Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.4 Lima Oct-Dec 2020 Epub Nov 04, 2020
http://dx.doi.org/10.17843/rpmesp.2020.374.5073
Original articles
Use of antibiotics in inpatients from a national hospital in Lima, Peru
1 Servicio de Enfermedades Infecciosas y Tropicales, Hospital Nacional Dos de Mayo, Lima, Perú.
2 Universidad Peruana de Ciencias Aplicadas (UPC), Lima, Perú.
3 Departamento de Epidemiología, Hospital Nacional Dos de Mayo, Lima, Perú.
4 Universidad Nacional Mayor de San Marcos, Lima, Perú.
5 Facultad de Ciencias de la Salud, Universidad Científica del Sur, Lima, Perú.
INTRODUCTION
Antibiotic use has significantly increased the life expectancy of the population 1. However, antibiotic resistance (AR) is a growing problem and one of the greatest challenges for public health today 2. Irrational or indiscriminate use of antibiotics is the most important cause for antibiotic resistance, and also results in other adverse consequences, such as toxicity, interactions, infection by Clostridioides (Clostridium) difficile and prolonged hospital stay; which finally increases morbidity, mortality and costs for the health system 3 , 4.
AR rates are particularly high in hospitals 5. It has been recognized for several decades that up to 50% of prescribed antibiotics have unnecessary or inappropriate indications 6 ) and, additionally, the small number of recently approved antibiotics reduces therapeutic options, forcing the increasingly prudent use of antibiotics. Therefore, to address this problem, the World Health Organization (WHO) promotes the establishment of Antimicrobial stewardship (AMS) programs. The AMS are an effective strategy that ensures the correct use of antibiotics, according to the available scientific evidence, to improve clinical outcomes, minimize adverse events and reduce hospital costs 7.
High-income countries have more resources and experience in developing AMS programs, therefore they have data available on antimicrobial use ( 8. On the other hand, in low- and middle-income countries, such as Peru, AMS programs have been implemented in recent years despite the scarcity of trained human resources and the economic limitations of health systems (9, 10).
Accordingly, actions have been taken in Peru to curb antimicrobial resistance, through a Multi-sector Plan (2019-2021) 11 and the development of the AMS Technical Standard. However, we still have limited information about the quality and characteristics of antibiotic prescriptions that can serve as a reference for optimizing antimicrobial control programs. For this reason, we present a work of point prevalence in the use of antibiotics, which will provide basic information about the patterns of antibiotic prescription in a third-level hospital in Lima, Peru.
KEY MESSAGES
Motivation for the study: The rational use of antibiotics is necessary to prevent the progression of antimicrobial resistance.
Main findings: We conducted a study to evaluate antibiotic use at a national referral hospital. Half of the patients received some antibiotic. In 2 out of 5 patients, antibiotic use was no justified. In 9 out of 10 cases, the antibiotic treatment was empirical and not directed.
Implications: It is necessary to reinforce the recommendations for the adequate rational use of antibiotics based on clinical practice guidelines and directed therapies.
MATERIALS AND METHODS
Population and sample
Hospital Nacional Dos de Mayo (HNDM) is a national reference teaching hospital, located in Lima, Peru. It is equipped with 605 beds and has the main medical and surgical specialties, except for transplant and burn units. The hospitalization areas include six medical rooms, one pediatric/surgical room, four surgical rooms, one trauma and orthopedic room, one urology room, one obstetrics room, one neonatal room, one pediatric intensive care unit (ICU) room, three adult ICU rooms, one cardiovascular ICU room, one infectious disease and tropical medicine room, and one pneumology room. Areas for high-risk adults include the hematology and medical oncology rooms. The term “other rooms” refers to the areas for pediatric surgery, neonatology, neonatal ICU, and adult ICU. In addition, we used inpatient records (at the time of the survey) from the medicine, surgery, OB/GYN, and pediatrics departments, as well as from the intensive care unit at HNDM.
We collected the information from medical records, and carried out a brief interview with the treating physician or nurse when a particular fact needed to be clarified. Also, information from patients within the same room was obtained in a single day. Patients who were hospitalized at 8:00 a.m. on the day on which the study was conducted were included, regardless of whether or not they used antibiotics. We excluded patients who were ready for discharge, outpatients, emergency room patients, and hemodialysis patients.
