.
In Peru, Oliveros reported 0.47 cases per 1,000 live births in 1993 6. However, in recent years an upward trend has been observed, ranging from 0.9 to 1.5 cases per 1,000 live births 5 - 7. This incidence could be greater in our population due to the high frequency of maternal-perinatal factors, such as insufficient prenatal control, sepsis, immaturity due to prematurity and factors inherent to neonatal intensive care 5.
NM is classified in 2 types, early and late 8. Early NM starts within the first 72 hours and is related to contamination through the birth canal with bacteria such as Escherichia coli, Streptococcus group B and Listeria monocytogenes 9 10. After 72 hours, late NM is associated with germs from the hospital environment, such as coagulase-negative Staphylococcus and gram-negative bacilli (Escherichia coli, Klebsiella pneumoniae, Enterobacter spp.) 9 - 11.
NM is a health emergency, and as soon as it is suspected, empirical antibiotic treatment should be indicated 12. However, diagnosis is complex due to the low specificity of signs and symptoms and the difficulty of isolating the germs by culture. So, when risk factors are detected, clinical suspicion is the only alternative 2 8.
Given the scarce information on NM in our country regarding aspects such as its frequency, impact on morbimortality and the prevalence of the pathogens involved 9 12, it is extremely important to know the epidemiological and clinical profile of the disease. For this reason, the objective of the study was to estimate the incidence, associated factors, clinical aspects and cerebrospinal fluid (CSF) characteristics, etiology, and complications of NM in hospitals in the city of Lima.
KEY MESSAGES
Motivation for the study: The frequency of neonatal meningitis in some hospitals and the absence of a treatment protocol motivated an epidemiological surveillance study in Lima.
Main findings: An incidence of 1.4 cases per 1,000 live births was found, preterm infants represented the highest proportion. Symptoms were non-specific, mainly respiratory distress in early NM, and fever and irritability in the late type. Cerebrospinal fluid showed moderate pleocytosis with hypoglycorrhachia and hyperproteinorrhachia. Escherichia coli and Listeria monocytogenes predominated.
Implications: There is a need to standardize the diagnostic and treatment criteria for NM. Likewise, epidemiological surveillance should continue in the neonatal units of our country.
MATERIALS AND METHODS
Design and population
Multi-center case series study carried out between 2017 and 2018, with the aim of carrying out hospital epidemiological surveillance of NM for 12 consecutive months in Lima hospitals, without intervening in the diagnosis and treatment processes.
To be included in the study, hospitals had to have neonatal units, neonatal physicians, specialized nursing staff, specialists in neurology or neuropediatrics, neuroimaging equipment and a clinical laboratory suitable for processing general analysis and cytochemical and bacteriological examination of CSF. For this purpose, 12 hospitals were selected, from which 6 met the inclusion criteria: Hospital Cayetano Heredia (HCH), Hospital Nacional Docente Madre Niño San Bartolomé (HSB), Hospital Nacional Arzobispo Loayza (HNAL), Instituto Nacional Materno Perinatal (INMP), Hospital Nacional Guillermo Almenara Irigoyen (HNGAI) and Hospital Nacional Daniel Alcides Carrión (HNDAC). All were level III health facilities.
A research team was organized with physicians representing each of the six hospitals, who were trained in the process of inclusion, follow-up and collection of clinical and laboratory data. All centers had a neonatologist and a neurologist. A new-case alert system was developed. The possibility of a case, was recorded, communicated and confirmed by the representant of each hospital. The monitoring and data collection continued until discharge. An ad hoc clinical file was created, with data about filiation, sex, age, gestational age, prenatal, birth and postnatal data, CSF characteristics and bacteriological data. There was no interference in management decisions. In all hospitals the objectives of the project were presented to the pediatric medical team.
In order to estimate hospital incidents, the number of births during the observation period was recorded, according to the perinatal register and statistics office of each hospital. Finally, premature births were recorded by gestational age and sex.
Variables
All full-term infants under 28 days or pre-term infants under 44 weeks corrected gestational age were entered into the study. The inclusion criteria for all cases of NM were infants who were symptomatic or at risk of infection; pleocytosis ≥ 30 leukocytes/μL in CSF, diagnosis and care at the hospital of birth. The hospital follow-up concluded with the discharge of the patient. Neonates with severe cerebral malformations and spinal dysraphism were excluded.
NM categorization was confirmed (germ identified), probable (high bacterial suspicion), and possible (low bacterial suspicion) 5 11. NM was confirmed when the germ was identified in the CSF, by culture, polymerase chain reaction (PCR), coagglutination or blood culture. Probable NM was defined by hypoglycorrhachia (glycorrhachia ≤50% of serum glucose or absolute glycorrhachia of ≤40 mg/dL) and hyperproteinuria (proteinuria ≥60 mg/dL) 5 8. Cases of possible NM had any level of glycorrhachia or proteinorrhachia or normal biochemical values. The viruses were identified by PCR or viral indirect immunofluorescence (viral IIF) in the CSF. The fungi were identified by CSF culture/PCR. In the case of lumbar punctures (LP), a leukocyte was discounted for every 500 red blood cells in CSF.
