Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Medicina Experimental y Salud Publica
versión impresa ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.3 Lima jul-sep 2020
http://dx.doi.org/10.17843/rpmesp.2020.373.5157
Original articles
Cytotoxic activity of the chloroform fraction of Piper aduncum and its effect on the cell cycle in gastric cancer cell lines
1 Laboratorio de Cultivo Celular e Inmunología, Universidad Científica del Sur, Lima, Perú.
2 Instituto de Medicina Regenerativa, Universidad Científica del Sur, Lima, Perú.
3 Laboratorio de Química y Bioquímica de los Productos Naturales, Universidad Científica del Sur, Lima, Perú.
INTRODUCTION
Gastric cancer is the third most frequent cause of cancer death worldwide 1 and the leading cause in Peru 2. It is usually detected in advanced stages where effective treatment is almost impossible. Since chemotherapy side effects are highly toxic, it has become urgent to seek new drugs with greater specificity against cancer cells, greater overall effectiveness and fewer side effects.
Medicinal plants are being studied as a source of new chemotherapeutic products. Nowadays, about 60% of the drugs used to treat cancer are derived from plants, for example, paclitaxel, initially obtained from Taxus brevifolia Nutt. 3; camptotecin, from Captotheca acuminata 4; etoposide, from Podophyllum species 5; vincristine, from Catharanthus roseus 6; and colchicine, from Colchicum autumnale 7.
The Piper genus consists of 700 species found in different places across the world. Most of these species have several positive effects on health, such as gastrointestinal and liver protection 8. In addition, many species of the Piper genus have shown cytotoxic activity against cell lines from breast, prostate, ovarian, pancreas, liver, colon and cervical cancer among others 9.
It has been reported in previous studies that the ethanolic extract from the Piper aduncum species presents cytotoxic activity against MCF-7 (breast cancer) and NCI-H460 (lung carcinoma) cells 10. However, the effect of the chloroform fraction from the Piper aduncum methanolic extract (PAMoCl) on gastric cancer cells has not been reported yet. Therefore, the aim of this study was to evaluate the cytotoxic activity of PAMoCl and its effect on the cell cycle in two gastric cancer cell lines: AGS and KATO III.
KEY MESSAGES
Motivation for the study: Due to the high mortality rate of gastric cancer in Peru, it is important to know if metabolites with cytotoxic activity against this type of cancer can be found in our environment.
Main findings: The chloroform fraction of Piper aduncum has cytotoxic activity, which causes the cell cycle to stop in the G2/M phase in 2 gastric cancer cell lines, one of them being the metastatic cell line.
Implications: Since the chloroform fraction has cytotoxicity against a metastatic gastric cancer cell line, identification of the metabolites responsible for this activity would be necessary for new treatments against metastatic gastric cancer.
MATERIALS AND METHODS
Preparation of the chloroform fraction from Piper aduncum
Piper aduncum leaves were collected from the lower Kimiri area, district of La Merced, province of Chanchamayo, department of Junin, at coordinates 11°02'16.5 "S 75°18'54.0 "W. Leaves were collected in the wild, and one sample was taxonomically identified as Piper aduncum L. by the natural history museum of the Universidad Nacional Mayor de San Marcos. The preparation of the Piper aduncum methanolic extract (PAMo) and the Piper aduncum chloroform fraction (PAMoCI) was completed under standardized protocols at the laboratory of Chemistry and Biochemistry of Natural Products of the Universidad Científica del Sur 11.
The leaves were cleaned and dried at 40 °C during two days, then crushed and sieved with a 1 mm mesh. Then 70 g of powdered leaves were weighed, 300 mL of methanol was added and filtered using a Whatman No. 1 filter paper. This procedure was repeated eight times, from which the last three were sonicated for 2 hours. The entire PAMo was filtered using a 0.44 µm membrane. To obtain the PAMoCI, the PAMo was concentrated to 200 mL and extracted by adding 300 mL of chloroform in a 1 L decanting tube. This process was repeated 8 times. The PAMoCl was concentrated at reduced pressure with a rotary evaporator. The PAMo and the PAMoCl were analyzed by thin-layer chromatography (TLC) using silica gel 60 for the stationary phase and benzene-acetone 8:1 as the mobile phase; and it was then developed with iodine and ultraviolet (UV) light 12. Complementary tests were conducted to identify chemical groups for both PAMo and PAMoCl according to protocols described by Lock 13. Finally, the PAMoCl was treated to a concentration of 32 mg/mL in dimethyl sulfoxide (DMSO) and stored at -80 °C for later use. The analytical solvents were purchased from Merck.
