Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.17843/rpmesp.2020.373.4918
Brief report
Immunological and biochemical response of older adults with urinary tract infection to uropathogenic Esherichia coli virulence factors
INTRODUCTION
Escherichia coli (E. coli) is the most frequent cause of bacteremia in men and women 1, and the urinary tract is the main way of infection in geriatric patients 2. This is partially due to the fact that the older adult population (OAP) presents particular immunological characteristics, and a subclinical state of chronic inflammation, known as immunosenescence, where the polymorphonuclear lineage (main line of defense in urinary tract infections (UTIs)) 3 has limited function ( 4. Several E. coli virulence factors have been described, mainly associated with bacteremia and sepsis, which include cell adhesion molecules, iron uptake systems and exotoxins that form a protein system that allows the bacteria to elude or injure the patient’s immune system 5 , 6.
The interaction between the immune system of patients with UTIs and the genetic ability of the bacteria to form virulence factors determines the bacterial clearance in the urinary tract 6, so it is necessary to explore the difference in the immune and biochemical response of older adults with UTIs to the various virulence factors associated with sepsis in uropathogenic E. coli (UPEC).
KEY MESSAGES
Motivation for the study: Older adults have an immunosenescent immune system, so it is convenient to identify virulence factors in the bacteria that can alter immune response.
Main findings: The Escherichia coli carrier of the pap GII gene induce greater tissue damage, which increases the concentration of iron and red blood cells. Besides, there is a generalized presence of nanA gene, which is important for older adults during sepsis stages.
Implications: The findings contribute to the study of the immunological response in older adults with UTI, and it is the first report in Peru about the frequency of virulence factors in uropathogenic Escherichia coli in gerontological nursing homes.
THE STUDY
Between April and July 2018, the urine of 24 older adults with UTIs, of both sexes, residing in private gerontological nursing homes in Lima, was evaluated. The diagnostic criteria for UTIs were defined as: a microscopic count of >5 leukocytes per field of 400x magnification, the conversion of nitrites by the Griess method and a count of more than 100,000 colony-forming units in the blood agar culture medium. Urine sediment analysis was standardized according to the recommendations of the Chilean Health Institute 7.
The etiological agent was identified by using traditional biochemical methods and the bacteria identified as E. coli were preserved in tryptic soy broth and 20% glycerol. Polymicrobial cultures were excluded from the study. Subsequently, the bacterial DNA was extracted using the GeneJet Genomic kit (Thermo Scientific®, Massachusetts, USA) and the presence of 11 virulence genes was evaluated: aer, α-hly, cnf-1, sfa, chuA, TcpC, nanA, pap GI, GII, GIII and iucC by end-point polymerase chain reaction.
The urine samples were centrifuged at 3,000 g (Sigma, 3-30KS) for 10 minutes, preserving the supernatant. Concentrations of TNF-α, IL-1β and iron were determined; besides, the total antioxidant capacity in urine was evaluated by ABTS•+ and FRAP methods, which acted as immune response markers through the increase of reactive oxygen substances (ROS). All analytes were measured using the Multiskan Go spectrophotometer (Thermo Scientific®, Massachusetts, USA).
The statistical analysis was carried out with the Epidat version 4.1 program. The description of the qualitative variables was made through frequency tables. Shapiro Wilk’s normality test was applied to determine the distribution of quantitative variables and Levene’s test was used to evaluate the variance homogeneity of the variables. The quantitative variables with normal distribution were analyzed by means of the Student’s T test. p values <0.05 were considered significant.
This study was approved by the Ethics Committee of the Faculty of Medicine of the Universidad Nacional Mayor de San Marcos, act 1812 with project code 0013.
FINDINGS
Twenty-four urine samples from older adults with UTIs by E. coli, from gerontological nursing homes, were analyzed. The most frequent virulence genes were nanA, pap GII, aer, chuA and iucC. Genes α-hly and cnf-1 were found in low proportion and the following genes were not found: TcpC, pap GI, pap GIII and sfa (Figure 1).
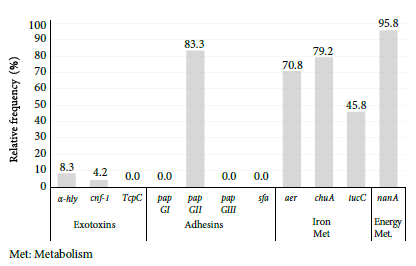
Figure 1 Relative frequency of virulence genes in uropathogenic Escherichia coli in older adults from nursing homes.
