Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.17843/rpmesp.2020.373.4465
Original articles
In vitro inhibitory effect of aluminum phthalocyanine tetrasulfonate chloride against Leishmania (Viannia) Peruviana and Leishmania (Viannia) Braziliensis
1 Laboratorio de Ecología Microbiana, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Lima, Perú.
2 Laboratorio de Referencia Nacional de Leishmaniasis, Instituto Nacional de Salud, Lima, Perú.
3 Facultad de Ciencias de la Salud, Universidad Autónoma de Ica, Perú
4 Laboratorio de Investigación y Desarrollo de Química Orgánica, Universidad Nacional Mayor de San Marcos, Lima, Perú.
INTRODUCTION
Leishmaniasis is a vector-borne disease caused by a protozoan of the Leishmania genus; in the Americas, it is transmitted to mammals, including humans, by a phlebotomine of the genus Lutzomyia known as “white blanket” or “Titira” 1. Depending on the infecting Leishmania species and the host immune system, this disease produces ulcerative lesions, mucosal metastasis, and liver, spleen and pancreas damages, which can cause death when not treated in a timely manner 2.
The Peruvian Ministry of Health reported 5,808 cases of tegumentary leishmaniasis in 2018, of which 94% and 6% represented cutaneous and mucocutaneous cases, respectively, caused mainly by Leishmania (Viannia) peruviana, Leishmania (V.) braziliensis, Leishmania (V.) guyanensis, and Leishmania (Leishmania) amazonensis. Other unusual clinical forms reported in our country are disseminated cutaneous leishmaniasis caused by species such as Leishmania (Viannia) braziliensis, Leishmania (V.) guyanensis, Leishmania (V.) peruviana, and Leishmania (L.) amazonensis, and diffuse cutaneous leishmaniasis caused by Leishmania (L.) amazonensis. In Peru there are no reports of visceral leishmaniasis cases, which can be lethal if not detected on time.
The first line therapy for all Leishmania species are pentavalent antimonials, and the second line is amphotericin B therapy, both administered parenterally in long sessions. Due to the nature of these drugs, their use causes adverse effects, such as nausea, vomiting, myalgia, nephrotoxic effects, etc., which could result in treatment abandonment and increase the reporting of therapeutic failures due to incomplete or inadequate doses. Furthermore, their use is not recommended in pregnant women or in patients with arrhythmias 3 - 5. Likewise, miltefosine used as an oral treatment and recommended by the World Health Organization also presents side effects, in addition to increasing the cost of treatment for this disease ( 5 , 6. These limitations have stimulated the search for more effective therapeutic alternatives with fewer side effects.
Photodynamic therapy (PDT) can induce cell death by apoptosis and necrosis mediated by singlet oxygen and highly reactive toxic radicals (reactive oxygen species), which are produced by the interaction of the photosensitizing agent, a specific wavelength and molecular oxygen 7; these characteristics make it a potential alternative for the treatment of infectious diseases, including leishmaniasis 8 - 10. This study aims to evaluate the in vitro effect of a new photosensitizer against the promastigote and amastigote stages of Leishmania (V.) peruviana and Leishmania (V.) braziliensis, species of epidemiological relevance for public health in Peru.
KEY MESSAGES
Motivation for the study: Pentavalent antimonials used in the treatment of leishmaniasis cause therapeutic failures due to incomplete or inadequate doses, or treatment abandonment. Therefore, it is necessary to look for new less toxic therapeutic agents to replace or complement the current treatment.
Main findings: In an in vitro model, the photosensitizer aluminum phthalocyanine tetrasulfonate chloride (AlPcClS4) inhibits the growth of promastigotes and amastigotes of Leishmania (V.) peruviana and Leishmania (V.) braziliensis.
Implications: The inhibitory effect of photodynamic therapy on AlPcClS4 against Leishmania motivates to keep researching on the selectivity index through new formulations and to evaluate the appropriate vector that allows the highest absorption in in vivo models.
MATERIALS AND METHODS
A descriptive observational study was carried out in the laboratories of Microbial Ecology (Faculty of Biological Sciences) and Organic Chemistry (Faculty of Chemistry) of the Universidad Nacional Mayor de San Marcos, and in the National Reference Laboratory of Leishmaniasis of the Instituto Nacional de Salud del Perú (INS).
Macrophages and promastigotes cell line
The cell line trials were performed in 24-well plates (Corning, Costar Cat. COR-3524), to which a circular plate was added at the base. The DH82 dog macrophage cell line was provided by the INS Cell Culture Laboratory, cultured in a minimum essential medium (MEM) (Gibco Cat. 61100-061), supplemented with 15% inactivated fetal bovine serum (IFBS) (Gibco, Cat. 1079255) and antibiotics (Gibco, Cat. 15240-062), incubated at 37 °C, 5% CO2 and maintained by successive tapping every 72 hours.