Cell line culture
The human gastric cancer cell lines AGS (primary) and KATO III (metastatic) were acquired from the European Authenticated Cell Culture Collection (ECACC 89090402 and 86093004), and cell line 293T (ATCC® CRL-3216TM) from the laboratory of Molecular Genetics of the Universidad Cientifica del Sur.
DMEM-F12 medium (Biowest), supplemented with 10% fetal bovine serum and 1% antibiotic-antifungal solution (full DMEM-F12), was used to culture AGS and 293T cells. RPMI medium (Biowest) supplemented with 20% fetal bovine serum and 1% antibiotic-antifungal solution (Biowest) was used to culture the KATO III cell line. All cells were incubated at 37 °C with 5% CO2 and subcultured when confluence was 70-80%.
Cellular viability assay
The cell viability assay was performed as previously described 14. AGS and 293T cells were counted using a Neubauer chamber and 5 × 103 cells/well were seeded in 96-well plates. KATO III cells were seeded in a quantity of 104 cells/well. All cells were incubated for 12 hours, then treated with 1.25 µg/mL, 2.5 µg/mL, 5 µg/mL, 10 µg/mL, 20 µg/mL, 40 µg/mL, 80 µg/mL, and 160 µg/mL of PAMoCl, and incubated again for 24 and 48 hours. In addition, a control group with the vehicle (DMSO at 0.5%), not the PAMoCl, was included. For cell viability assay, 20 µL of resazurine (0.15 mg/mL) was added to each well and incubated for 3 hours. Finally, the 96-well plates were read with a Synergy LX (Biotek) multimodal plate reader by spectrophotometry at wavelengths of 570 nm and 600 nm. The PAMoCl concentration, that causes 50% cell growth inhibition (GI50) with respect to the control group growth, was calculated.
Cellular morphology observation
Changes in cell morphology after incubation of AGS, KATO III and 293T cells with PAMoCl were observed and photographed on an inverted phase-contrast microscope (Nikon Eclipse TI) after 24 and 48 hours.
Cellular cycle evaluation by flow cytometry
The Darzynkiewicz et al. protocol 15, with slight modifications, was used to evaluate the effect of PAMoCl on the cell cycle. A total of 350,000 AGS and 500,000 KATO III cells were seeded in 100 × 15 mm petri dishes with DMEM-F12 and RPMI media. After 24 hours, the culture medium was replaced with a new DMEM-F12 medium and a complete RPMI containing PAMoCl at concentrations of 19.62 and 39.23 µg/mL for AGS cells and 87.49 and 160 µg/mL for KATO III cells; these concentrations were obtained from the cell viability assay. All plates were incubated for 24 hours at 37 °C and 5% CO2. After 24 hours, the plates were washed twice with phosphate buffer saline (PBS) 1X and trypsinized for 5 minutes. The resuspended cells were collected by centrifugation and fixed with cold 70% ethanol; then they were incubated at 4 °C for 30 minutes. Afterwards, the cells were stained with a propidium iodide solution (50 µg/mL) and RNase (100 µg/mL) for an additional 30 minutes and immediately analyzed in the Guava EasyCyte flow cytometer (Merck).
Data analysis
The absorbance data obtained from the cell viability assay were exported to a Microsoft Excel file and expressed as percentages with respect to the control group. For the dose-response relationship and the calculation of GI50, a non-linear regression model was used. The significant differences between groups were compared using the one-way ANOVA test with the Tukey test, as a post hoc test (p < 0.05), using the GraphPad Prism program. The cell cycle results were analyzed using the FCS 7 Express program (DeNovo solutions). The analyzed data are the result of three independent experiments.