Regarding the immune and biochemical response of older adults to the various virulence genes evaluated, a concentration of 37.6 red blood cells/μL and 193.4 μg/L of iron was observed in patients carrying the pap GII gene, values were significantly higher in patients infected with E. coli, but without this gene. In addition, patients with strains carrying the pap GII gene showed a positive tendency towards having a higher urine antioxidant capacity regarding the ABTS•+ test (1348.7 vs. 634.8 Eq-real Vit C μg/mL) (Table 1). On the other hand, a higher concentration of leukocytes (p = 0.070) was observed in patients infected with E. coli carriers of the three genes related to iron metabolism evaluated (iucC, aer and chuA) (Table 2).
Table 1. Immunological and biochemical markers compared to the presence of the pap GII gene.
| Immunological and biochemical markers | Presence of pap GII a | Absence of pap GII a | p value b |
|---|---|---|---|
| Leucocytes/μL | 270.1 ± 240.7 | 225.8 ± 126.0 | 0.729 |
| Red blood cells/μL | 37.6 ± 37.4 | 12 ± 8.2 | 0.010 |
| Iron (μg/L) | 193.4 ± 139.6 | 85.3 ± 26.2 | 0.004 |
| IL-1β (pg/mL) | 375 ± 293.2 | 147 ± 98.8 | 0.144 |
| TNF- α (pg/mL) | 65.2 ± 35.0 | 94.5 ± 61.5 | 0.195 |
| ABTS Eq- real Vit C (μg/mL) | 1348.7 ± 1455.7 | 634.8 ± 299.0 | 0.059 |
| FRAP (mM) | 1.006 ± 0.416 | 0.801 ± 0.332 | 0.366 |
a media ± desviación estándar; b prueba T de Student
FRAP: Recuperación de fluorescencia después de photobleaching, ABTS: Ácido 2,2’-azino-di-(3-etilbenzotiazolina)-6-sulfónico
Table 2 Immunological and biochemical markers compared with the presence of iucC+aer+chuA genes.
| Immunological and biochemical markers | Presence of iucC+aer+chuA a | Absence of iucC+aer+chuA a | p value b |
|---|---|---|---|
| Leucocytes/μL | 391.7 ± 226.6 | 209.6 ± 206.5 | 0.070 |
| Red blood cells/μL | 35.3 ± 36 | 32.6 ± 36.4 | 0.903 |
| Iron (μg/L) | 210.6 ± 140.2 | 160.9 ± 132.6 | 0.418 |
| IL-1β (pg/mL) | 421.1 ± 280.5 | 302.4 ± 284.4 | 0.359 |
| TNF - α (pg/mL) | 82.8 ± 24.2 | 64.9 ± 45 | 0.332 |
| ABTS Eq- real Vit C (μg/mL) | 1763.7 ± 2349.6 | 1009.9 ± 626.8 | 0.435 |
| FRAP (mM) | 0.957 ± 0.307 | 0.978 ± 0.447 | 0.911 |
a Mean ± standard deviation; b Student’s T-test.
FRAP: Fluorescence recovery after photobleaching, ABTS: 2,2'-Azino-di-(3-ethylbenzothiazoline)-6-sulfonic acid.
DISCUSSION
This study evaluated the immune and biochemical response in the urine of hospitalized older patients infected by UPEC (the main etiological agent of UTIs). One of the main findings was that the presence of the pap GII gene induced hematuria and a higher iron concentration in the urine. The frequency of virulence genes important in the pathogenicity process of UTIs was also evaluated. Another important finding was that 95.8% of UPEC presented the nanA gene.
N-acetylneuraminic acid, an important substrate in the energetic production of E. coli, is split by the N-acetylneuraminic lyase (nanA) enzyme into pyruvate and N-acetyl-D-manosamine 8. The presence of nanA enzyme generates high competitiveness in the bacteremia-producing UPEC; and although its function in UTI pathogenesis in murine models is less relevant 8, results indicate the existence of a high risk of developing sepsis in older adults with bacteremia.
On the other hand, exotoxins have a role in the pathogenesis of UTIs. Neutrophils, the main line of defense in UTIs, are lysed by high doses of α-hemolysin (HlyA) and, in addition, bladder cells in low doses are exfoliated 9, which deteriorates the body’s two main lines of defense 3. Nevertheless, we obtained a frequency of 8.3% in the studied UPEC, which is lower than what was found in other studies carried out in geriatric population 10 , 11. Likewise, the exotoxins, such as the necrotizing cytotoxic factor type 1 (CNF1) genetically related to HlyA, induce a rearrangement of the neutrophilic cytoskeleton through the activation of the Rho GTPase type enzymes 9. In accordance with what was reported by α-hly, we found a UPEC carrier of the cnf-1 gene.