Cultures of Leishmania (V.) peruviana and Leishmania (V.) braziliensis strains, cryopreserved at -70 ± 5 °C, were also used. For reactivation, Leishmania strains were defrosted and immediately transferred to a biphasic medium on blood agar with 15% rabbit blood, then to Schneider’s Drosophila liquid medium at pH 6.65-6.75 (Gibco, Cat. 21720024) supplemented with 20% IFBS 11 . 12, plus 150 µg/mL gentamicin and then incubated at 24 ± 1 °C, to obtain higher parasite mass. The reactivated strains were kept in Schneider’s medium at 10% with IFBS. The in vitro infection process in macrophages was carried out with metacyclic promastigotes obtained by induction at an acid pH of 5.5 with 10% IFBS and antibiotics.
The process of macrophage infection took place in 24-well plates of 1 × 105 macrophages/mL, in MEM with IFBS at 15%, and incubated at 37 °C and 5% of CO2 for 15 hours. Metacyclic promastigotes were added in a 20:1 ratio (parasite: macrophage) and incubated at 33 °C and 5% of CO2 for 5 hours. Finally, three washes were performed with 2% MEM and IFBS to eliminate non-phagocytic parasites.
Inhibitory activity assessment
A solution of chlorinated tetrasulfonate aluminum phthalocyanine (AlPcClS4) photosensitizer (Frontier Scientic) was prepared at an initial concentration of 1 mM plus 0.5% dimethyl sulfoxide (DMSO) (Applichem) in phosphate buffer saline (PBS) at pH 7.4 and filtered at 0.45 µm.
To determine the mean inhibitory concentration (IC50) of AlPcClS4, two independent trials were conducted, each one of them in triplicate. Regarding promastigotes, they were evaluated at the compound concentrations of 0; 25; 50; 75; 100; 200 and 350 μM in 3 mL of liquid medium with 5 × 105 parasites (T0) and incubated during 24 hours at 26 °C in a dark room. At the end, LED light was irradiated once at a specific wavelength of 675 nm, 30 J/s*m2 of power and at a distance of 10 cm for 30 minutes, allowing an irradiation power of approximately 5.4 J/cm2. Also, the inhibitory effect of phthalocyanine PDT on Leishmania promastigotes at 0, 24, 48, 72 and 96 hours was indirectly determined by the colorimetric MTT (Methyl Thiazole Tetrazolium) assay 13.
Regarding intracellular amastigotes, after the infection and washing process, 1 mL of MEM with IFBS at 15% and AlPcClS4 at concentrations of 50, 75, 100 and 200 µM was added, then the samples were incubated for 24 hours at 37 °C and 5% of CO2. Afterwards, they were irradiated with LED light and incubated again for 72 hours post-irradiation. The effect of the compound on the amastigote forms was determined by quantitative PCR.
The effect of AlPcClS4 with and without exposure to light was independently evaluated. Promastigotes and DH82 macrophages were used as growth control; and stibogluconate (Pentostam) at concentrations of 100, 200 and 2 × 104 μM for promastigote trials and 200 μM for intracellular amastigotes, 0.01% triton was used as treatment control.
MTT colorimetric assay
The MTT colorimetric assay was developed according to the methodology described by Mesa et al. 14 with the following modifications: the promastigotes treated at different concentrations of phthalocyanine and then irradiated were collected in sterile conical tubes of 1.5 mL; the parasites were centrifuged at 3,500 rpm; the supernatant was carefully discarded; then 450 μL of liquid medium and 50 μL of MTT (5 mg/mL) were added, and then he samples were incubated for 4 hours at 32 °C. Finally, to stop the formation of formazan crystals, a 10% solution of sodium dodecyl sulfate (SDS), 0.01 N HCl 14 was added to the wells. The samples were read at an absorbance of 570 nm in the Eon microplate spectrophotometer (Biotek).
DNA extraction and quantification of the parasitic load
The infected and treated macrophages, 72 hours post-irradiation, were washed with sterile PBS at pH 7.2 to remove remnants from the culture medium and from the DNA extraction. 100 μL of trypsin-EDTA was added to the well and incubated for 10 minutes to detach the cells from the slides. Then, 100 μL of PBS was added, the cell suspension was collected and placed in 1.5 mL conical tubes to extract nucleic acids, by using the PureLink® Genomic DNA Kit (Invitrogen, Cat. K1820-02), and the samples were eluted in a final volume of 60 μL.
The inhibitory effect of photodynamic treatment in infected macrophages was measured by quantifying the parasitic load by qPCR 15 , 16. Oligonucleotides Leis.L1 5'-GACGCACCCCTCCAA-3' and Leis.U1 5'-AAGTGCTTCCCATCGCAACT-3' were used as PCR initiators, and Leis.P1 FAM 5'-CGGTTCGGTGTGTGCGCC-3' TAMRA as a marked probe; the methodology is described in Wortmann et al. 15
The standard curve was made using Leishmania (V.) braziliensis genomic DNA at a concentration of 415.75 ng equivalent to 5 × 106 parasites/µL (120 parasites = 10 pg of DNA) and dilutions equivalent to 106, 105, 104, 103, 102, 10, 10-1 parasites. The final volume was 20 µL, and consisted of 1X Kapa Probe Fast qPCR Master Mix (Kapa Biosystems, Cat. KK4701), 0.2 µmol/µL of each primer, 0.04 µmol/µL of the probe and 5 µL of DNA from the samples. DNA was amplified by an initial denaturation of 95 °C for 30 seconds to activate the enzyme, followed by 40 denaturation repeats at 95 °C for 30 seconds and hybridization/extension at 60 °C for 30 seconds.
