Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Medicina Experimental y Salud Publica
versión impresa ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.4 Lima oct-dic 2020 Epub 07-Sep-2020
http://dx.doi.org/10.17843/rpmesp.2020.374.6330
Original articles
Chlorine dioxide and chlorine derivatives for the prevention or treatment of COVID-19: a systematic review
1 Instituto de Evaluación de Tecnologías en Salud e Investigación - IETSI, EsSalud, Lima, Perú.
2 Instituto de Efectividad Clínica y Sanitaria (IECS), Buenos Aires, Argentina.
INTRODUCTION
In March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic. The first cases were reported in December 2019 in the Chinese city of Wuhan, and a new type of coronavirus was subsequently identified as the causative agent (SARS-CoV-2) 1. As of August 9, more than 19 million confirmed cases of COVID-19 have been reported worldwide and 471,012 cases and 20,844 deaths caused by this disease have been confirmed in Peru 2.
Person-to-person is the main transmission route, via droplets expelled by an infected person when sneezing, coughing, talking, and even indirectly through fomites. In addition, airborne transmission by aerosol could occur under specific circumstances 3. In mild to moderate cases, symptoms are like a common cold and may or may not result in mild pneumonia; sometimes neurological and gastrointestinal symptoms occur, usually without the need for hospitalization. Dyspnea and hypoxia occur in severe cases, in which more than 50% of the lung tissue is compromised. In these cases, oxygen therapy or mechanical ventilation is required. Critical patients have multiorgan involvement, as well as an intense inflammatory response, and may present sepsis and multiorgan dysfunction syndrome which can lead to death 4 - 6.
There is not enough scientific evidence to support the use of any drug as treatment and/or preventive therapy against SARS-CoV-2 7 , 8. Now, only symptomatic treatments are available. Thus, because of the lack of established treatment guidelines, different drugs with not enough scientific evidence are used worldwide, 9. In addition, some drugs offered in the Peruvian market, besides not having scientific support for use against COVID-19, lack sanitary authorization to be used in humans as medical treatment 10. However, the distributors and manufacturers of these products assure their effectiveness and safety against COVID-19, as preventive, curative, and symptomatic drugs 11.
The products mentioned previously are chlorine dioxide, sodium chlorite and other chlorine derivatives. Commercially chlorine dioxide is known as CDS (chlorine dioxide solution) and is advertised as a derivative of the compound initially sold as MMS (miracle mineral supplement or mineral miracle solution) 12, which contains sodium chlorite. Sodium chlorite is converted to chlorous acid when mixed in water with an acid, as indicated by its distributors, and then becomes chlorine dioxide. On the other hand, CDS is the chlorine dioxide gas in solution. Chlorine dioxide and other chlorine derivatives (e.g., sodium chlorite, sodium hypochlorite, etc.) are used as disinfecting agents in various industrial processes, due to their strong oxidizing power ( 13 , 14. The oxidizing effect ends up denaturing organic compounds, however, this effect is not specific against a particular type of organism.
In this scenario, it is necessary to gather scientific evidence to contrast the widespread claims and the hypothesis in favor of using CDS or MMS as a preventive agent and as a curative or control treatment against COVID-19 15. Therefore, this study aimed to systematically review the efficacy and safety of the use of chlorine dioxide and chlorine derivatives, in the prevention or treatment of COVID-19.
KEY MESSAGES
Motivation for the study: In the absence of an effective medicine to prevent or cure COVID-19, an increasing number of people are ingesting chlorine dioxide or chlorine derivatives, chemical compounds not authorized for human consumption.
Main findings: To date, there is no scientific evidence to support the use of chlorine dioxide or chlorine derivatives to prevent or treat COVID-19.
Implications: In the absence of evidence, these chemicals cannot be considered effective or safe. That said, the scientific and medical community has expressed concern about the harm that consumption of chlorine dioxide or chlorine derivatives may cause to people.
MATERIALS AND METHODS
For this systematic review we followed the statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 16 and the Cochrane Manual for systematic reviews of interventions 17. The protocol was registered in PROSPERO with the reference number CRD42020200641 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=200641).
Eligibility criteria
Randomized clinical trials, quasi-experimental studies, cohort studies, case-control studies, cross-sectional studies, and case reports evaluating chlorine dioxide and chlorine derivatives to prevent or treat COVID-19 in people of all ages were included. No language or publication status restrictions were applied. Studies on other coronavirus infections (e.g., MERS-CoV, SARS-CoV, etc.) were also considered if no studies on SARS-CoV-2 infections were found. Animal or in vitro studies were excluded.