The TcpC protein recently described as interfering in the production of proinflammatory cytokines in the UTI 12 was not found in any of the evaluated UPEC, which may reflect the absence of the type IV pathogenicity island, dependent on its horizontal transfer, or the recent evolutionary acquisition of the gene in extra-intestinal E. coli 12.
Regarding the immunological and biochemical response observed in the older adults, iron is scarce in the urinary fluid and is indispensable for bacterial metabolism 13 , 14, the microorganisms that infect the urinary tract must have the capacity to capture and compete for iron assimilation 14. UPECs have three systems of iron uptake (siderophores, hemophores and direct iron uptake in its ferrous state), which are reported to have increased expression in vivo 15. On the other hand, iron restriction is an immune defense mechanism used by the host to limit bacterial proliferation 16. Although we could not find significant differences between immunological and biochemical markers and genes associated with iron metabolism, it can be observed that patients with UPEC, carriers of the iucC, aer and chuA genes, tend to have higher leukocyte concentrations, which may indicate a greater immune stress. Besides, the presence of chuA gene and other siderophore systems are highly concentrated in strains that cause recurrent UTI 17, a common situation in gerontological resting centers.
Finally, the pap GII gene is a virulence factor whose function is to ensure the adhesion of UPEC to renal tissue. The association of pap GII gene with pyelonephritis stages has been documented 6, besides, it has been proposed that it confers competitive advantage during bacteremia stages by UTI 8. Some evidence indicates that its presence is not determinant in stages of pyelonephritis, therefore, its function is not yet conclusive in the process of infection in the urinary tract 18.
Results obtained show that patients with UPEC positive for the pap GII gene had higher iron concentration compared to patients with UPEC negative for the pap GII gene. It has been found that the interaction of pap GII with its receptor induces rapid transcription of the airS gene in UPEC in urine, which has a fundamental role in the activation of the siderophore systems (aerobactin/enterobactin) 19 by decreasing the concentration of iron in urine. In the results obtained we did not find this association. However, this could be explained by the higher concentration of red blood cells and hemolysis in this group of patients. The adhesion of the pap GII protein activates the synthesis of cytokines 20, causing the rupture of blood vessels located in the lamina itself. On the other hand, the ABTS•+ test describes that in patients with positive pap GII UPEC there is a tendency to have greater antioxidant capacity, which could be due to higher iron concentration, which interferes with the assay, although, this tendency could also be explained by the deficiency of neutrophils to produce reactive oxygen substances. Recent studies have described an increase in the neutrophil subpopulation CD16/CD62L in the OAP, which presents a lower level of response to cytokine stimuli 4.
This study has several limitations. First, it was not possible to record information from the patients regarding their clinical condition, infection stage, use of antibiotics, infection recurrence. Second, the low number of urine samples analyzed, which increases the probability of obtaining a type II error. Third, we do not complement our studies with gene expression analysis, so we do not know if the genes evaluated are indeed expressed by the bacteria.
In conclusion, E. coli carrying the pap GII gene can induce greater tissue damage that possibly favors a higher iron concentration in the urine, which stimulates its increase. In addition, patients infected with E. coli with pap GII show a positive tendency to have greater antioxidant capacity, which may be due to the deficiency of neutrophils recruited in the OAP during the production of ROS. Finally, the generalized presence of nanA gene is important due to its high relevance in sepsis stages.