Statistical analysis
The results mean and standard deviation were determined with Microsoft Excel 2010. Likewise, the inhibition percentage was calculated from the optical densities found in the MTT assay, using the formula 17:
Additionally, the QQ plot and Shapiro-Wilk test were done to evaluate the normality and homogeneity of the residual values of the data using a scatter plot. FDT measurements were compared according to 0, 25, 50, 75, 100, 200 and 350 µM AlPcClS4 concentrations on Leishmania (V.) peruviana and Leishmania (V.) braziliensis using ANOVA and Tukey’s test, with a α = 0.05. IC50 was also determined against Leishmania promastigote.
RESULTS
The effect of FDT on Leishmania was quantified by the MTT assay. For Leishmania (V.) peruviana, control therapy presented an OD570 of 0.27 ± 0.04; for 100 µM the OD570 was 0.22 ± 0.05, for 200 µM the OD570 was 0.15 ± 0.03, and for 350 µM the OD570 was 0.14 ± 0.02 (Figure 1). After 24 hours of 50 µM photosensitizer radiation, this species presented a 26% inhibition of parasitic growth compared to the control sample; at concentrations of 100, 200 and 350 µM it showed a parasitic growth inhibition of 65.6%, 72.9% and 73.9%, respectively.
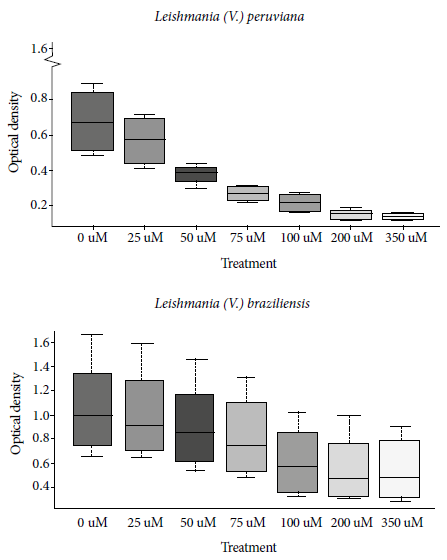
Figure 1 Mean optical density and standard deviation of the formazan crystals’ quantification according to the MTT (Methyl Thiazole Tetrazolium) assay, at different concentrations of phthalocyanine in Leishmania (V.) peruviana and Leishmania (V.) braziliensis
At 96 hours post-radiation the inhibition percentage reached 68.9%; 78.8% and 80.1%, respectively. At 24, 48, 72 and 96 hours was, the IC50 was 56.5; 50; 44; and 39.7 µM respectively (Figure 2). Regarding the intracellular form, at 72 hours post-exposure the photosensitizer concentrations of 50, 75, 100 and 200 µM reached an inhibition of the Leishmania (V.) peruviana amastigotes of 20.5%; 46.5%; 65.2% and 57.8%, respectively (Table 1).
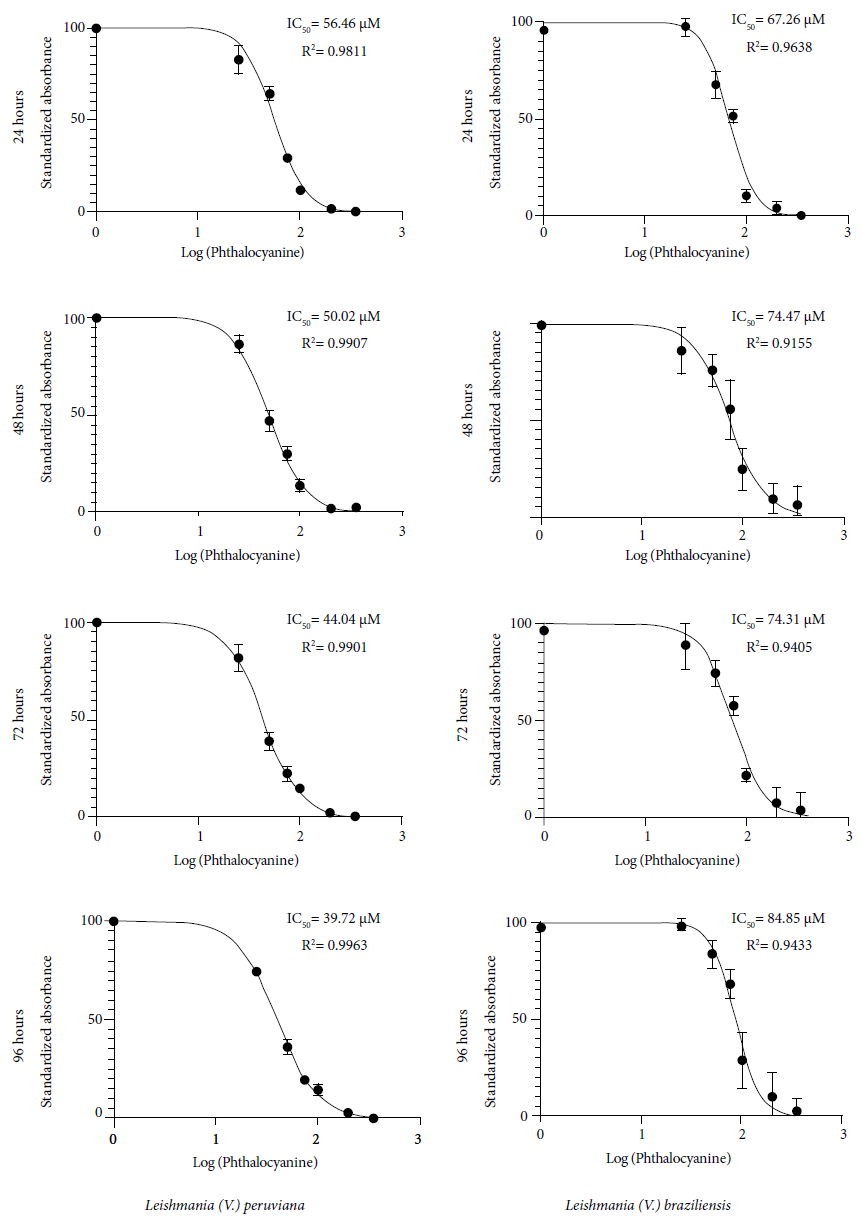
Figure 2 Mean inhibitory concentration (IC50) of aluminum phthalocyanine chloride in photodynamic therapy on Leishmania (V.) peruviana and Leishmania (V.) braziliensis, evaluated at 24, 48, 72 and 96 hours post-irradiation.