Procedure
Instrument
A 3-week cross-sectional study was carried out (December 3-21, 2018). The research team, composed of infectious disease specialists and residents, received training on conducting the survey according to the WHO methodology for estimating point prevalence of antibiotic use in hospitals 12. A preliminary pilot was carried out to determine the time for collecting information, errors in reporting, and doubts in filling out the questionnaire. Information was collected electronically, and most of the questions in the questionnaire were multiple choice.
The survey questionnaire was validated and used in previous studies in Spanish 12. It was structured as follows: 1) patient-related information (hospitalization unit, demographic data, date of admission, and recent surgeries during hospitalization), which was completed for all patients, whether or not they were prescribed antibiotics on the evaluation day; 2) information on antibiotic management (diagnosis, microbiology, use of antibiotics, and adherence to clinical guidelines). Information was obtained from medical records, the nursing kardex, and health personnel on duty in the medical hospitalization rooms (doctors and nurses).
Variables
1) Type of indication for the use of antibiotics, according to the type of infection: community-acquired or acquired 48 hours after the patient’s hospitalization. 2) Reason for prescribing antibiotics: treatment (evidence or suspicion of infection), prophylaxis (no evidence of infection), or unknown. 3) Type of treatment: empirical (no evidence of infectious agent or infection), directed (evidence of infectious agent). 4) Missed dose: when the dose indicated by the physician, was not administered to the patient (verified in the nursing kardex) and was considered omitted due to lack of supply or another cause (error in administration, non-tolerance by the patient, unknown). 5) Availability of results (culture with antimicrobial sensitivity) in the clinical record. 6) Informing the treating physician of the results: the treating physician reports that the results were received. 7) Compliance with suggestions from Clinical Practice Guidelines (CPG).
The prescription was considered adequate when it was according to an international or local guideline or had the advice of an infectious disease specialist. Non-compliance was considered when the treating physician reported that he or she did not use any international or local CPG to decide on the antibiotic used; or if such a decision was not supported by an infectious disease physician through assessment or consultation.
Statistical analysis
The variables were compiled and analyzed in a database in Microsoft Excel 2018. The analysis was descriptive, and absolute and relative (percentage) frequencies were presented in frequency tables as well as in bar and pie charts.
Ethical aspects
This study has been approved by the HNDM Ethics Committee (Reg. 014261). Given the observational nature of the study and the anonymous way in which the data from the clinical records were collected, the ethics committee decided that informed consent from the patients was not required.
RESULTS
We identified 358 patients distributed in different hospitalization rooms of the HNDM. The mean age was 49 (standard deviation [SD ]: 25.3) years (<18 years: 25.7%; 18-65 years: 53.9%; >65 years: 34.1%). The distribution by gender was similar (49.2% males, 50.5% females, and one transgender patient 0.3%). Most patients were from adult medical rooms (62.5%), adult surgery (17%), neonatology (5%), high-risk adults (4.7%), pediatric medicine (4.2%) and other rooms (high-risk adults [3.3% ], pediatric ICU [1.1% ], obstetrics-gynecology [1.1% ], pediatric surgery [0.8% ]).
Invasive devices were present in 341 cases (95.2%). The most frequent was the peripheral venous catheter (90.5%), followed by the bladder (16.5%) and the central venous catheter (10.9%), while hemodialysis, peritoneal and other catheters accounted for 7%. Orotracheal intubation was performed in 8.4% of patients, and some surgical procedure was performed in 23.5% of patients during their hospitalization.
Antibiotics were used in 51.7% of the patients; most frequently in pediatric (73.3%), pediatric surgery (66.7%), adult medicine (57.1%), neonatology (50%), obstetrics-gynecology (50%) and adult surgery rooms (42.6%); and less frequently in adult ICUs (29.4%), pediatric ICUs (25%) and high-risk adult hospitalization (8.3%) (Table 1).