Early MN was defined as, confirmed, probable or possible cases diagnosed before 72 hours of age. Late NM was defined as cases diagnosed after 72 hours of age 5 8. Early neurological complications were defined within the first seven days of detection. The complications considered were hydrocephalus, ventriculitis, subdural effusion and cerebral infarction, identified by cerebral ultrasound or cerebral magnetic resonance.
In order to measure the burden of disease, out-of-hospital cases were recorded. Out-of-hospital cases are defined as cases of NM born in other hospitals and admitted during the study period.
A set of prenatal, natal and postnatal variables were recorded and analyzed. Numerical variables were: maternal age, antenatal control, gestational age, birth weight; and categorical variables were: maternal urinary infection, maternal fever, chorioamnionitis, presence of meconium amniotic fluid, pre-eclampsia / eclampsia, asphyxia, intraventricular hemorrhage, sepsis, anemia, meconium aspiration, fever, respiratory distress, hypoactivity, irritability, vomiting. Likewise, CSF characteristics and germ frequencies, treatment, complications and lethality were recorded and analyzed.
Ethical considerations
The identity of the patients was protected by numerical codes. The project was also approved by the Institutional Ethics Committee of Universidad Peruana Cayetano Heredia and by the ethics committees of each of the participating hospitals.
Statistical Analysis
The information was collected and stored in a Microsoft Excel 2016 © database. The accumulated incidence during one year of observation in each hospital was determined. The project started in several successive months in 2017 and ended sequentially in 2018. The cumulative incidence was estimated from the sum of confirmed cases, probable cases and possible cases divided by the number of live births. Out-of-hospital cases were not considered for the incidence calculation.
The frequencies of clinical and laboratorial variables are presented for early, late and out-of-hospital NM. Numerical variables were summarized with medians and their interquartile range. Logistic regression was performed to determine the influence of some factors on early meningitis with respect to late meningitis, by analyzing all cases. Homogeneity was determined by the Levene and Forsythe-Browne tests. The only few missing data were from the prenatal control variable, so no replacement technique was necessary.
RESULTS
Patient Enrollment
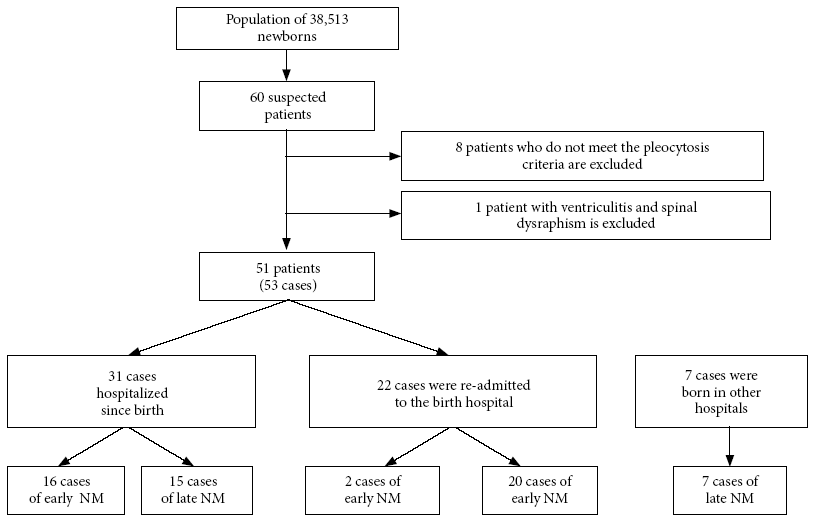
The project started in 2017. Given that the enrollment in hospitals was carried out gradually, the study was completed in 2018. During this period a total of 38,513 live neonates were registered in the six hospitals, of which 51 patients were included who developed 53 cases of NM, one patient presented three episodes of NM. From the reported cases, 41.5% (22/53) were neonates who, having left the hospital in good condition, were readmitted on suspicion of an infectious process. During the study period, seven out-of-hospital cases were admitted (Figure 1), considered only for the profile of clinical, etiological and laboratorial analysis.
The average maternal age was 27.2 years and parity was 2.3 per woman. The number of prenatal controls was also insufficient in 58.8% (30/51) of the mothers. From the neonates, 54.7% (29/53) were born prematurely before 37 weeks. The population studied was homogeneous among the hospitals included.
Epidemiological characteristics
The hospital incidence was 1.4 cases per 1,000 live births, with a wide variation among hospitals, from 0 to 3.2 cases per 1,000 live births. HCH and HNDMNSB had the highest incidence. In pre-term infants under 37 weeks, the NM incidence was 7.5 cases per 1,000 live births and 0.7 cases per 1,000 live full-term births (Table 1).