Search Strategies
A systematic electronic search for articles published up to July 24, 2020 was conducted in the following databases: PubMed, Embase (Excerpta Medica Database), CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane Library, Web of Science, LILACS (Latin American and Caribbean Literature in the Health Sciences), and SciELO (Scientific Electronic Library Online). A search strategy was initially designed for PubMed and was adapted to the other databases; it combined the terms “coronavirus”, “SARS-CoV-2”, and “Chlorine” with synonyms and other medical descriptors.
A librarian (DC) created the search strategies, which were then validated by two of the authors (AB and AHV). The search terms used for the databases are detailed in the Appendix 1 of the supplementary material. The electronic search was complemented by manual searches of the reference lists of relevant articles to identify possible studies not found in the electronic search (snowball strategy), Google Scholar (first ten pages of results), and pre-print repositories (medRxiv and bioRxiv) using a combination of the following terms: “coronavirus”, “SARS-CoV-2”, and “Chlorine”. In addition, we reviewed records from clinical trials in progress or with unpublished data from the China Clinical Trials Registry (ChiCTR), the Netherlands Trials Registry (NTR), ClinicalTrials.gov, and the International Standardized Randomized Controlled Trial Number (ISRCTN). The search was conducted without restrictions regarding study design, publication status, publication date or language.
Study selection
In the first stage, results from the electronic and manual search were imported into the reference management software EndNote X9 (license 3061914708). Then, all duplicate records were eliminated following the methodology described by Bramer et al. The identified records were evaluated to verify if they complied with the inclusion criteria; those that did not meet the criteria were excluded from the review. Two independent reviewers (AB and AHV) participated in the evaluation of the eligibility of all records by means of the Rayyan web application (https://rayyan.qcri.org/) 19. Any disagreements were resolved between the two reviewers, and if no decision could be made, a third reviewer participated in the discussion (VP).
Extraction and synthesis of results
We planned to report any outcome of using chlorine dioxide and chlorine derivatives to prevent or treat COVID-19, such as the cure rate, resolution time, reduction in the severity of the disease, hospitalization period, mortality rate, adverse events, among others. We also planned to obtain the general characteristics of each study and to evaluate the quality of each one according to the study type. However, no study was found to meet the inclusion criteria for these processes.
RESULTS
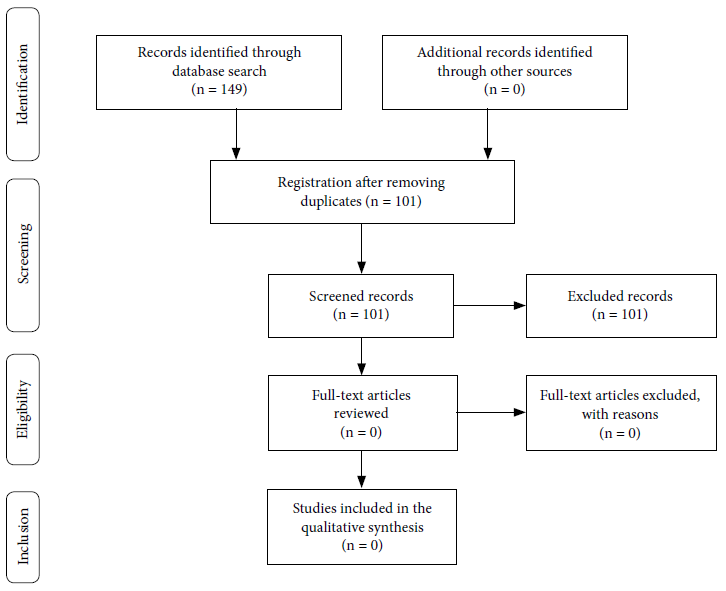
The search of the databases and other sources included 101 records after removing duplicates. From these 101 records, no published or pre-published studies were identified to have evaluated the use of chlorine dioxide and chlorine derivatives to prevent or treat COVID-19 or other coronavirus infections (Figure 1).