Table 1 Determination of mean inhibitory concentration (IC50) and selectivity index of aluminum phthalocyanine tetrasulfonate chloride and sodium stibogluconate against promastigote and amastigote stages of Leishmania (V.) peruviana and Leishmania (V.) braziliensis.
| Species | Hours | Inhibition % | IC50 | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 µM | 50 µM | 75 µM | 100 µM | 200 µM | 350 µM | ||||
| Phthalocyanine | |||||||||
| Promastigotes | |||||||||
| 24 | 12.89 | 26.09 | 52.47 | 65.60 | 72.88 | 73.90 | 56.46 | 53.49 - 59.36 | |
| L. (V.) peruviana | 48 | 10.24 | 40.89 | 53.53 | 66.15 | 75.97 | 76.51 | 50.02 | 48.30 - 51.75 |
| 72 | 14.53 | 48.67 | 61.81 | 67.71 | 78.40 | 79.77 | 44.04 | 42.42 - 45.69 | |
| 96 | 20.86 | 51.33 | 64.86 | 68.91 | 78.78 | 80.61 | 39.72 | 38.75 - 40.70 | |
| 24 | 0.81 | 16.45 | 25.68 | 49.60 | 53.43 | 55.50 | 67.26 | 63.60 - 70.94 | |
| L. (V.) braziliensis | 48 | 5.43 | 14.12 | 26.13 | 44.00 | 52.93 | 54.70 | 74.47 | 67.86 - 81.47 |
| 72 | 2.73 | 11.24 | 19.84 | 37.75 | 44.87 | 47.00 | 74.31 | 69.13 - 79.66 | |
| 96 | 3.21 | 10.09 | 17.40 | 34.75 | 42.56 | 44.38 | 75.88 | 71.74 - 80.07 | |
| DH82 Macrophages | |||||||||
| L. (V.) peruviana | 72 | ND | 20.52 | 46.45 | 65.16 | 57.78 | ND | 60.28 | 56.78 - 63.80 |
| L. (V.) braziliensis | 72 | ND | 4.15 | 9.04 | 20.31 | 36.90 | ND | 94.92 | 90.80 - 99.63 |
| Sodium stibogluconate | |||||||||
| Promastigotes | |||||||||
| 24 | ND | ND | ND | 35.75 | 45.70 | 98.13 a | 216.4 | 196.3 - 240.8 | |
| L. (V.) peruviana | 48 | ND | ND | ND | 35.25 | 41.94 | 98.36 a | 250.1 | 215.4 - 295.6 |
| 72 | ND | ND | ND | 33.25 | 48.58 | 98.43 a | 202.1 | 182.7 - 228.3 | |
| 96 | ND | ND | ND | 46.45 | 49.04 | 98.35 a | 152.3 | 127.4 - 181.3 | |
| 24 | ND | ND | ND | 21.40 | 31.73 | 96.88 a | 389.4 | 325.2 - 471.2 | |
| L. (V.) braziliensis | 48 | ND | ND | ND | 21.39 | 30.38 | 96.02 a | 408.4 | 288.4 - 604.2 |
| 72 | ND | ND | 27.01 | 37.19 | 96.36 a | 310 | 257.9 - 381.3 | ||
| 96 | ND | ND | ND | 30.93 | 33.24 | 97.52 a | 355.1 | 276.0 - 470.8 | |
| DH82 Macrophages | |||||||||
| L. (V.) peruviana | 72 | ND | ND | ND | ND | 54.56 | ND | ND | ND |
| L. (V.) braziliensis | 72 | ND | ND | ND | ND | 50.23 | ND | ND | ND |
a Evaluated at 2 × 104 μM pentavalent antimonials. IC50: mean inhibitory concentration; 95% CI: 95% confidence interval. ND: not determined.