RESULTS
The presence of metabolites in PAMo and PAMoCl was evidenced by TLC (Figure 1). The identification of the chemical groups are detailed in Table 1.
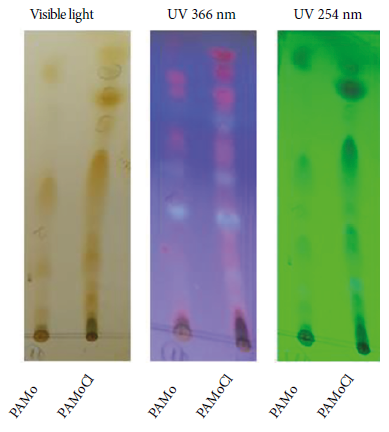
Figure 1 Thin layer chromatography profile of Piper aduncum methanolic extract and chloroform fraction of Piper aduncum methanolic extract revealed with iodine (visible light) and UV light. Mobile phase: benzene-acetone 8:1. Stationary phase: silica gel 60.
Table 1 Metabolite groups present in Piper aduncum methanolic extract and its chloroform fraction
| Metabolite group | Test | PAMo | PAMoCl |
|---|---|---|---|
| Phenolics | FeCl3 | Positive | Positive |
| Flavonoids | Shinoda | Positive | Positive |
| Anthocyanins | Rosenhein | Positive | Positive |
| Triterpenoids and steroids | Lieberman-Buchard | Positive | Positive |
PAMoCl: chloroform fraction of Piper aduncum methanolic extract
PAMo: methanolic extract of Piper aduncum
Effect of the chloroform fraction of the Piper aduncum methanolic extract on cell viability
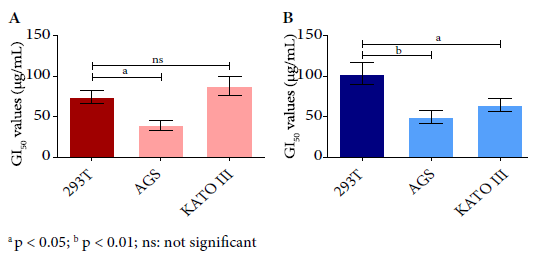
In the cell viability assay, all evaluated cell lines showed cytotoxic activity of PAMoCl with the following GI50 results at 24 hours: 39.23 µg/mL, 87.49 µg/mL and 74.10 µg/mL; and at 48 hours 49.47 µg/mL; 64.68 µg/mL and 101.8 µg/mL for AGS, KATO III and 293T cell lines, respectively (Figure 2). At 48 hours, PAMoCl GI50 was significantly lower for AGS and KATO III lines compared to 293T (cytotoxicity control) with values of p < 0.01 and p < 0.05, respectively (Figure 3).
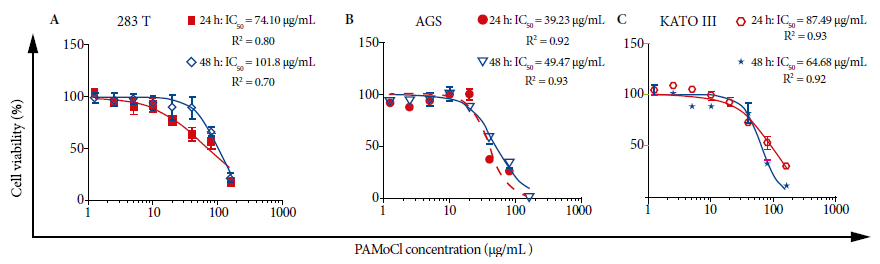
Figure 2 Effect of the chloroform fraction of Piper aduncum methanolic extract (PAMoCl) on cell viability. Cell lines 293T, AGS and KATO III were treated at different concentrations of PAMoCl (1.25; 2.5; 5; 10; 20; 40; 80 and 160 µg/mL) during 24 and 48 hours. GI50 graphs are shown, which are log10 dependent cell viability curves (%) of PAMoCl concentration (µg/mL) for each cell line. In the upper right part of each graph, GI50 values are shown at 24 and 48 hours.