Additionally, as a result of the review of clinical trial records, a single study was identified in ClinicalTrials.gov (NCT04343742) entitled “Determination of the Effectiveness of Oral Chlorine Dioxide in the Treatment of COVID 19”, it was registered as an observational study that plans to include 20 Colombian participants to evaluate the efficacy of oral chlorine dioxide in the treatment of patients with COVID-19 infection 20.
DISCUSSION
Based on the results of the systematic review, no published or unpublished scientific evidence has evaluated the use of chlorine dioxide or chlorine derivatives as a preventive or therapeutic agent against COVID-19 administered by respiratory, oral, or parenteral route. Only one study was identified as observational in ClinicalTrials.gov and has no results to date. Likewise, due to the recent occurrence of this type of coronavirus, the search was expanded to include other types of coronavirus, for which no evidence was identified either. This lack of evidence has also been reported by the Instituto Nacional de Salud del Perú in a technical document of evidence synthesis 21.
Prior to the emergence of the COVID-19 pandemic, the use of chlorine products was already commercialized in some European countries and the United States. Specifically, sodium chlorite, under the name of MMS, whose distributors claimed that after mixing with an acid, it had antimicrobial, antiviral and antibacterial effects, and was supposed to be a treatment for various unrelated diseases, such as autism spectrum disorder, neoplasms, hepatitis and HIV/AIDS 22. There are several communications before and after the current COVID-19 pandemic issued by regulatory entities, whose purpose is to denounce and demand the withdrawal of this product from the market within those countries 23 - 25.
However, within the Americas, chlorine dioxide and sodium chlorite have continued to be offered with special relevance due to the SARS-CoV-2 pandemic; their use is promoted not only as a treatment for the previously mentioned diseases, but also as a preventive and treatment agent for SARS-CoV-2 infection. The Pan American Health Organization (PAHO) 26 does not recommend the use of chlorine dioxide or sodium chlorite by oral or parenteral route in patients suspected or diagnosed with COVID-19. It also mentions that it should not be used in any other type of illness, since there is no evidence of its efficacy; on the contrary, the ingestion or inhalation of these products would generate serious adverse effects. The U.S. Food and Drug Administration (FDA) has received reports of adverse events caused by these products, including respiratory failure due to methemoglobinemia, cardiac arrhythmia due to prolongation of the QT interval, hypotension due to hydro-electrolyte imbalance, acute liver failure, hemolytic anemia, vomiting, and severe acute diarrhea 24 , 27.
The concept of “first do no harm” is a fundamental principle in health and in life. As previously mentioned, chlorine dioxide and chlorine derivatives are routinely used in industrial processes, for example, in the purification of water for human consumption. It should be noted that the health effects of a substance will always depend on the dose, duration and form of exposure, the presence of other substances, personal characteristics and habits, and the individual’s health status 13. That is why there are maximum permitted limits for the amount of chlorine dioxide and chlorite per volume of water in the drinking water treatment process. However, the products offered to prevent and treat COVID-19, in addition to not having scientific evidence of their effectiveness, lack sanitary registration, so it is not possible to standardize a maximum dose that at least ensures that such substance can be safe in order to prevent adverse events. It has been observed that the concentrations of these products exceed the maximum limits allowed in drinking water in countries where chlorine dioxide or sodium chlorite have been marketed as therapeutic agents 23. In other words, these products marketed as preventive agents or treatments by oral or parenteral route not only do not have clinical scientific evidence that has demonstrated benefit for any type of disease but could also cause serious harm.
In Peru, the Dirección General de Medicamentos, Insumos y Drogas released a statement against the use of these substances to treat any disease, such as COVID-19, warned that the promotion and commercialization of these types of products is illegal and reported that their consumption could cause potentially fatal damage 10.
The purpose of this research was to try to identify any published or to-be-published scientific study regarding the efficacy and safety of the administration by respiratory, oral, or parenteral route of chlorine dioxide, sodium chlorite or chlorine derivatives. Therefore, as demonstrated in the results of this systematic review, no academic, research or health entity in the world has seen reasonable to study the potential preventive or therapeutic effects of this substances, attributed by its distributors, despite the fact that to date there is a therapeutic void regarding treatment for COVID-19.
To spread arguments trying to use an apparent scientific language can cause confusion for the consumer, therefore, it is necessary to understand the mechanism of action of oxidizing agents, such as chlorine dioxide, sodium chlorite, sodium hypochlorite, among others. These substances serve as disinfectants since they can oxidize other compounds by means of an oxidation-reduction reaction (also known as REDOX), in which the oxidizing agent is reduced by gaining electrons, while the reducing agent is oxidized by losing electrons 28. This oxidizing effect denatures the organic compounds 14 , 29. However, its effect is not specific to a particular organism, since all living organisms are composed of organic molecules, our cells, like other microorganisms, are also affected 30.