Table 1 Cumulative incidence of neonatal meningitis according to hospital institution.
| Institution | Total | Pre-term | ||||
|---|---|---|---|---|---|---|
| Live births | Cases | Cumulative incidence(per 1,000 live births) | Live births | Cases | Cumulative incidence(per 1,000 live births) | |
| Hospital Nacional Cayetano Heredia | 4,436 | 14 | 3.2 | 826 | 7 | 8.5 |
| Hospital Nacional Docente Madre Niño San Bartolomé | 6,155 | 20 | 3.2 | 395 | 8 | 20.3 |
| Instituto Nacional Materno Perinatal | 18,138 | 17 | 0.9 | 1,634 | 13 | 8.0 |
| Hospital Nacional Arzobispo Loayza | 2,765 | 1 | 0.4 | 226 | 0 | 0.0 |
| Hospital Nacional Daniel Alcides Carrión | 3,915 | 1 | 0.3 | 481 | 1 | 2.1 |
| Hospital Guillermo Almenara Irigoyen | 3,104 | 0 | 0.0 | 280 | 0 | 0.0 |
| Total | 38,513 | 53 | 1.4 | 3,842 | 29 | 7.5 |
Surveillance during a 1-year period.
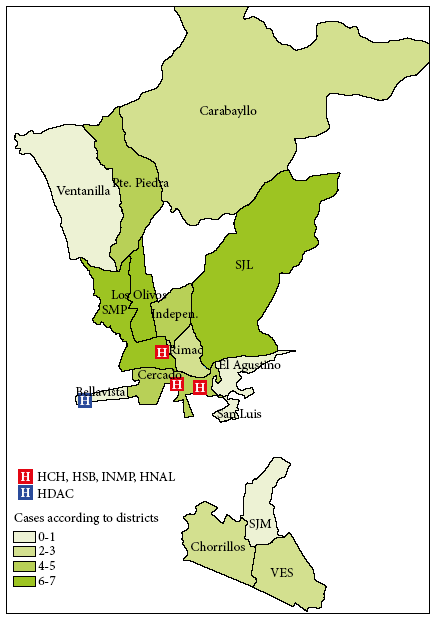
The male/female ratio was 1.4; males represented the 58.3% (35/60) and females 41.7% (25/60). The majority of patients were from northern Lima at 42% (25/60), mainly from the districts of San Martín de Porres and Los Olivos, followed by districts of eastern Lima at 15% (9/60). Figure 2 shows that the cases came from areas surrounding the hospitals.
From the total, 34% (18/53) were early NM cases and 66% (35/53) were late NM cases. Cases of confirmed NM were 58.5% (31/53), of which 25.8% (8/31) were early and 74.2% (23/31) late. Bacterial NM occurred in 87.1% (27/31) of the confirmed cases and viral NM in 12.9% (4/31). Probable NM was 22.6% (12/53) of the total of cases and possible NM was 18.9% (10/53). Regarding outpatients, 4 had confirmed NM, 2 had probable NM and 1 had possible NM.
Clinical characteristics
For early NM, the associated prenatal factors were meconial amniotic fluid (38.9%), urinary tract infection (33.3%), maternal fever (27.8) and chorioamnionitis (22.2%). However, in the late NM, these factors did not seem to have a major influence (Table 2).
Table 2 Prenatal, natal and postnatal characteristics, according to the type of meningitis
| Characteristic | Early NM (n = 18) | Late NM (n = 35) | Out-of-hospital NM (n = 7) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Cesarean section delivery | 11 (61.1) | 18 (51.4) | 3 (42.9) |
| Prenatal controls | |||
| <6 | 10 (55.5) | 17 (54.8) | 3 (42.9) |
| 6 or more | 4 (22.3) | 14 (45.2) | 3 (42.9) |
| Prenatal and natal factors | |||
| Medications during pregnancy | 0 (0.0) | 1 (2.9) | 1 (14.3) |
| Maternal fever | 5 (27.8) | 1 (2.9) | 0 (0.0) |
| Premature rupture of membranes >18 h | 3 (16.7) | 9 (25.7) | 0 (0.0) |
| Urinary tract infection | 6 (33.3) | 7 (20.0) | 2 (28.6) |
| Vaginal infection | 0 (0.0) | 2 (5.7) | 1 (14.3) |
| Pelvic-uterine surgery | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| Meconium-stained amniotic fluid | 7 (38.9) | 9 (25.7) | 1 (14.3) |
| Chorioamnionitis | 4 (22.2) | 5 (14.3) | 0 (0.0) |
| Prolonged labor | 2 (11.1) | 1 (2.9) | 0 (0.0) |
| Preeclampsia/eclampsia | 2 (11.1) | 5 (14.3) | 1 (14.3) |
| Intrauterine growth restriction | 0 (0.0) | 2 (5.7) | 0 (0.0) |
| Male gender | 11 (61.1) | 20 (57.1) | 4 (57.1) |
| Gestational age (weeks) | |||
| <37 | 11 (61.1) | 18 (51.4) | 3 (42.9) |
| ≥37 | 7 (38.9) | 17 (48.6) | 4 (57.1) |
| Weight (grams) | |||
| <1,500 | 4 (22.2) | 9 (25.7) | 2 (28.6) |
| 1,500 to 2,499 | 7 (38.9) | 10 (28.6) | 1 (14.3) |
| ≥2,500 | 7 (38.9) | 16 (45.7) | 4 (57.1) |
| Age at the onset of symptoms (days) a | 0.9 (1.8) | 18.6 (20.1) | 11.9 (11.6) |
| Postnatal factors | |||
| Sepsis | 9 (50.0) | 7 (20.0) | 3 (42.9) |
| Asphyxia | 1 (5.6) | 0 (0.0) | 2 (28.6) |
| Meconium aspiration | 0 (0.0) | 1 (2.9) | 1 (14.3) |
| Intraventricular hemorrhage | 4 (22.2) | 2 (5.7) | 1 (14.3) |