The main reason for using antibiotics was community-acquired infections (66.1%), followed by healthcare-associated infections (19%). The most frequent diagnoses for antibiotic use were pneumonia (19.6%), skin and soft tissue infection without non-surgical osteomyelitis (12.1%), lower urinary tract infection (11.1%), intra-abdominal infection (8.9%), clinical sepsis (7.4%), upper urinary tract infection (4.2%). The least frequent diagnoses were acute or chronic exacerbated bronchitis, asymptomatic bacteriuria, and systemic response inflammatory syndrome, which received antibiotics in 2.6%, 2.1%, and 0.5% of cases, respectively (Table 1).
Table 1 Point prevalence of antibiotic use (n = 358)
| Characteristics | n (%) |
|---|---|
| Overall prevalence | 185 (51.7) |
| Distribution by rooms | |
| Pediatric medicine hospitalization | 11 (73.3) |
| Pediatric surgery hospitalization | 2 (66.7) |
| Adult general medicine hospitalization | 128 (57.1) |
| Neonatology hospitalization | 9 (50.0) |
| Obstetrics and gynecology hospitalization | 2 (50.0) |
| Adult surgery hospitalization | 26 (42.6) |
| Adults UCI | 5 (29.4) |
| Pediatric UCI | 1 (25.0) |
| High-risk adults | 1 (8.3) |
| Type of indication | |
| HCRI | 36 (19.0) |
| Community-acquired infections | 125 (66.1) |
| Unknown | 7 (3.7) |
| Other | 21 (11.1) |
| Reason for prescribing antibiotics | |
| Surgical prophylaxis | 15 (7.9) |
| Treatment | 166 (87.8) |
| Unknown | 8 (4.2) |
| Length of surgical prophylaxis | |
| Single dose | 0 |
| Multiple doses in a day | 15 (100) |
| Multiple doses in more than a day | 0 |
| Unknown | 0 |
ICU: intensive care unit; HCRI: health care related infections.
The use of antibiotics was mostly indicated as treatment (87.8%), surgical prophylaxis (7.9%) and for unknown reasons (4.2%). All indications for surgical prophylaxis received multiple doses of the drug on the same day (Table 1).
Regarding the number of antibiotics used per patient, 49.7% received one antibiotic, 44.9% two antibiotics and 5.4% three or more antibiotics. Only 57.3% of patient prescriptions followed guidelines based on local or international CPGs for antibiotic indication, while 28.5% did not follow any standardized recommendations. The frequency of antibiotic use in accordance with local or international CPGs was as follows: high-risk adults (100%), neonatology (94.4%), adult medicine (77.7%), pediatric medicine (71.4%), pediatric surgery (66.7%), adult surgery (48.7%), obstetrics-gynecology (25%) and adult ICU (20%) (Figure 1).
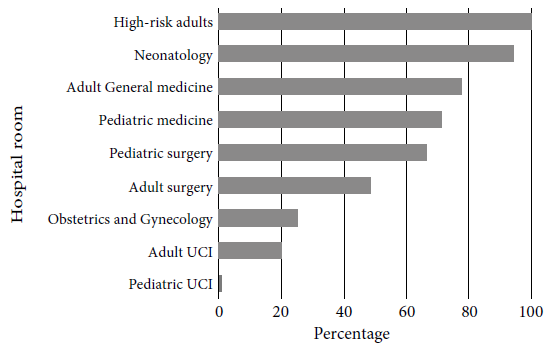
Figure 1 Compliance with local or international clinical practice guidelines by hospital room at Hospital Nacional Dos de Mayo, 2018.
In 141 cases (49.8%), the initial antibiotic prescriber was the specialist physician, while in the remaining 50.2% the resident physician was the prescriber. We also found that 86.8% of the therapies were empirical. Likewise, 37% of the patients had some biological sample collected for microbiological culture (blood [22.7% ], urine [43.3% ], sputum [11.3% ], wound drainage [6.3% ], sterile fluids [7.5% ] and others [8.8% ]). Finally, in 71% of the cases, the culture results were reported to the treating physician (Table 2).