The values regarding the PDT effect on Leishmania promastigotes were analyzed independently for each species. Each assay was composed by 4 evaluation times performed in triplicate at 7 different concentrations, a total of 28 treatments per species were analyzed. Data obtained for Leishmania (V.) peruviana and Leishmania (V.) braziliensis presented a normal distribution (p = 0.1041 and 0.1036, respectively). Comparison of means by ANOVA allowed to determine that there is a statistically significant difference between treatments in both Leishmania species (p = 0.0001).
PDT in Leishmania (V.) peruviana with 200 and 350 µM of AlPcClS4 did not present statistically significant differences (p > 0.05) by using Tukey’s test (α = 0.05), evaluating each one at 24 and 48 hours. Similarly, these same concentrations evaluated at 72 and 96 hours post-exposure did not present statistically significant differences either (p > 0.05) (Figure 3).
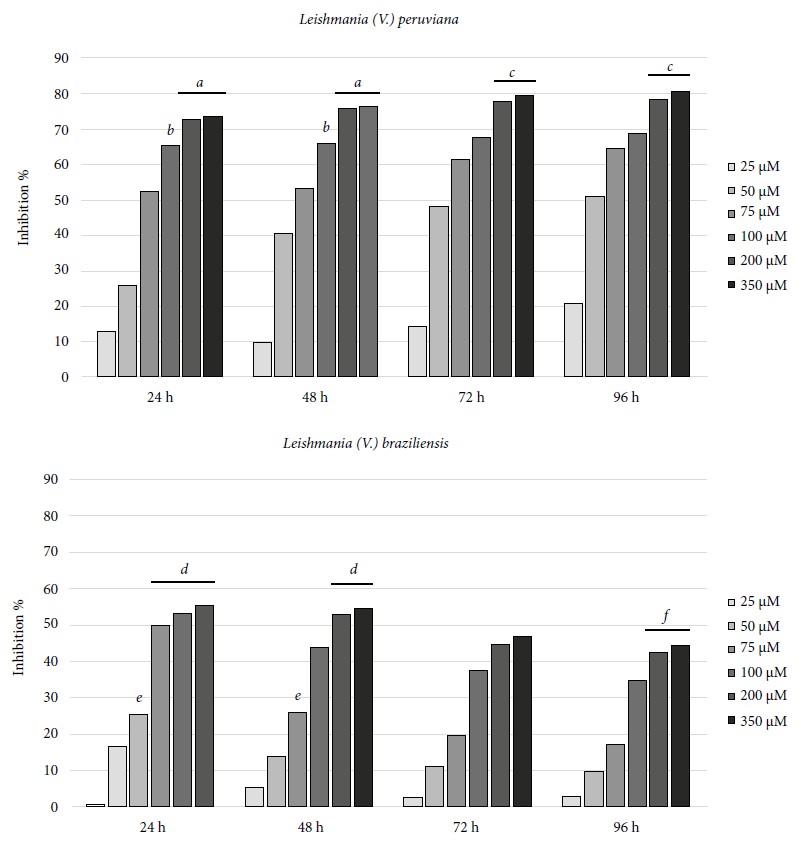
Figure 3 Inhibition of Leishmania parasitic growth, by exposure to photodynamic therapy with aluminum phthalocyanine tetrasulfonate chloride (a, b, c, d, e, and f, represent treatments that do not present statistically significant differences, Tukey p test > 0.05).
Treatment only with irradiation at 670 nm and at 5.4 J/cm2, with and without exposure to the photosensitizing agent, did not show any phototoxic effect compared to the growth control.
DISCUSSION
This study evaluated the phototoxic effect of aluminum phthalocyanine tetrasulfonate chloride in an in vitro phototherapy assay on Leishmania (V.) braziliensis and Leishmania (V.) peruviana promastigotes, by using the MTT assay, which allowed the quantification of formazan crystals as a result from the enzymatic activity of the mitochondrial dehydrogenase enzyme of the living parasites 13. The photodynamic treatment consisted in LED light irradiation at a wavelength of 670 nm and potence of 5.4 J/cm2 on Leishmania promastigotes in culture medium with AlPcClS4 concentrations of 25; 50; 75; 100; 200 and 350 µM, according to the methodology described by Amin et al. 7 on neoplastic cells. Regarding amastigotes, the assessment of the effect by PDT was carried out with quantitative PCR.