| Anemia | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Connatal pneumonia | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| Pathological jaundice | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| Symptoms | |||
| Fever | 7 (38.9) | 19 (54.3) | 5 (71.4) |
| Irritability | 7 (38.9) | 20 (57.1) | 3 (42.9) |
| Hypoactivity | 7 (38.9) | 17 (48.6) | 4 (57.1) |
| Breathing difficulty | 13 (72.2) | 10 (28.6) | 3 (42.9) |
| Weak sucking | 4 (22.2) | 12 (34.3) | 3 (42.9) |
| Vomiting | 1 (5.6) | 2 (5.7) | 4 (57.1) |
| Jaundice | 4 (22.2) | 4 (11.4) | 5 (71.4) |
| Apnea | 5 (27.8) | 7 (20.0) | 1 (14.3) |
| Convulsions | 2 (11.1) | 6 (17.1) | 1 (14.3) |
| Bulging fontanelle | 3 (16.7) | 2 (5.7) | 3 (42.9) |
| Hypotonia | 5 (27.8) | 6 (17.1) | 3 (42.9) |
| Hypertonia | 3 (16.7) | 2 (5.7) | 2 (28.6) |
| Hyperreflexia | 1 (5.6) | 3 (8.6) | 1 (14.3) |
| Hyporreflexia | 2 (11.1) | 1 (2.9) | 2 (28.6) |
| Lethality | 1 (5.6) | 1 (2.9) | 0 (0.0) |
NM: neonatal meningitis
a Mean (SD)
Sepsis was the most important factor related to NM; and according to the NM types, 50% (9/18) were early meningitis cases, 20% (7/35) were late meningitis cases and 42.9% (3/7) were of out-of-hospital meningitis cases. The average age for the onset of symptoms in early NM cases was 0.9 days; 18.6 days in late NM; and 11.9 days in the out-of-hospital cases. Symptoms such as respiratory distress, were more common in the early NM. In late NM, fever, irritability and hypoactivity predominated (Table 2). Table 5 shows the risk factors associated with early NM in relation to late NM.
Table 5 Factors associated with early neonatal meningitis compared to late neonatal meningitis.
| Factor | OR | p-value | 95% CI |
|---|---|---|---|
| Maternal fever | 18.51 | 0.021 | 1.56-219.87 |
| Sepsis | 5.10 | 0.040 | 1.08-24.07 |
| Breathing difficulty | 4.59 | 0.043 | 1.05-20.11 |
| Cesarean section delivery | 4.12 | 0.079 | 0.85-20.01 |
OR: Odds Ratio, 95% CI: 95% confidence interval
Cytochemical and bacteriological characteristics of the CSF
On average, 2.5 and 2 LPs were performed for early and late NM, respectively. In most cases of early NM, the LP was performed on the first day of hospitalization; and in cases of late NM, it could take until the third day of illness.
In Table 3, the cytochemical characteristics of the CSF are presented. The median value for pleocytosis was 225 leukocytes/μL for early NM and 202 leukocytes/μL for late NM, the median value for polymorphonuclears (PMN) was of 57% and 30%, respectively. Hypoglycorrhachia was similar in both types of meningitis and hyperproteinorrhachia was higher in early NM. In the out-of-hospital type, there was less pleocytosis and more glycorrhachia (Table 3).
Table 3 Cerebrospinal fluid characteristics, according to the type of meningitis.
| Variable | Early NM (n = 18) | Late NM (n = 35) | Out-of-hospital NM (n = 7) | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Leucocytes (cells/μL) | 225 | 130-1912 | 202 | 45-530 | 150 | 32-866 |
| PMN (%) | 57 | 30-70 | 30 | 10-52 | 60 | 35-60 |
| Glucose (mg/dL) | 36 | 24-42 | 32 | 25-44 | 43 | 34-46 |
| Proteins (mg/dL) | 188 | 115-499 | 125 | 81-201 | 139 | 62-266 |
| Erythrocytes (cells/μL) | 100 | 10-500 | 3 | 0-100 | 32 | 5-50 |
NM: neonatal meningitis, IQR: interquartile range (25th and 75th percentiles), PMN: polymorphonuclears
A total of 35 germs were identified, including bacteria, viruses and a case of Candida albicans. In all clinical types, Escherichia coli and Listeria monocytogenes predominated. In 17.1% (6/35) of the cases the germ was isolated both in blood culture and in the CSF. Escherichia coli was found in four of those cases, group B Streptococcus and coagulase negative Staphylococcus, in one case each. Cases of influenza B, coronavirus and adenovirus were identified by indirect immunofluorescence (IIF). In a single case, PCR was performed, isolating herpes virus VI (Table 4).