The use of products that have not been proven to be safe and effective against COVID-19 not only poses a potential health risk to those who use them, but also to the entire population. When a false sense of security is generated, due to the supposed unproven beneficial effect of the product, prevention and control measures against COVID-19 that have been proven to be effective may be abandoned, such as the use of masks, social distancing, hand hygiene and respiratory etiquette 3 , 31. Therefore, it is a collective duty to be responsible for the prevention and control measures that each individual uses.
Although a thorough review of the literature was conducted, there may be additional sources in other bibliographic databases or regional repositories. However, this study included the largest and most important databases in the biomedical area, gray literature and pre-print repository, without applying any restriction to the inclusion of studies according to design, publication status, date or language of publication, as long as they were conducted in humans.
It is concluded that currently, there is no scientific evidence to support the use of chlorine dioxide or chlorine derivatives to prevent or treat COVID-19. This is possibly due to the fact that there would be no biological plausibility or preliminary clinical evidence to support the development of coherent hypotheses for the use of chlorine dioxide or chlorine derivatives as therapeutic or preventive agents, and to the concern of the medical community about the toxicity of these products.
REFERENCES
1. Coronavirus: the first three months as it happened. Nature. 2020;10.1038/d41586-020-00154-w. doi: 10.1038/d41586-020-00154-w. [ Links ]
2. Brote de enfermedad por el Coronavirus (COVID-19) [Internet]. OPS; 2020 [citado el 10 de agosto de 2020]. Disponible en: https://www.paho.org/es/temas/coronavirus/brote-enfermedad-por-coronavirus-covid-19. [ Links ]
3. Meselson M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N Engl J Med. 2020;382(21):2063. doi: 10.1056/NEJMc2009324. [ Links ]
4. Bourgonje AR, Abdulle AE, Timens W, Hillebrands J-L, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020. doi: 10.1002/path.5471. [ Links ]
5. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. doi: 10.1001/jama.2020.2648. [ Links ]
6. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. NIH; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://www.covid19treatmentguidelines.nih.gov/. [ Links ]
7. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) [Internet]. CDC; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. [ Links ]
8. Clinical management of COVID-19 [Internet]. OMS; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://www.who.int/publications/i/item/clinical-management-of-covid-19. [ Links ]
9. Bendezu-Quispe G, Rodríguez-Zúñiga MJM, Roman YM, Mori-Llontop LM, Peralta V, Fiestas F. Agentes potencialmente terapéuticos contra el SARS-CoV-2: revisión rápida de la evidencia. Rev Peru Med Exp Salud Pública. 2020;37(2):320-6. doi: 10.17843/rpmesp.2020.372.5409. [ Links ]
10. Dirección General de Medicamentos, Insumos y Drogas. Consumo de dióxido de cloro o clorito de sodio es peligroso para la salud [Internet]. DIGEMID; 2020 [citado el 11 de agosto de 2020]. Disponible en: http://www.digemid.minsa.gob.pe/main.asp?Seccion=3&IdItem=2215. [ Links ]
11. Chuquillanqui F. Coronavirus en Perú [Internet]. RPP noticias; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://rpp.pe/lima/actualidad/coronavirus-en-peru-dioxido-de-cloro-es-vendido-ilegalmente-a-traves-de-redes-sociales-como-cura-de-la-covid-19-noticia-1282416. [ Links ]
12. Reporte Breve N° 34. Uso de dióxido de cloro para el tratamiento de pacientes con diagnóstico de COVID-19 [Internet]. EsSalud: IETSI; 2020 [citado el 11 de agosto de 2020]. Disponible en: http://www.essalud.gob.pe/ietsi/pdfs/covid_19/RB34_dioxidodecloro_19Julio_editado.pdf. [ Links ]
13. Agencia para Sustancias Tóxicas y el Registro de Enfermedades. Resúmenes de Salud Pública. Dióxido de cloro y clorito (Chlorine Dioxide and Chlorite) [Internet]. ATSDR; 2019 [citado el 11 de agosto de 2020]. Disponible en: https://www.atsdr.cdc.gov/es/phs/es_phs160.html. [ Links ]