Observation of cell morphology
PAMoCl caused dose-dependent changes in the morphology of AGS, KATO III and 293T cells (Figure 4). After a 24-hour treatment with PAMoCl, starting at 20 µg/mL, AGS cells began to contract and others resuspended. At higher concentrations, such as 80 µg/mL and 160 µg/mL, more cell fragments, few resuspended cells and none attached were observed. After 48 hours the effect was similar. In the KATO III cell line, dead cells (Figure 4) are observed starting from 80 µg/mL, at both 24 and 48 hours. After a 24-hour treatment with PAMoCl, 293T cells began to contract at 80 µg/mL. At 160 µg/mL more contracted and resuspended cells were observed, however, cells could still be found adhering to the culture plate. At 48 hours, the observed effect was similar.

Figure 4 Effect of the chloroform fraction of Piper aduncum methanolic extract at 5 µg/mL, 20 µg/mL and 80 µg/mL concentrations on the morphology of gastric cancer cell lines AGS, KATO III and human kidney 293T. A control group of cells without treatment but with the vehicle (DMSO 0.5%) is also shown for each cell line. T.M. 100X. Red arrow: resuspended cells; blue arrow: attached cells; white arrow: contracted cells; and black arrow: dead cells.
Effect on the cell cycle
Results showed an effect on the cell cycle from the gastric cancer lines. The percentage of AGS cells in G2/M phase was 31.8%; 44.1% and 52.7% for the concentrations of 0 µg/mL; 19.62 µg/mL and 39.23 µg/mL of PAMoCl, respectively (Figure 5 A-C). The percentage of KATO III cells in G2/M phase was 30.9%; 29.3% and 49.0% for the concentrations of 0 µg/mL; 87.49 µg/mL and 160 µg/mL of PAMoCl, respectively (Figure 5 D-F).
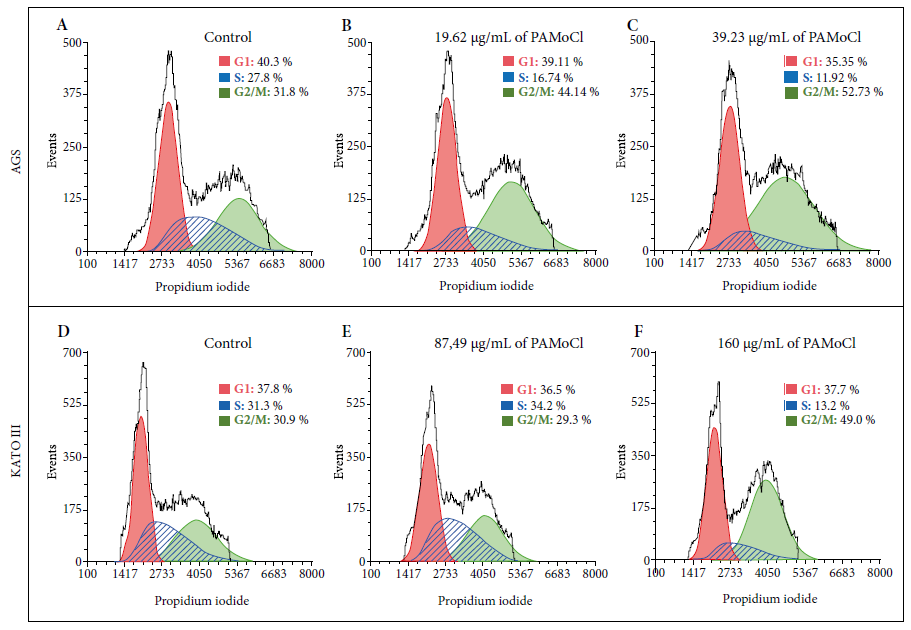
Figure 5 Flow cytometry analysis for the cell cycle of gastric cancer cell lines AGS and KATO III that were treated with the chloroform fraction of Piper aduncum methanolic extract (PAMoCl). Histograms are shown indicating the amount of DNA marked by propidium iodide. Three groups are considered for each cell line: A) control group with AGS cells without PAMoCl, but with the vehicle; B) AGS cells treated with 19.62 μg/mL of PAMoCl; C) AGS cells treated with 39.23 μg/mL of PAMoCl; D) control group with KATO III cells without treatment but with the vehicle; E) KATO III cells treated with 87.49 μg/mL of PAMoCl; F) KATO III cells treated with 160 μg/mL of PAMoCl. Raw data from flow cytometry (black curve), G1: Gap 1 (red curve), S: synthesis (blue curve), and G2/M: Gap 2/mitosis (green curve), cell cycle phases.