Table 4 Isolation of the infectious agent according to the type of meningitis.
| Infectious agent | Cultivated fluid | Early NM | Late NM | Out-of-hospital NM | Total |
|---|---|---|---|---|---|
| Escherichia coli | CSF | 3/18 | 2/35 | 0/7 | 10/60 a |
| Blood | 2/18 | 7/35 | 0/7 | ||
| Listeria monocytogenes | CSF | 1/18 | 2/35 | 0/7 | 8/60 |
| Blood | 3/18 | 2/35 | 0/7 | ||
| Coagulase-negative Staphylococcus | CSF | 0/18 | 1/35 | 1/7 | 3/60 a |
| Blood | 1/18 | 1/35 | 0/7 | ||
| Group B Streptococcus | CSF | 0/18 | 1/35 | 1/7 | 2/60 a |
| Blood | 0/18 | 0/35 | 1/7 | ||
| Enterococo faecium | CSF | 0/18 | 0/35 | 0/7 | 2/60 |
| Blood | 1/18 | 1/35 | 0/7 | ||
| Staphilococus epidermidis | CSF | 0/18 | 0/35 | 0/7 | 2/60 |
| Blood | 0/18 | 2/35 | 0/7 | ||
| Serratia marcescens | CSF | 0/18 | 0/35 | 0/7 | 1/60 |
| Blood | 0/18 | 1/35 | 0/7 | ||
| Serratia liquecies | CSF | 0/18 | 0/35 | 0/7 | 1/60 |
| Blood | 0/18 | 1/35 | 077 | ||
| Staphilococus hominis | CSF | 0/18 | 0/35 | 0/7 | 1/60 |
| Blood | 0/18 | 0/35 | 1/7 | ||
| Influenza B (IIF) | CSF | 0/18 | 1/35 | 0/7 | 1/60 |
| Adenovirus (IIF) | CSF | 0/18 | 1/35 | 0/7 | 1/60 |
| Coronavirus (IIF) | CSF | 0/18 | 1/35 | 0/7 | 1/60 |
| Herpes virus VI (PCR) | CSF | 0/18 | 1/35 | 0/7 | 1/60 |
| Candida albicans (PCR) | CSF | 0/18 | 0/35 | 0/7 | 1/60 |
| Blood | 0/18 | 0/35 | 1/7 |
NM: neonatal meningitis; CSF: cerebrospinal fluid; PCR: polymerase chain reaction; IIF: indirect immunofluorescence
a Isolation of the germ in both blood and CSF
The total represents the burden of disease addressed by all the hospitals.
Treatment and special conditions
Treatment schedules were highly variable. The mean duration for cases of early NM was 21 days and 19.5 days for late meningitis. Before the first LP was performed, 62% of children received antibiotics. The most commonly used drugs were ampicillin (60%), cefotaxime (38%), vancomycin (28%), meropenem (33%) and gentamicin (22%), in different schedules.
In the specific analysis of late meningitis, not including outpatients, two groups were differentiated (Figure 1). In the first group, 86.7% (13/15) of patients were pre-term infants, 46.6% (7/15) had respiratory distress, most were diagnosed at seven days of age, and their CSF was characterized by increased pleocytosis. In the second group, 75% (15/20) were full-term infants, fever and irritability were the most frequent symptoms and the diagnosis was made within the first two days of hospitalization.
Three patients had special presentations. One had minor pleocytosis and urinary-related bacteremia by Escherichia coli; another had normal initial cytochemistry, but with a CSF culture positive for Escherichia coli that later developed pleocytosis; and the third one had three episodes of meningitis (recurrent) by extended-spectrum beta-lactamase-producing Escherichia coli.
Complications and lethality
At least one neurological complication was observed in 25% (15/60) of the cases, from which, 73.3% (11/15) were pre-term infants. Early and late neurological complications were ventriculitis and hydrocephalus respectively.
From the cases with late NM, 95.2% (40/42) were discharged with favorable evolution, early NM had a favorable evolution in 77.8% (14/18) of the cases. Four cases were referred to a hospital with a higher complexity level. Two neonates died (3.3%), one presenting early NM and the other, the late type.