Phthalocyanines are chemically stable organic compounds that have received particular attention in phototherapy as a sensitizing agent mainly in the treatment of infectious diseases due to their low toxicity and their biochemical properties that improve the humoral and cellular response to the infectious agent or target cells in case of neoplasia. They also participate in the production of oxidative molecules such as reactive oxygen species and singlet oxygen, which act directly on aminoacid residues of proteins and enzymes, such as cysteine, methionine, tryptophan, among others, related to the virulence of the pathogen 10. Studies related to PDT as an alternative to leishmaniasis treatment have found promising levels of IC50 when evaluating several photosensitizers such as zinc phthalocyanine (ZnPc), aluminum phthalocyanine (AlPc) and aluminum tetrasulfonate (AlPcS4), among others, on amastigotes and promastigotes of Leishmania (V. ) braziliensis, Leishmania (V.) panamensis, Leishmania (L.) amazonensis, Leishmania (L.) chagasi, Leishmania (L.) major and Leishmania (L.) tropica 8 , 9 , 18 - 20. These species cause cutaneous and mucocutaneous lesions 1.
Pinto et al. 9 found that in vitro photodynamic therapy evaluated on the fourth day post-irradiation, that used AlPcClS4 concentrations of 1 and 10 μM and irradiated at a length of 659 nm on Leishmania (V.) braziliensis promastigote, achieved an inhibition of approximately 42% and 49%, respectively. On the ninth day post-irradiation, an inhibition of 34% and 41.3%, respectively, was achieved. In Leishmania (L.) major, evaluated under the same conditions, the inhibition effect at the fourth day post-irradiation was 50% for both concentrations, while at the ninth day post-irradiation inhibition was 32% and 41% corresponding to concentrations of 1 and 10 μM, respectively.
Hernandez 21 evaluated aluminum phthalocyanine chloride (AlPcCl) and aluminum phthalocyanine disulfonate chloride (AlPcClS2) on Leishmania (L.) amazonensis by using an irradiation of 3 J/cm2 and reported an IC50 of 0.046 µM ± 0.018 and an IC50 of 4.101 µM ± 0.136, respectively. Likewise, Escobar et al. 20 used AlPcCl with an irradiation of 10.0 J/cm2 at 670 nm and, at 24 hours post-irradiation, and reported an IC50 of 0.0033 and 0.17 μM in promastigotes of Leishmania (L.) chagasi and Leishmania (V.) panamensis, respectively. Also, Zinc phthalocyanine (ZnPc) under the same conditions and with the same evaluated species, presented an IC50 of 6.45 μM and 6.05 μM, respectively. In our study, we have used the same time interval and the IC50 found was 56.5 µM and 67.26 µM for Leishmania (V.) peruviana and Leishmania (V.) braziliensis, respectively.
It is likely that the heterogeneous PDT results are due to the time and fluency of irradiation, the wavelength used, the nature of the compound used as photosensitizer (isomers), the physicochemical properties related to cell internalization and molecular charge, these variables together with the quantum yield of singlet oxygen, the subcellular location, the sulfonation degree, the size of the molecule, the variety of metal ions, the absorption of light, the affinity for the target tissue, the selectivity by cellular compartments, such as the mitochondria and the irradiation power achieved in the cells, could influence the ability of the compound to cross the lipid bilayer of the parasite’s cell membrane and reduce the effects and efficiency of the photodynamic treatment 22.
In this study, similar to Amin et al. 7 description, the irradiation alone had no effect on promastigotes, infected macrophages or free macrophages. Likewise, the photosensitizer without exposure to LED light radiation was not toxic in any of the concentrations used, which is consistent with the principle of PDT, where the independent application of the compound or irradiation has no effect on cell viability 23.
It was not possible to determine the selectivity index (SI), a useful parameter to estimate the effectiveness of a compound on a given pathogen 24, neither the mean lethal concentration (LD50) on the cell line containing the intracellular form of Leishmania, important parameter for the determination of the SI, which constitutes the main limitation of the study.
Likewise, PDT could improve the performance of conventional therapy, which implies several possibilities regarding the management of tegumentary leishmaniasis, such as dose reduction, application time of conventional therapies, species-specific therapy, etc.; as a result, side effects could be reduced 25 , 26. However, it is still necessary to carry out studies that determine the risk of disease reactivation or relapse and the inadequate response to treatment; it is also important to conduct studies related to evaluating strategies that allow improving the efficiency of treatment through exposure to more than one cycle of irradiation. In this study, a single irradiation with LED light for 30 minutes was used, which is probably not sufficient to affect all parasites. In addition, it is important to carry out research studies related to formulating, developing, and evaluating the effectiveness and selectivity index in cell lines and animal models to estimate the compound efficiency on the parasite.
In conclusion, AlPcClS4 as a PDT photosensitizer showed a constant anti-Leishmania effect mainly on Leishmania (V.) peruviana promastigotes and, in a smaller proportion in Leishmania (V.) braziliensis, the latter started a recovery process after 72 hours post-treatment, unlike Leishmania (V.) peruviana that showed a higher sensitivity to treatment, without a recovery process until 96 hours post-treatment. In the amastigote forms the phototoxic effect was lower, but the response of DH82 macrophages infected with Leishmania (V.) peruviana was higher compared to macrophages infected with Leishmania (V.) braziliensis.