DISCUSSION
This is the first reported study in which the cytotoxic activity of PAMoCl and its effect on the cell cycle is demonstrated in two gastric cancer cell lines: AGS and KATO III.
The GI50 values in this study for both cell lines are lower than those reported by Herrera et al. for the ethanolic extract of P. aduncum in the MCF-7, HT-29, K-562 and H-460 cell lines 10. It is important to mention that the GI50 values found for the AGS and KATO III lines are significantly lower than those of the 293T line, which is an embryonic human kidney cell line and was used as toxicity control. This shows that PAMoCl has a greater effect on gastric cancer cells than on noncancerous cells.
Various metabolites found in plants from the Piper genus have shown biological activity, especially alkaloids. For example, piperine has been shown to inhibit tumor growth and metastasis of lung cancer cells 16. Pipernonalin shows activity against human prostate cancer cells 17. Amides may also inhibit growth in cancer cell lines 18.
In this study, it was observed that PAMoCl has a concentration-dependent effect on the cell cycle, which causes the treated cells to stop in the G2/M phase. This effect was observed in both AGS and KATO III cells. These results coincide with those reported for other plant metabolites that have shown the same effect in G2/M phase. For example, roscovitin (purines) inhibited the expression of proteins p53, CDK7, cyclin A, cyclin E and CDK2 in non-small cell lung cancer 19, lymphoma 20 and breast cancer 21. Sulforaphane (isothiacyanates) increased the expression of cyclin B1 and p21 22. Quercetin (flavonols) in hepatocellular carcinoma cells overexpressed p53, p21 and decreased the expression of cyclin D1, CDK2 and CDK7 23. Finally, berberine (alkaloids) inhibited the expression of cyclin B1 and increased the expression of Wee1 in leukemia cells 24.
Similar to what was found in this study, several species from Piper genus have metabolites with effects on the G2/M phase. For example, piperin from P. nigrum and P. longum in osteosarcoma cells 25, flavkawaina from P. methysticum 26, hinokinine from P. cubeba 27, hydroxychavicol from P. betle 28; and piperlongumina from P. longum L. showed decreased expression of cyclin D1 in AGS cells 29.
In the phytochemical analysis, 4 metabolite groups were detected in PAMoCl: phenolic groups and derivatives, flavonoids, anthocyanins, triterpenoids and steroids. This is why we consider important to study the identification of secondary metabolites using analytical techniques, such as chromatography, infrared spectroscopy, mass spectrometry and nuclear magnetic resonance that could allow us to elucidate the chemical structure of the active compounds.
However, it should be noted that there are limitations to this study. For example, the time and place for sample collection, the method of extraction, among others, factors that can generate variability in the quantity of metabolites and, consequently, in their biological activity.
In conclusion, it is reported that PAMoCl contains secondary metabolites with cytotoxic activity that causes the cell cycle to stop in the G2/M phase in two gastric cancer cell lines both primary and metastatic. The results from this study will allow to further search for active principles present in PAMoCl that have greater efficacy in eliminating gastric cancer cells, but with less toxicity for healthy cells.