Table 2 Characteristics of antibiotic use at Hospital Nacional Dos de Mayo, 2018 (n = 358)
| Characteristics | n (%) |
|---|---|
| Type of prescriber | |
| Specialist | 141 (49.9) |
| Medical resident | 142 (50.1) |
| Type of treatment | |
| Empirical | 145 (86.8) |
| Directed | 22 (13.2) |
| Unknown | 0 |
| Any missed antibiotic doses | |
| Yes | 64 (22.7) |
| No | 202 (71.6) |
| Unknown | 16 (5.6) |
| Average number of doses missed (mean, IQR) | 2.9 (1-1.6) |
| Causes for missed doses | |
| All doses missed due to shortage | 4 (6.3) |
| No dose missed due to shortage | 1 (1.6) |
| Some doses missed due to shortage | 27 (42.2) |
| Unknown | 32 (50.0) |
| Sample collection for microbiological diagnosis | |
| Yes | 70 (37.0) |
| No | 86 (45.5) |
| Unknown | 33 (17.5) |
| Sample type | |
| Blood | 18 (22.7) |
| Urine | 34 (43.3) |
| Sputum | 9 (11.3) |
| Wound drainage | 5 (6.3) |
| Sterile fluids | 6 (7.5) |
| Other | 7 (8.8) |
| Availability of culture results | 51 (72.9) |
| Availability of antimicrobial sensitivity testing | 37 (53.3) |
| Results reported to the treating physician | 49 (71.0) |
IQR: Interquartile range
In 64 patients (22.7%), at least one required antibiotic dose was missed, with a median of missed doses of 2.9 (interquartile range: 1-16). Missing a dose was mainly as a consequence of shortage in 48.5% of the cases. Meropenem, vancomycin, and ceftriaxone were the most frequently used antibiotics for community and healthcare-related infections (Table 3).
Table 3 Antibiotic distribution by type of indication at Hospital Nacional Dos de Mayo, 2018 (n = 189).
| Antibiotic | HCRI (n = 36) | CAI (n = 125) | Unknown (n = 7) | Other (n =21) |
|---|---|---|---|---|
| Amikacin | 1 | 3 | 3h | 5 |
| Azithromycin | 0 | 4e | 0 | 0 |
| Ceftazidime | 5 | 12f,g | 2h | 1 |
| Ceftriaxone | 9 | 49d,e | 2d | 3 |
| Ciprofloxacin | 3a | 13a,g | 1a | 1 |
| Clindamycin | 2a | 17a,d,f | 2a,d | 3 |
| Colistin | 2b | 0 | 0 | 0 |
| Ertapenem | 2 | 0 | 0 | 0 |
| Imipenem | 3c | 4 | 0 | 0 |
| Meropenem | 8b,c | 27c | 0 | 3 |
| Vancomycin | 8c | 18c | 1 | 5 |
The number of antibiotics is higher than the total number of each indication type due to the combined antibiotic therapy.
HCRI: healthcare-related infections; CAI: community-acquired infections.
a ciprofloxacin + clindamycin (HCRI: 1; CAI: 4; unknown: 1); b colistin + meropenem (HCRI: 2); c carbapenem + vancomycin (HCRI: 4; CAI: 5); d ceftriaxone + clindamycin (CAI: 3; unknown: 1); e ceftriaxone + azithromycin (CAI: 3); f ceftazidime + clindamycin (CAI: 2); g ceftazidime + ciprofloxacin (CAI: 5); h ceftazidime + amikacin (unknown: 2).
DISCUSSION
The overall frequency of antibiotic use we found (51.7%) was similar to that of other point prevalence studies 13 - 15. Studies from other parts of the Americas show data similar to our reality. For example, in 2011, a study took place in acute care hospitals in ten U.S. states and reported that nearly 50% of the patients had received some antibiotic 13. In 2015, another study that included four Latin American countries (Argentina, Brazil, Chile, and Mexico) described that 47.5% of the patients recruited used antibiotics, of which 90% were for systemic use 14. Another study on the point prevalence of antibiotic use was carried out in Mexico between August and September 2016, and it described that 51% of 260 patients received at least one antibiotic 15.
On the other hand, studies on European population present some differences with respect to our reality. In 2016, a study carried out by the national center for nosocomial infection surveillance in Germany found a 26% prevalence of antibiotic use in 64,412 patients from 218 hospitals 16. Additionally, a study conducted on acute care hospitals in Europe found that one third of the patients received at least one antibiotic (70%, one antibiotic; 24%, two antibiotics; 4.5%, three; and 1.3%, four or more antibiotics) in 2017 17. This reinforces the concept that in the last decade, the frequency of antibiotic use (by using point prevalence) is lower in regions of Europe 16 , 17 than in critical care units in America 13 - 15.