Acknowledgements
To the National Innovation Program for Competitiveness and Productivity - INNOVATE-PERU (Agreement No. 177-PNICP-PIAP-2015) for funding this research. To the National Public Health Center of the Instituto Nacional de Salud, for the facilities provided.
REFERENCES
1. Ministerio de Salud. Leishmaniasis. Módulos Técnicos. Serie de Documentos Monográficos [Internet]. Lima: MINSA; 2000. 83 p. Disponible en: http://bvs.minsa.gob.pe/local/OGEI/795_MS-OGE106.pdf. [ Links ]
2. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [ Links ]
3. Murray HW. Leishmaniasis in the United States: Treatment in 2012. Am J Trop Med Hyg. 2012;86(3):434-40. doi: 10.4269/ajtmh.2012.11-0682. [ Links ]
4. Kedzierski L. Leishmaniasis vaccine: Where are we today?. J Glob Infect Dis. 2010;2(2):177-85. doi: 10.4103/0974-777X.62881. [ Links ]
5. De Menezes JPB, Guedes CES, De Oliveira Almeida Petersen AL, Fraga DBM, Veras PST. Advances in development of new treatment for leishmaniasis. Biomed Res Int. 2015;2015:815023. doi: 10.1155/2015/815023. [ Links ]
6. Soto J, Soto P. Miltefosina oral para el tratamiento de la leishmaniasis. Biomedica. 2006;26 Suppl 1:207-17. [citado el 15 de abril de 2019];26(1):10.7705/biomedica.v26i1.1514. Disponible en: https://revistabiomedica.org/index.php/biomedica/article/view/1514/1645. [ Links ]
7. Amin RM, Hauser C, Kinzler I, Rueck A, Scalfi-Happ C. Evaluation of photodynamic treatment using aluminum phthalocyanine tetrasulfonate chloride as a photosensitizer: New approach. Photochem Photobiol Sci. 2012;11(7):1156-63. doi: 10.1039/c2pp05411f. [ Links ]
8. Dutta S, Kolli BK, Tang A, Sassa S, Chang KP. Transgenic Leishmania model for delta-aminolevulinate-inducible monospecific uroporphyria: Cytolytic phototoxicity initiated by singlet oxygen-mediated inactivation of proteins and its ablation by endosomal mobilization of cytosolic uroporphyrin. Eukaryot Cell. 2008;7(7):1146-57. doi: 10.1128/EC.00365-07. [ Links ]
9. Pinto JG, Soares CP, Mittmann J. Assessment of Leishmania major and Leishmania braziliensis promastigote viability after photodynamic treatment with aluminum phthalocyanine tetrasulfonate (AlPcS4). J Venom Anim Toxins Incl Trop Dis. 2011;17(3). doi: 10.1590/S1678-91992011000300010. [ Links ]
10. Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections-State of the art. Photodiagnosis Photodyn Ther. 2009;6(3-4):170-88. doi: 10.1016/j.pdpdt.2009.10.008. [ Links ]
11. Ponte-Sucre A, Díaz E, Padrón-Nieves M. Drug Resistance in Leishmania Parasites [Internet]. Viena: Springer Verlag Wien; 2013 [citado el 12 de abril de 2019]. Disponible en: https://link.springer.com/book/10.1007/978-3-7091-1125-3#toc. [ Links ]
12. Gamboa D, Torres K, De Doncker S, Zimic M, Arévalo J, Dujardin J-C. Evaluation of an in vitro and in vivo model for experimental infection with Leishmania (Viannia) braziliensis and L . (V.) peruviana. Parasitology. 2008;135(3):319-26. doi: 10.1017/S0031182007003848. [ Links ]
13. Brito S, Crescente O, Fernández A, Coronado A, Rodriguez N. Eficacia de un ácido kaurénico extraído de la planta venezolana Wedelia trilobata (Asterácea ) contra Leishmania (Viannia) braziliensis. Biomedica. 2006; 26 Suppl 1:180-7. [citado el 12 de junio de 2019];26:180-7. Disponible en: http://www.scielo.org.co/pdf/bio/v26s1/v26s1a19.pdf. [ Links ]
14. Mesa AM, Molano PA, Seon B, Figadere B, Robledo SM, Muñoz DL, et al. Síntesis y actividades Leishmanicida y citotóxica in vitro de análogos 2-Arilquinolonas. Rev la Fac Química Farm [Internet]. 2008 [citado el 12 de abril de 2019];15(2):259-66. Disponible en: http://www.scielo.org.co/pdf/vitae/v15n2/v15n2a08.pdf. [ Links ]
15. Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J, et al. Rapid diagnosis of Leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg. 2001;65(5). doi: 10.4269/ajt-mh.2001.65.583. [ Links ]
16. Gomes LI, Gonzaga FM, Morais-Teixeira E de, de Souza-Lima BS, Freire VV, Rabello A. Validation of quantitative real-time PCR for the in vitro assessment of antileishmanial drug activity. Exp Parasitol. 2012;131(2):175-9. doi: 10.1016/j.exppara.2012.03.021. [ Links ]