14. Chlorine Dioxide, Chlorite and Chlorate in Drinking-water [Internet]. OMS; 2016 [citado el 11 de agosto de 2020]. Disponible en: https://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/chlorine-dioxide-chlorite-chlorate-background-jan17.pdf. [ Links ]
15. Kály-Kullai K, Wittmann M, Noszticzius Z, Rosivall L. Can chlorine dioxide prevent the spreading of coronavirus or other viral infections? Medical hypotheses. Physiol Int. 2020;107(1):1-11. doi: 10.1556/2060.2020.00015. [ Links ]
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [ Links ]
17. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) [Internet]. Cochrane; 2019 [citado el 6 de agosto de 2020]. Disponible en: www.training.cochrane.org/handbook. [ Links ]
18. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240-3. doi: 10.3163/1536-5050.104.3.014. [ Links ]
19. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [ Links ]
20. Determination of the Effectiveness of Oral Chlorine Dioxide in the Treatment of COVID 19 [Internet]. NIH; 2020 [citado el 6 de agosto de 2020]. Disponible en: https://clinicaltrials.gov/ct2/show/NCT04343742. [ Links ]
21. Eficacia y seguridad del dióxido de cloro para el tratamiento de COVID-19 [Internet]. INS; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://web.ins.gob.pe/sites/default/files/Archivos/authenticated%2C%20administrator%2C%20editor/publicaciones/2020-08-04/SE_24_dioxido%20de%20cloro.pdf. [ Links ]
22. Office of the Commissioner. Peligro: No beba la solución mineral milagrosa o productos similares [Internet]. FDA; 2019 [citado el 11 de agosto de 2020]. Disponible en: https://www.fda.gov/consumers/articulos-en-espanol/peligro-no-beba-la-solucion-mineral-milagrosa-o-productos-similares. [ Links ]
23. European Chemicals Agency. Stopping products sidestepping enforcement through rebranding [Internet]. ECHA; 2019 [citado el 11 de agosto de 2020]. Disponible en: https://newsletter.echa.europa.eu/home/-/newsletter/entry/stopping-products-sidestepping-enforcement-through-rebranding. [ Links ]
24. Office of the Commissioner. Coronavirus (COVID-19) Update: FDA Warns Seller Marketing Dangerous Chlorine Dioxide Products that Claim to Treat or Prevent COVID-19 [Internet]. FDA; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-warns-seller-marketing-dangerous-chlorine-dioxide-products-claim. [ Links ]
25. Miracle Mineral Solution and Sodium chlorite solutions [Internet]. Food Standards Agency; 2017 [citado el 11 de agosto de 2020]. Disponible en: https://www.food.gov.uk/business-guidance/miracle-mineral-solution-and-sodium-chlorite-solutions. [ Links ]
26. Organización Panamericana de la Salud. La OPS no recomienda tomar productos que contengan dióxido de cloro, clorito de sodio, hipoclorito de sodio o derivados, 16 de julio del 2020 [Internet]. OPS; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://iris.paho.org/handle/10665.2/52484. [ Links ]
27. Hagiwara Y, Inoue N. First case of methemoglobinemia caused by a ClO2-based household product. Pediatr Int. 2015;57(6):1182-1183. doi: 10.1111/ped.12708. [ Links ]
28. Chapter 8 Chemical Oxidation and Reduction. In: Studies in Environmental Science. Elsevier; 1979. p. 97-113. [ Links ]
29. Aieta EM, Marco Aieta E, Berg JD. A Review of Chlorine Dioxide in Drinking Water Treatment. American Water Works Association. 1986;78:62-72. doi: 10.1002/j.1551-8833.1986.tb05766.x. [ Links ]
30. Couri D, Abdel-Rahman MS, Bull RJ. Toxicological effects of chlorine dioxide, chlorite and chlorate. Environ Health Perspect. 1982;46:13-7. doi: 10.1289/ehp.824613. [ Links ]
31 Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) - Transmission [Internet]. CDC; 2020 [citado el 11 de agosto de 2020]. Disponible en: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. [ Links ]
Funding: This study was funded by the Instituto de Evaluación de Tecnologías en Salud e Investigación (IETSI), EsSalud, Perú.
Received: August 11, 2020; Accepted: September 02, 2020











 texto en
texto en