REFERENCES
1. Nicolle LE. Urinary Tract Infections in the Older Adult. Clin Geriatr Med. 2016;32(3):523-38. doi: 10.1016/j.cger.2016.03.002. [ Links ]
2. Mylotte JM, Tayara A, Goodnough S. Epidemiology of Bloodstream Infection in Nursing Home Residents: Evaluation in a Large Cohort from Multiple Homes. Clin Infect Dis. 2002;35(12):1484-90. doi: 10.1086/344649. [ Links ]
3. Luna-Pineda VM, Ochoa S, Cruz-Córdova A, Cázares-Domínguez V, Vélez-González F, Hernández-Castro, R, et al. Infecciones del tracto urinario, inmunidad y vacunación. Bol Med Hosp Infant Mex. 2018; 75(2): 67-78. doi: 10.24875/BMHIM.M18000011. [ Links ]
4. Sauce D, Dong Y, Campillo-Gimenez L, Casulli S, Bayard C, Autran B, et al. Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset. J Gerontol A Biol Sci Med Sci. 2017;72(2):163-72. doi: 10.1093/gerona/glw062. [ Links ]
5. Daga AP, Koga VL, Gabriel J, Soncini M, Matos CM De, Regina M, et al. Escherichia coli Bloodstream Infections in Patients at a University Hospital : Virulence Factors and Clinical Characteristics. Front Cell Infect Microbiol. 2019;9:191. doi: 10.3389/fcimb.2019.00191. [ Links ]
6. Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991; 4(1):80-128. doi: 10.1128/cmr.4.1.80. [ Links ]
7. Gómez R, Pellegrini P. Recomendaciones para el Ánalisis del Sedimento urinario. Departamento Laboratorio Biomédico Nacional y de Referencia. Instituto Nacional de Salud Pública de Chile. 2013 [citado el 03 de mayo de 2020]. Disponible en: https://www.araucaniasur.cl/wp-content/uploads/2019/11/sedimento-urinario 10052013A.pdf. [ Links ]
8. Smith SN, Hagan EC, Lane MC, Mobley HLT. Dissemination and Systemic Colonization of Uropathogenic Escherichia coli in a Murine Model of Bacteremia. MBio. 2010;1(5):1-9. doi: 10.1128/mBio.00262-10. [ Links ]
9. Lüthje P, Brauner A. Virulence Factors of Uropathogenic E. coli and Their Interaction with the Host. Adv Microb Physiol. 2014;65:337-72. doi: 10.1016/bs.ampbs.2014.08.006. [ Links ]
10. Santo, E, Macedo C, Marin JM. Virulence factors of uropathogenic Escherichia coli from a University Hospital in Ribeirão Preto, São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2006;48(4):185-88. doi: 10.1590/s0036-46652006000400002. [ Links ]
11. Croxall G, Hale J, Weston V, Manning G, Cheetham P, Achtman M, et al. Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. J Antimicrob Chemother. 2011;66(11):2501-8. doi: 10.1093/jac/dkr349. [ Links ]
12. Xiao T, Waldhuber A, Svanborg C, Snyder G, Römmler F, Miethke T, et al. A Comparative Analysis of the Mechanism of Toll-Like Receptor-Dis-ruption by TIR-Containing Protein C from Uropathogenic Escherichia coli. Pathogens. 2016;5(1):25. doi: 10.3390/pathogens5010025. [ Links ]
13. Pfrimer K, Micheletto RF, Marchini JS, Padovan GJ, Moriguti J, Ferriolli E. Impact of Aging on Urinary Excretion of Iron and Zinc. Nutr Metab Insights. 2014;7:47-50 doi: 10.4137/NMI.S12977. [ Links ]
14. Alteri CJ, Smith SN, Mobley HLT. Fitness of Escherichia coliduring urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5(5):e1000448. doi: 10.1371/journal.ppat.1000448. [ Links ]
15. Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HLT. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 2010; 11;6(11): e1001187. doi: 10.1371/journal.ppat.1001187. [ Links ]
16. Bullen JJ. The Significance of Iron in Infection. Clin Infect Dis. 1981;3(6):1127-38. doi: 10.1093/clinids/3.6.1127. [ Links ]
17. Ejrnæs K, Stegger M, Reisner A, Ferry S, Monsen T, Holm SE, et al. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: Phylogenetic groups, virulence factors and biofilm formation. Virulence. 2011;2(6):528-37. doi: 10.4161/viru.2.6.18189. [ Links ]
18. Mobley HLT, Jarvis KG, Elwood JP, Whittle DI, Lockatell CV, Russell RG, et al. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10(1):143-55. doi: 10.1111/j.1365-2958.1993.tb00911.x. [ Links ]
19. Zhao R, Shi J, Shen Y, Li Y, Han Q, Zhang X, et al. Phylogenetic distribution of virulence genes among ESBL-producing uropathogenic escherichia coli isolated from long-term hospitalized patients. J Clin Diagnostic Res. 2015;9(7):1-4. doi: 10.7860/JCDR/2015/13234.6157. [ Links ]
20. Svensson M, Duan R, Svanborg C. Role of the Ceramide-sig-naling Pathway in Cytokinr Responses to P-fimbriated. J Exp Med. 1996;183(3):1037-44. doi: 10.1084/jem.183.3.1037. [ Links ]
Funding sources: Funding for research groups from the Universidad Nacional Mayor de San Marcos (project code A17011681) and internal funding from the Universidad de Piura.
Cite as: Gonzales-Rodriguez AO, Infantes Varillas SF, Barrón Pastor HJ, Llimpe Mitma Y, Huerta Canales D, Wong Chero PA, et al. Immunological and biochemical response of older adults with urinary tract infection to uropathogenic Escherichia coli virulence factors. Rev Peru Med Exp Salud Publica. 2020;37(3):527-31. doi: https://doi.org/10.17843/rpmesp.2020.373.4918.
Received: October 24, 2019; Accepted: May 06, 2020











 text in
text in 