In our study, 66% of the patients received antibiotic therapy for community-acquired infections; other studies report 76.5% (Mexico, 2016) 15, 70% (USA, 2011) 13, and 60.5% (Latin America, 2015) 14, which are similar to our results. In Europe, less patients (45%) received antibiotic therapy for community-acquired infections 17. The studies mentioned, including ours, agree that the most frequent diagnosis for antimicrobial prescription was lower respiratory infections.
The studies we cited show that the most frequently used antibiotics for community acquired infections were penicillins combined with beta-lactamase inhibitors, followed by cephalosporins and fluoroquinolones 16 , 17 , 21 , 23; to prescribe these drugs is related to a rational use of antibiotics and shorter hospital stays ( 18. Some series like ours, mainly from Latin American countries 14, describe the use of broad-spectrum antibiotics in cases where they were not necessarily needed, such as in hospitalization outside the critical care unit 19 and community-acquired infections 13 , 15.
Even though 88% of the patients who used antibiotics (for treatment of an infectious pathology), about a third of them did not follow an indication based on any CPG. Similar findings are shown in two American studies, such as the one from Ohio University Hospital, where 30% of the antibiotic indications were considered unnecessary 20. A Mexican study (2016) 15 ) found that antibiotic use was not justified in 21% of the patients. Similarly, in Latin America (Argentina, Brazil, Chile, Mexico) it was only 64%. In contrast, in Australian hospitals (2014) 21, the percentage of inappropriate prescriptions was about a quarter of the total prescriptions and non-guideline use was observed in 27% of the patients. Another multicenter study (2015) 14 reported a similar percentage of adherence to CPG (77.4%), and finally a study in Singapore 23 showed that the use of antibiotics without justification was infrequent (0.4%). The causes described for the unjustified use of antibiotics were prolonged therapy times, non-infectious processes, and treatment of colonizing microorganism findings 20.
Antibiotic prophylaxis in surgery represented 8% of the total prescriptions in our study, which highlights its irrational use, as it exceeded the number of days for antibiotic treatment. So, it is important to point out that multiple doses with a duration of more than 24 hours were prescribed in almost all the indications. In other countries, such as Australia, this behavior is observed in 40% of the prescriptions 21 and in European countries between 54% and 56% 16 , 17. In contrast, a study carried out in 13 hospitals in Singapore showed that only 8.4% of the prescriptions for surgical prophylaxis lasted more than one day 23. These data reveal that there is much to be done to raise awareness among professionals in surgical specialties regarding the duration of antibiotic prophylaxis, since this causes an increase in antibiotic resistance.
Regarding microbiological isolation, our study highlights that only 37% of cases had a microbiological sample taken to find the possible causal agent; most of these samples (77.3%) consisted of blood, urine and sputum. In a retrospective study carried out at the University Medical Center of the University of Amsterdam, Netherlands, with 733 hospital beds, about 7,500 blood cultures are carried out by year, which contrasts with the figures we obtained 24. In a study of hospitals in Singapore (2015-2016) 23, 45.5% showed some positive microbiological culture.
Due to the limited number of beds, the pediatric and adult ICUs represented a small percentage (1.1% and 4.7%, respectively) of the total number of patients evaluated. However, there was a lower percentage of antibiotic use in those units (25% in pediatric ICU and 29.4% in adult ICU), which suggests a greater intervention of the AMS of the HNDM in the mentioned rooms. The latest consensus promoted by the Joint Programming Initiative on Antimicrobial Resistance (2019) standardizes suggestions for optimal follow-up and monitoring of antimicrobial control programs, and emphasizes the item referring to the measurement of drug use, where it is specified that the defined daily dose (DDD) or days of therapy (DOT) are the best parameters for evaluation 25.
The most frequently used antibiotics were ceftriaxone (22.2%), meropenem (12.8%), vancomycin (10.8%), clindamycin (8%), ceftazidime (6.7%) and ciprofloxacin (6%). It is important to note the remarkably similar percentages of the use of meropenem and vancomycin for both nosocomial and community infections, as well as the percentage of ceftriaxone use for nosocomial infections. This reflects the unwise use of antibiotics in our reality.