DISCUSSION
NM had a hospital incidence of 1.4 cases per 1,000 live births, with a higher risk in pre-term than in full-term infants. The wide variability in incidence leads to the suspicion that diagnostic protocols for NM were not standarized across hospitals. A variety of causal germs, mostly bacteria, were identified, with the frequency of Eschericha coli and Listeria monocytogenes being particularly noteworthy.
We present a larger NM clinical scenario than those known. In this scenario the early type is related to birth conditions and the late type is related to prolonged stay of pre-term infants in neonatal units 9. We provide a new viewpoint, derived from the community, which occurs more in full-term infants near the second week, related to a higher proportion of viral agents.
Neonatal meningitis is an under-diagnosed and under-recorded prevalent disease in our country 5 13. In 2016, Zea et al., noted that LP is often deferred in confirmed sepsis 14. Likewise, in a similar population it has been observed that medical criteria may vary depending on the level of medical specialization 15.
We present an epidemiological surveillance study according the management protocols of each hospital. The incidence of 1.4 per 1,000 live births is an average value worldwide 3, and is initially taken as a reference. This value will have to be adjusted in the future when the diagnostic criteria are standardized. However, the high incidence in premature infants alerts us about the need for vigilance in neonatal units (2, 16).
This study was characterized by the inclusion of cases with defined pleocytosis. This was made to meet the inflammatory criteria for meningitis, classified as confirmed, probable and possible, according to the definition of neonatal sepsis. Therefore, more positive isolates were found in blood cultures. Less were found to be positive in blood and CSF cultures, and only a few cases were observed solely in CSF culture. We believe that PCR could have helped to reduce the number of probable and possible cases, and to identify cases of pleocytosis as just an inflammatory phenomenon 8 9 17.
All known risk factors for neonatal sepsis are related to NM 12 18. For the early type, peripartum fever and incomplete prenatal controls were found to be risk factors. These factors suggest the risk of microbial invasion from the vaginal flora, the subsequent placental inflammatory response, initiation of labor and consequently sepsis and meningitis 8. However, other clinical and sociocultural factors may not be considered.
Classically, NM is divided into early and late according to its mechanism of contamination 12 16 19. However, we have identified a third group of patients who come from their homes, from the community environment, are term infants, febrile and irritable with less pleocytosis, contaminated with common respiratory tract agents, both bacterial and viral, and in some cases by germs that colonize maternal secretions.
The age of symptoms onset for both types of NM was found to be within the expected ranges, 0.9 days for early NM cases and 18.6 days for late NM cases. This was found to be in accordance with other series, and clearly associated with the type of birth and neonatal unit stay 12, 16, 20. The group of children of out-of-hospital origin also behaved as late NM at 11.9 days.
The symptoms were more frequent in early NM than in the late type, being very nonspecific and related to sepsis. Among them, respiratory difficulty stood out in 70% of early cases, perhaps due to lung immaturity in the premature group or to respiratory acidosis 3 12 16. In late NM more neurological symptoms were observed 1 3. However, identification of these symptoms depends on the experience of the examiner 14 21. Maternal fever, sepsis, and respiratory distress were three factors found to be more likely to develop in early NM than in the late type. These were probably generated by maternal infections, urinary tract infections and chorioamnionitis 1 8. It will remain for future studies to ensure the diagnosis of chorioamnionitis by pathological examination of the placenta.
In most cases, more than one LP was carried out, following international guidelines. Given that NM is a difficult to diagnose multi-symptomatic disease caused by many aggressive agents, the guidelines recommend that the LP be performed prior to the use of antibiotics. It is also recommended that a new control should be performed within 48-72 hours, especially if there is no clinical improvement, with the purpose of reducing the bacterial load or achieving sterilization of the CSF 14 21 22.
In both clinical types, moderate pleocytosis without PMN predominance was noted, they also presented hypoglycorrhachia and proteinorrhachia. This particular characteristic has already been observed in other national studies 6 7, perhaps, bacteriological factors, sample processing and patient’s immunological conditions are involved. In bacterial NM, hypoglycorrhachia and proteinorrhachia are common findings. These are explained by glucose consumption and increased detritus, their persistence for more than two weeks has been associated with poor prognosis 23. However, these indicators may be aggravated by the presence of intracranial hemorrhage. Also, on rare occasions, the first LP may not demonstrate pleocytosis, and a second sample may be required within 12 to 24 hours 21.
The microbiological behavior of NM has varied regarding time and different geographical areas 2 3 16 24 - 26. Streptococcus agalactie stands out mainly in developed countries and gram-negative bacteria in non-developed countries 3 24. In this series, Eschericha coli and Listeria monocytogenes were the prevalent germs in both types of NM, followed by a variety of gram-negative and gram-positive bacteria, fewer virus cases and one case of Candida albicans, all described in different case series.
NM by Eschericha coli has been known for many decades 1 2 to be a part of early neonatal sepsis cases. It can also cause late NM, usually associated with severe acute and mid-term complications such as hydrocephalus, subdural effusions, cerebral infarctions and abscesses 2 27. In recent years, the increased frequency of beta-lactamase strains and their antimicrobial resistance has being notable 24 26. Therefore, their presence in this series alerts about early identification and treatment.