Acknowledgements
To Dr. Juan Manuel Iglesias, head of the Molecular Genetics Laboratory of the Universidad Científica del Sur, for the donation of the 293T cell line used in this study.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [ Links ]
2. Torres-Román JS, Grados-Sánchez O. Cáncer gástrico en el Perú: una realidad susceptibilidad de cambio. Rev Gastroenterol del Perú [Internet]. 2015;35(3):276. Disponible en: http://www.revistagastroperu.com/index.php/rgp/article/view/118/115. [ Links ]
3. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1-2):179-92. doi: 10.1016/s0378-5173(01)00986-3. [ Links ]
4. Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: current perspectives. Bioorg Med Chem. 2004;12(7):1585-604. doi: 10.1016/j.bmc.2003.11.036. [ Links ]
5. Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11(18):2443-66. doi: 10.2174/0929867043364531. [ Links ]
6. Moore A, Pinkerton R. Vincristine: Can its therapeutic index be enhanced?. Pediatr Blood Cancer. 2009;53(7):1180-7. doi: 10.1002/pbc.22161. [ Links ]
7. Lin Z-Y, Kuo C-H, Wu D-C, Chuang W-L. Anticancer effects of clinically acceptable colchicine concentrations on human gastric cancer cell lines. Kaohsiung J Med Sci. 2016;32(2):68-73. doi: 10.1016/j.kjms.2015.12.006. [ Links ]
8. Kumar N, Misra P, Dube A, Bhattacharya S, Dikshit M, Ranade S. Piper betle Linn. a maligned Pan-Asiatic plant with an array of pharmacological activities and prospects for drug discovery. Curr Sci. 2010;99(7):922-32. Disponible en: https://www.jstor.org/stable/24066069. [ Links ]
9. Wang YH, Morris-Natschke S, Yang J, Niu HM, Long CL, Lee KH. Anticancer principles from medicinal Piper (Hú Jiāo) plants. J Tradit Complement Med. 2014;4(1):8-16. doi: 10.4103/2225-4110.124811. [ Links ]
10. Herrera-Calderon O, Alvarado-Puray C, Arroyo-Acevedo J, Rojas-Armas J, Chumpitaz-Cerrate V, Hañari-Quispe R, et al. Phytochemical screening, total phenolic content, antioxidant, and cytotoxic activity of five peruvian plants on human tumor cell lines. Pharmacognosy Res. 2018;10(2);161-165. doi: 10.4103/pr.pr_109_17. [ Links ]
11. Amiel-Pérez J, Fukusaki A, Enciso N, Altamirano C, Herrera AM, Marcelo Á, et al. Interferencia de pigmentos vegetales al aplicar la técnica XTT a extractos de Buddleja globosa, Senecio tephrosiodes Turcz. Y Equisetum giganteum. Científica. 2016;13(1):9-26. doi: 10.21142/cient.v13i1.318. [ Links ]
12. Bladt S. Plant Drug Analysis: A thin layer chromatography atlas. Springer Science & Business Media; 2009. doi: 10.1007/978-3-642-00574-9. [ Links ]
13. Lock OR. Invetigacion Fitoquimica, Metodos en el estudio de productos naturales. 1ra. edición. Lima: Pontificia Univ Catolica Peru; 2016. [ Links ]
14. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, et al. Cell Viability Assays. Assay Guid Man [Internet]. 2004;(Md):1-25. Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/23805433. [ Links ]
15. Crowley LC, Chojnowski G, Waterhouse NJ. Measuring the DNA content of cells in apoptosis and at different cell-cycle stages by propidium iodide staining and flow cytometry. Cold Spring Harb Protoc. 2016;2016(10):pdb-prot087247. doi: 10.1101/pdb.prot087247. [ Links ]
16. Lai L, Fu Q, Liu Y, Jiang K, Guo Q, Chen Q, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012;523. doi: 10.1038/aps.2011.209. [ Links ]
17. Lee W, Kim K-Y, Yu S-N, Kim S-H, Chun S-S, Ji J-H, et al. Pipernonaline from Piper longum Linn. induces ROS-mediated apoptosis in human prostate cancer PC-3 cells. Biochem Biophys Res Commun. 2013;430(1):406-12. doi: 10.1016/j.bbrc.2012.11.030. [ Links ]
18. Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MAS, Elmiro FJM, et al. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Zeitschrift für Naturforsch C. 2005;60(7-8):539-43. doi: 10.1515/znc-2005-7-805. [ Links ]
19. Zhang F, Zhang T, Gu Z-P, Zhou Y-A, Han Y, Li X-F, et al. Enhancement of radiosensitivity by roscovitine pretreatment in human non-small cell lung cancer A549 cells. J Radiat Res. 2008;49(5):541-8. doi: 10.1269/jrr.08024. [ Links ]
20. Lacrima K, Rinaldi A, Vignati S, Martin V, Tibiletti MG, Gaidano G, et al. Cyclin-dependent kinase inhibitor seliciclib shows in vitro activity in diffuse large B-cell lymphomas. Leuk Lymphoma. 2007;48(1):158-67. doi: 10.1080/10428190601026562. [ Links ]
21. Wesierska-Gadek J, Gritsch D, Zulehner N, Komina O, Maurer M. Interference with ER-a enhances the therapeutic efficacy of the selective CDK inhibitor roscovitine towards ER-positive breast cancer cells. J Cell Biochem. 2011;112(4):1103-17. doi: 10.1002/jcb.23024. [ Links ]
22. Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, et al. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48(2):198-206. doi: 10.1207/s15327914nc4802_10. [ Links ]
23. Li Y, Duan S, Jia H, Bai C, Zhang L, Wang Z. Flavonoids from tartary buckwheat induce G2/M cell cycle arrest and apoptosis in human hepatoma HepG2 cells. Acta Biochim Biophys Sin. 2014;46(6):460-70. doi: 10.1093/abbs/gmu023. [ Links ]
24. Khan M, Giessrigl B, Vonach C, Madlener S, Prinz S, Herbaceck I, et al. Berberine and a Berberis lycium extract inactivate Cdc25A and induce a-tubulin acetylation that correlate with HL-60 cell cycle inhibition and apoptosis. Mutat Res Mol Mech Mutagen. 2010;683(1-2):123-30. doi: 10.1016/j.mrfmmm.2009.11.001. [ Links ]
25. Zhang J, Zhu X, Li H, Li B, Sun L, Xie T, et al. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int Immunopharmacol. 2015;24(1):50-8. doi: 10.1016/j.intimp.2014.11.012. [ Links ]
26. Ji T, Lin C, Krill LS, Eskander R, Guo Y, Zi X, et al. Flavokawain B, a kava chalcone, inhibits growth of human osteosarcoma cells through G2/M cell cycle arrest and apoptosis. Mol Cancer. 2013;12(1):55. doi: 10.1186/1476-4598-12-55. [ Links ]
27. Cunha NL, Teixeira GM, Martins TD, Souza AR, Oliveira PF, Símaro GV, et al. (-)-Hinokinin Induces G2/M Arrest and Contributes to the Antiproliferative Effects of Doxorubicin in Breast Cancer Cells. Planta Med. 2016;82(06):530-8. doi: 10.1055/s-0042-101761. [ Links ]
28. Guha Majumdar A, Subramanian M. Hydroxychavicol from Piper betle induces apoptosis, cell cycle arrest, and inhibits epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Pharmacol. 2019;166:274-291. doi: 10.1016/j.bcp.2019.05.025. [ Links ]
29. Song B, Zhan H, Bian Q, Gu J. Piperlongumine inhibits gastric cancer cells via suppression of the JAK1, 2/STAT3 signaling pathway. Mol Med Rep. 2016;13(5):4475-80. doi: 10.3892/mmr.2016.5091. [ Links ]
Funding: This work was co-funded by the Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica (CONCYTEC) and the Universidad Científica del Sur, via the "Evaluation of the effect of extracts and fractions of three medicinal plants on gastric cancer stem cells" project, contract No. 134-2017-FONDECYT.
Cite as: Mayanga-Herrera A, Tapia-Rojas S, Fukusaki-Yoshizawa A, Marcelo-Rodríguez A, Amiel-Pérez J. Cytotoxic activity of the chloroform fraction of Piper aduncum and its effect on the cell cycle in gastric cancer cell lines. Rev Peru Med Exp Salud Publica. 2020;37(3). doi: https://doi.org/10.17843/rpmesp.2020.373.5157.
Received: January 24, 2020; Accepted: May 21, 2020











 texto en
texto en