This study did not evaluate antibiotic consumption in a DDD, patient outcome, and duration of antibiotic therapy, due to the design of the cross-sectional study that sought to describe the characteristics of antibiotic use in hospitalized patients at a given time. Therefore, these variables could be considered in future studies.
In conclusion, this study describes a high frequency of irrational use of antibiotics in hospitalized patients. We found that 15-25% of the cases had an unjustified use of cephalosporins, clindamycin and amikacin, in addition 13% used carbapenems for community acquired infections. In about 90% of patients who received antibiotics, the prescription was empirical and not directed. Our hospital requires urgent measures to strengthen the institutional AMS, in order to decrease the rates of unnecessary and inadequate use of antibiotics, as well as to improve prescriptions according to CPGs, grant timely access to antibiotics and lower the figures of bacterial resistance.
REFERENCES
1. Hollis A, Ahmed Z. Preserving Antibiotic: Rationally. N Engl J Med. 2013;369(26):2474-6. doi: 10.1056/NEJMp1311479. [ Links ]
2. World Health Organization. Antimicrobial resistance: global report on surveillance [Internet]. Geneva, Switzerland; 2014 [citado el 27 de mayo de 2020]. Disponible en: http://www.who.int/iris/handle/10665/112642. [ Links ]
3. Shaughnessy MK, Amundson WH, Kuskowski MA, DeCarolis DD, Johnson JR, Drekonja DM. Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109-16. doi: 10.1086/669089. [ Links ]
4. Beardsley JR, Williamson JC, Johnson JW, Luther VP, Wrenn RH, Ohl CC. Show me the money: long-term financial impact of an antimicrobial stewardship program. Infect Control Hosp Epidemiol. 2012;33(4):398-400. doi: 10.1086/664922. [ Links ]
5. Nasr P. Genetics, Epidemiology and Clinical Manifestations of Multidrug-Resistant Acinetobacter baumannii. J Hosp Infect. 2020;104(1):4-11. doi: 10.1016/j.jhin.2019.09.021. [ Links ]
6. Centers for Disease Control and Prevention, Office of Infectious Disease [Internet]. Antibiotic resistance threats in the United States, 2013; 2013. [citado el 27 de mayo de 2020]. Disponible en: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [ Links ]
7. Cisneros J, Pérez-Moreno M, Gil-Navarro M. Política de antibióticos. Comisión de infecciones y uso de antimicrobianos. Enferm Infecc Microbiol Clin. 2014;32(8):533-6. doi: 10.1016/j.eimc.2014.01.008. [ Links ]
8. Barlam T, Cosgrove S, Abbo L, MacDougall C, Schuetz A, Septimus E, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi: 10.1093/cid/ciw118. [ Links ]
9. World Health Organization. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit [Internet]. WHO; 2019 [citado el 27 de mayo de 2020]. Disponible en: https://apps.who.int/iris/handle/10665/329404. [ Links ]
10. Van Dijck C, Vlieghe E, Cox J. Antibiotic stewardship interventions in hospitals in low-and middle income countries: a systematic review. Bull World Health Organ. 2018;96(4):266-80. doi: 10.2471/BLT.17.203448. [ Links ]
11. Ministerio de Salud. DS N°010-2019-SA Aprueban el "Plan Multisectorial para enfrentar la Resistencia a los Antimicrobianos 2019 - 2021" que como Anexo forma parte integrante del presente Decreto Supremo [Internet]. MINSA; 2019 [citado el 27 de mayo de 2020]. Disponible en: https://www.gob.pe/institucion/minsa/normas-legales/276868-010-2019-sa. [ Links ]
12. Global PPS Development Group. Point Prevalence Survey of Antimicrobial Consumption and Resistance [Internet]. GLOBAL-PPS; 2017 [citado el 27 de mayo de 2020]. Disponible en: http://www.global-pps.com/wp-content/uploads/GLOBAL-PPS-2017-Protocol.pdf. [ Links ]