Listeria monocytogenes is a pathogen that has become more important in Peruvian series in recent years 5 7. It has been observed to be 5-20% 26 of the early and late types reported, and it usually produces a moderate or severe disease, according to some international and national reports. However, its infectious mechanism is not clearly identified, but it is understood that the invasion is by genitourinary route and related to the maternal intestinal flora.
The lethality rate by NM in national reports has been decreasing over time. In 1993, Oliveros et al. reported 20% death in a series of 24 cases 6, and in 2017, Lewis reported 3.8% in a series of 53 patients 5. Such decrease may be related to early diagnosis and treatment. However, the frequency of neurological complications was 25%, and the high morbidity in premature infants (75%) was noteworthy 28 29. Consequently, the use of cerebral ultrasound as a diagnostic tool for hydrocephalus, ventriculitis and cerebral infarction is very important in premature infants 30.
Not including certain variables such as prenatal steroid use, intrauterine infections, histological chorioamnionitis, invasive procedures, recording of sepsis cases without meningitis, antimicrobial sensitivity and resistance, community contacts, and not involving more hospitals are among the main limitations of this study. However, the strengths of the study were to demonstrate that NM is frequent, that pre-term infants are at greater risk, that the disease can present itself in different ways and that a wide spectrum of causal infectious agents exists. With these considerations we contribute to the national knowledge of this disease.
In conclusion, the hospital incidence of NM was 1.4 cases per 1,000 live births, and even higher in premature infants. Respiratory distress was the most frequent symptom for early NM, while fever and irritability were the most frequent symptoms of late NM. Moderate pleocytosis, with hypoglycorrhachia and proteinorrhachia, was noted in the CSF. The most frequent pathogens isolated were Eschericha coli and Listeria monocytogenes. The most common neurological complications were ventriculitis and hydrocephalus. A new pathogenic scenario for NM is proposed, it consists of three infection types: vertical infection, by vaginal flora germs; nosocomial infection, by contamination in neonatal units; and infection from the community by common germs.
A national epidemiological surveillance study of NM is recommended. This study should standardize diagnostic criteria (clinical, cytochemical, culture, PCR), neuroimaging criteria (ultrasound and resonance) and criteria for identification of perinatal risk factors.
REFERENCES
1. Bell WE, McCormick WF, Murillo PL. Meningitis neonatales. Infecciones neurológicas en el niño. 2 ed. Barcelona: Salvat; 1979. [ Links ]
2. Ziai M, Haggerty RJ. Neonatal meningitis. N Engl J Med. 1958;259(7):314-20. doi: 10.1056/NEJM195808142590702. [ Links ]
3. Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial Meningitis in the Infant. Clin Perinatol. 2015;42(1):29-45. doi: 10.1016/j.clp.2014.10.004. [ Links ]
4. Holt DE. Neonatal meningitis in England and Wales: 10 years on. Arch Dis Child - Fetal Neonatal Ed. 2001;84(2):85F-89. [ Links ]
5. Lewis G, Schweig M, Guillén-Pinto D, Rospigliosi ML. Meningitis neonatal en un hospital general de Lima, Perú, 2008 al 2015. Rev Peru Med Exp Salud Pública. 2017; 34:233-8. doi: 10.17843/rpmesp.2017.342.2297. [ Links ]
6. Oliveros Donohue MA, Ramos Pianezzi R, León Cueto JL, Mazzini Pérez-Reyes J, Van Oordt, Bellido J, Livia Becerra C. Meningitis neonatal en la UCI del Hospital Edgardo Rebagliati Martins (IPSS) 1986-88. Diagnóstico. 1993;32(4/6):73-7. [ Links ]
7. Lazo E, Guillén D, Zegarra J. Meningitis neonatal en el Hospital Nacional Cayetano Heredia. Rev Peru Pediatr. 2008;61(3):157-164. [ Links ]
8. Volpe J. Bacterial and Fungal Intracranial Infections. Neurology of the Newborn. Fifth Edition. Philadelphia: Saunders Elsevier; 2013. [ Links ]
9. Devi U, Bora R, Malik V, Deori R, Gogoi B, Das JK, et al. Bacterial aetiology of neonatal meningitis: A study from north-east India. Indian J Med Res. 2017 Jan;145(1):138-143. doi: 10.4103/ijmr.IJMR_748_15. [ Links ]