17. George SA, Bhadran S, Sudhakar M, Harini BP. Comprehensive in vitro evaluation of pharmacological activities of selected mass spectrometry profiling of flacourtia jangomas flower extract. Asian J Pharm Clin Res. 2017 [citado el 12 de abril de 2019];10(5):237-44. Disponible en: https://innovareacademics.in/journals/index.php/ajpcr/article/view/17419/10818. [ Links ]
18. Peloi LS, Biondo CEG, Kimura E, Politi MJ, Lonardoni MVC, Aristides SMA, et al. Photodynamic therapy for American cutaneous leishmaniasis: The efficacy of methylene blue in hamsters experimentally infected with Leishmania (Leishmania) amazonensis. Exp Parasitol [Internet]. 2011;128(4). doi: 10.1016/j.exppara.2011.04.009. [ Links ]
19. Fink C, Toberer F, Enk A, Gholam P. Effective treatment of cutaneous leishmaniasis caused by Leishmania tropica with topical photodynamic therapy. JDDG. 2016;14(8). doi: 10.1111/ddg.13082. [ Links ]
20. Escobar P, Hernández IP, Rueda CM, Martínez F, Páez E. Photodynamic activity of aluminium (III) and zinc (II) phthalocyanines in Leishmania promastigotes. Biomedica. 2006 [citado el 27 de junio de 2017];26 Suppl 1(Iii):49-56. Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/17361841 [ Links ]
21. Hernández Peñaranda IP. Actividad fototóxica in vitro e in vivo de ftalocianina de aluminio clorada contra Leishmania amazonensis [Tesis]. Bucaramanga: Universidad Industrial de Santander; 2010. [ Links ]
22. Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part one - Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004 [citado el 6 de marzo de 2019];1(4):279-93. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4108220. [ Links ]
23. Aureliano DP, Ribeiro MS, Lauretta Lindoso JA, Pogliani FC, Sellera FP, Song D, et al. Treatment and Control of Leishmaniasis Using Photodynamic Therapy. En: Leishmaniasis - Trends in Epidemiology, Diagnosis and Treatment [Internet]. 2014 [citado el 17 de abril de 2019]. p. 393-412. Disponible en: https://www.intechopen.com/books/leishmaniasis-trends-in-epidemiology-diagnosis-and-treatment/treat-ment-and-control-of-leishmaniasis-using-photodynamic-therapy. [ Links ]
24. Sánchez-Suárez J, Albarracín D, Rojas M, Rincón J, Delgado G. Evaluación de la actividad citotóxica y leishmanicida de extractos y fracciones de Piper cumanense y Piper holtonii Resumen Evaluation of the cytotoxic and leishmanicidal activity of extracts Introducción. Rev Colomb Cienc Quím Farm. 2010 [citado el 20 de marzo de 2019] 39. Disponible en: https://revistas.unal.edu.co/index.php/rccquifa/article/view/22998. [ Links ]
25. Ribeiro JBP, Miranda-Vilela AL, Amorim AAS, Garcia RD, Moreira JR, Gomes CM, et al. Study of the efficacy of N-methyl glucamine antimoniate (SbV) associated with photodynamic therapy using liposomal chloroaluminium phthalocyanine in the treatment of cutaneous leishmaniasis caused by Leishmania (L.) amazonensis in C57BL6 mice. Photodiagnosis Photodyn Ther. 2019;26:261-9. doi: 10.1016/j.pdpdt.2019.04.004. [ Links ]
26. Ribeiro JBP, Miranda-Vilela AL, Graziani D, Gomes MR de A, Amorim AAS, Garcia RD, et al. Evaluation of the efficacy of systemic miltefosine associated with photodynamic therapy with liposomal chloroaluminium phthalocyanine in the treatment of cutaneous leishmaniasis caused by Leishmania (L.) amazonensis in C57BL/6 mice. Photodiagnosis Photodyn Ther. 2016;13:282-90. doi: 10.1016/j.pdpdt.2015.08.006. [ Links ]
Funding: The study was funded by the National Innovation Program for Competitiveness and Productivity - INNOVATE-PERU (Agreement No. 177-PNICP- PIAP-2015) of the Ministry of Production, Lima, Peru.
Cite as: Izarra-Rojas KV, Rojas-Palomino N, Gonzales-Medrano JL, Minaya-Gómez G, Berrocal-Huallpa A, Santiago-Contreras J, et al. In vitro inhibitory effect of aluminum phthalocyanine tetrasulfonate chloride against Leishmania (Viannia) peruviana and Leishmania (Viannia) braziliensis. Rev Peru Med Exp Salud Publica. 2020;37(3):462-70. doi: https://doi.org/10.17843/rpmesp.2020.373.4465.
10This study is part of the thesis: Izarra Rojas KV. Actividad fotodinámica in vitro de ftalocianina de aluminio tetrasulfonada clorada (AlPcClS4) frente a estadios extracelular e intracelular de Leishmania (Viannia) peruviana, L. (V.) braziliensis y L. (Leishmania) amazonensis, (Thesis). Lima: Universidad Nacional Mayor de San Marcos; 2018.
Received: April 16, 2019; Accepted: June 17, 2020











 text in
text in 