13. Magill SS, Edwards JR, Beldavs ZG, Dumyati G, Janelle SJ, Kainer MA, et al. Prevalence of Antimicrobial Use in US Acute Care Hospitals, May-September 2011. JAMA. 2014;312(14):1438-46. doi: 10.1001/jama.2014.12923. [ Links ]
14. Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, et al. Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619-e629. doi: 10.1016/S2214-109X(18)30186-4. [ Links ]
15. Soria-Orozco M, Padrón-Salas A, González-Mercado JJ, Villava-von der Heyde N, Valerdi-Contreras L, López-Iñiguez Á, et al. Prevalence of antimicrobial use among hospitalized patients in non-critical areas in a university hospital in Mexico. Salud Publica Mex. 2017;59(5):504-505. doi: 10.21149/8465. [ Links ]
16. Aghdassi SJS, Gastmeier P, Piening BC, Behnke M, Peña Diaz LA, Gropmann A, et al. Antimicrobial usage in German acute care hospitals: results of the third national point prevalence survey and comparison with previous national point prevalence surveys. J Antimicrob Chemother. 2018;73(4):1077-1083. doi: 10.1093/jac/dkx494. [ Links ]
17. Plachouras D, Kärki T, Hansen S, Hopkins S, Lyytikäinen O, Moro ML, et al. The Point Prevalence Survey Study Group. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23(46):1800393. doi: 10.2807/1560-7917.ES.23.46.1800393. [ Links ]
18. Ufer M, Radosevic N, Vogt A, Palcevski G, Francetic I, Reinalter SC, et al. Antimicrobial drug use in hospitalised paediatric patients: a cross-national comparison between Germany and Croatia. Pharmacoepidemiol Drug Saf. 2005;14(10):735-9. doi: 10.1002/pds.1108. [ Links ]
19. Stenehjem E, Hersh AL, Sheng X, Jones P, Buckel WR, Lloyd JF, et al. Antibiotic Use in Small Community Hospitals. Clin Infect Dis. 2016;63(10):1273-1280. doi: 10.1093/cid/ciw588. [ Links ]
20. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972-8. doi: 10.1001/archinte.163.8.972. [ Links ]
21. Turnidge JD, Thursky K, Chen CS, McNeil VR, Wilkinson IJ. Antimicrobial use in Australian hospitals: how much and how appropriate?. Med J Aust. 2016;205(10):S16-S20. doi: 10.5694/mja15.00899. [ Links ]
22. Kuster SP, Ruef C, Ledergerber B, Hintermann A, Deplazes C, Neuber L, et al. Quantitative antibiotic use in hospitals: comparison of measurements, literature review, and recommendations for a standard of reporting. Infection. 2008;36(6):549-59. doi: 10.1007/s15010-008-7462-z. [ Links ]
23. Cai Y, Venkatachalam I, Tee NW, Tan TY, Kurup A, Wong SY, et al. Prevalence of Healthcare-Associated Infections and Antimicrobial Use Among Adult Inpatients in Singapore Acute-Care Hospitals: Results From the First National Point Prevalence Survey. Clin Infect Dis. 2017;64(2):S61-S67. doi: 10.1093/cid/cix103. [ Links ]
24. Nannan Panday RS, Wang S, Van de Ven PM, Hekker TAM, Alam N, Nanayakkara PWB. Evaluation of blood culture epidemiology and efficiency in a large European teaching hospital. PLoS One. 2019;14(3):e0214052. doi: 10.1371/journal.pone.0214052. [ Links ]
25. Schweitzer VA, Van Werkhoven CH, Rodríguez Baño J, Bielicki J, Harbarth S, Hulscher M, et al. Optimizing design of research to evaluate antibiotic stewardship interventions: consensus recommendations of a multinational working group. Clin Microbiol Infect. 2019;26(1):41-50. doi: 10.1016/j.cmi.2019.08.017. [ Links ]
Cite as: Resurrección-Delgado C, Chiappe-Gonzalez A, Bolarte-Espinoza J, Martínez-Dionisio L, Muñante-Meneses R, Vicente-Lozano Y, et al. Use of antibiotics in inpatients from a national hospital in Lima, Peru. Rev Peru Med Exp Salud Publica. 2020;37(4).doi: https://doi.org/10.17843/rpmesp.2020.374.5073.
Received: December 31, 2019; Accepted: July 29, 2020











 text in
text in 