10. Pérez RO, Lona JC, Quiles M, Verdugo MÁ, Ascencio EP, Benítez EA. Sepsis neonatal temprana, incidencia y factores de riesgo asociados en un hospital público del occidente de México. Rev Chil Infectol. 2015;32(4):447-452. [ Links ]
11. Zea-Vera A, Turin CG, Ochoa TJ. Unificando los criterios de sepsis neonatal tardía: propuesta de un algoritmo de vigilancia diagnóstica. Rev Peru Med Exp Salud Pública. 2014;31(2):358-63. [ Links ]
12. Perlman JM, Cilio M. Neonatal Meningitis: Current Treatment Options. Neurology. Neonatology Questions and Controversies. Third Edition. Phyladelphia: Elsevier; 2019. [ Links ]
13. Medina, María del Pilar. Frecuencia de enfermedad neurológica en recién nacidos. Rev Peru Pediatr. 2007;60(1):11-9. [ Links ]
14. Zea-Vera A, Turín CG, Rueda MS, Guillén-Pinto D. Uso de la punción lumbar en la evaluación de sepsis neonatal tardía en recién nacidos de bajo peso. Rev Peru Med Exp Salud Pública. 2016;33(2):278-282. [ Links ]
15. Vera S. Variabilidad del criterio para indicar la punción lumbar en las unidades de cuidados intensivos neonatales [tesis de bachiller]. Lima: Facultad de Medicina, Universidad Peruana Cayetano Heredia; 2018. [ Links ]
16. Coto GD, López JB, Fernández B, Fraga JM, Fernández JR, Reparaz R, et al. Meningitis neonatal. Estudio epidemiológico del Grupo de Hospitales Castrillo. An Pediatr. 2002;56(6):556-63. [ Links ]
17. Marcilla C, Martínez A, Carrascosa M, Baquero M, Alfaro B. Meningitis víricas neonatales. Importancia de la reacción en cadena de la polimerasa en su diagnóstico. Rev Neurol. 2018; 67:484-490. [ Links ]
18. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770-80. doi: 10.1016/S0140-6736(17)31002-4. [ Links ]
19. Zhao Z, Yu J-L, Zhang H-B, Li J-H, Li Z-K. Five-Year Multicenter Study of Clinical Tests of Neonatal Purulent Meningitis. Clin Pediatr (Phila). 2018;57(4):389-97. doi: 10.1177/0009922817728699. [ Links ]
20. Olmedo I, Pallas CR, Miralles M, Simón de las Heras R, Rodriguez J, Chasco A. Meningitis neonatal: Estudio en 56 casos. An Esp Pediatr. 1997; 46:189-194. [ Links ]
21. Garges HP. Neonatal Meningitis: What Is the Correlation Among Cerebrospinal Fluid Cultures, Blood Cultures, and Cerebrospinal Fluid Parameters?. Pediatrics. 2006;117(4):1094-100. [ Links ]
22. Greenberg RG, Benjamin DK, Cohen-Wolkowiez M, Clark RH, Cotten CM, Laughon M, et al. Repeat lumbar punctures in infants with meningitis in the neonatal intensive care unit. J Perinatol. 2011;31(6):425-429. doi: 10.1038/jp.2010.142. [ Links ]
23. Tan J, Kan J, Qiu G, Zhao D, Ren F, Luo Z, et al. Clinical Prognosis in Neonatal Bacterial Meningitis: The Role of Cerebrospinal Fluid Protein. PLoS One. 2015 Oct 28;10(10):e0141620. doi: 10.1371/journal.pone.0141620. [ Links ]
24. Collaborative Study Group for Neonatal Bacterial Meningitis. A multicenter epidemiological study of neonatal bacterial meningitis in parts of South China. Zhonghua Er Ke Za Zhi Chin J Pediatr. 2018;56(6):421-428. doi: 10.3760/cma.j.issn.0578-1310.2018.06.004. [ Links ]
25. Berardi A, Lugli L, Rossi C, China MC, Vellani G, Contiero R, et al. Infezioni da Streptococco B Della Regione Emilia Romagna. Neonatal bacterial meningitis. Minerva Pediatr. 2010;62(3 Suppl 1):51-4. [ Links ]
26. Bentlin MR, Ferreira GL, Rugolo LMS de S, Silva GHS, Mondelli AL, Rugolo Júnior A. Neonatal meningitis according to the microbiological diagnosis: a decade of experience in a tertiary center. Arq Neuropsiquiatr. 2010;68(6):882-887. [ Links ]
27. Zhu M-L, Mai J-Y, Zhu J-H, Lin Z-L. Clinical analysis of 31 cases of neonatal purulent meningitis caused by Escherichia coli. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr. 2012;14(12):910-912. [ Links ]
28. Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert A-A, McDonald J, et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017;140(1):1-8. Pediatrics. 2017 Jul;140(1). doi: 10.1542/peds.2017-0476. [ Links ]
29. Krebs VLJ, Costa GAM. Clinical outcome of neonatal bacterial meningitis according to birth weight. Arq Neuropsiquiatr. 2007;65(4b):1149-1153. [ Links ]
30. Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, et al. Neonatal cranial sonography: ultrasound findings in neonatal meningitis-a pictorial review. Quant Imaging Med Surg. 2017 Feb;7(1):123-131. doi: 10.21037/qims.2017.02.01. [ Links ]
Received: August 30, 2019; Accepted: April 29, 2020











 text in
text in