Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Medicina Experimental y Salud Publica
versión impresa ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.4 Lima oct-dic 2020 Epub 06-Nov-2020
http://dx.doi.org/10.17843/rpmesp.2020.374.6198
Originales breves
Clinical and epidemiological characteristics of children with COVID-19 in a pediatric hospital in Peru
1 Instituto Nacional de Salud del Niño San Borja, Lima, Perú.
INTRODUCTION
COVID-19 is an infectious disease caused by the SARS-CoV-2 virus and was declared a pandemic on March 11, 2020. Children represent between 1 and 6% of the cases and show lower mortality rates than adults 1. In Peru, 683,687 cases of COVID-19 have been reported, as well as 29,687 deaths due to COVID-19, of which 125 were children and adolescents 2.
Children with COVID-19 show different characteristics than adults, for example, household contacts are identified more frequently, and most patients are asymptomatic or have mild symptoms 3. However, a severe onset presentation called SARS-CoV-2-associated multisystemic inflammatory syndrome has been reported and described only in children 4.
Most cases show normal counts of leukocytes, lymphocytes, and neutrophils in laboratory tests. Elevation of acute phase reactants is not frequent in children with COVID-19 5. Peribronchial cuffing in chest radiography and ground-glass opacification in chest tomography are the most frequent findings 6; however, they are unspecific.
The ventilatory support and the use of dexamethasone in patients with respiratory failure were both useful in the management of adult patients with COVID-19 7. There is no evidence for the use of other treatments in children.
There are no reports available on the most common presentations of COVID-19 in Peru. Therefore, our objective is to describe the clinical and epidemiological characteristics of children treated in a national referral pediatric hospital during the first months of the pandemic.
KEY MESSAGES
Motivation for the study: There are no reports on the most common clinical presentation of COVID-19 in children in Peru.
Main findings: In children, initial symptoms of COVID-19 are similar to those of other viruses, including fever, respiratory, digestive and dermal symptoms. These symptoms are nonspecific, so epidemiological contact is important for diagnosis in children. Disease progression in children with comorbidities was favorable in most cases.
Implications: Children with COVID-19 present mild symptoms in most cases and the treatment should be mainly symptomatic and supportive.
THE STUDY
We conducted a retrospective descriptive study on inpatients and outpatients under 18 years of age diagnosed with COVID-19 and treated at Instituto Nacional del Niño San Borja (INSNSB) from March to May 2020; children admitted to intensive care were excluded. We used consecutive non-probabilistic convenience sampling.
We reviewed the medical records of the hospitalized patients and the chest images were examined by a pediatric radiologist. We registered the laboratory test results and the images that were obtained closest to the symptom onset or up to 72 hours before or after diagnosis.
Outpatients were contacted prior to the study as part of the healthcare monitoring program approved by the institution as a strategy to detect warning signs in patients with COVID-19 treated in the triage installed since the beginning of the pandemic. We created a follow-up system with a structured questionnaire for recording information. Patient monitoring was made via telephone calls, which included the identification of epidemiological data and alarm symptoms, as well as education on prevention if no symptoms were detected. Subsequently, telephone follow-up was done every 72 hours until the resolution of symptoms. Emergency care was recommended if any alarm signs were detected.
Children with a positive real-time polymerase chain reaction (RT-PCR) test for SARS-CoV-2 or a positive serological test (IgM and IgG, or IgM) were considered COVID-19 cases. Samples were obtained from serum and we used the qualitative cassette format (Coretests®) as the serological test.
The data analysis was performed with the Stata 15.0 statistical package. We used absolute and relative frequencies for qualitative variables and measures of central tendency and dispersion for the quantitative variables.
This study was approved by the INSNSB Ethics Committee (code PI-437). We requested a waiver of informed consent because we worked exclusively with the medical records, there was no contact with patients for study purposes. The data collected were kept anonymous and the database will be stored for three years. A code was assigned to each patient, without the possibility of identification, which ensured confidentiality.
FINDINGS
We evaluated 33 children with COVID-19, 57.6% were boys and the median age was 4.8 years (range: 2 months - 17 years); 63.6% (n = 21) were inpatients; 60.6% had comorbidities, the median number of comorbidities was 1 (range: 0-5) and neurological comorbidities were the most frequent. Only three children who were outpatients had comorbidities. Of the total patients, 81.8% had contact with a person with COVID-19 and, most of the time, the contact occurred within the household. The median incubation period was seven days (interquartile range [IQR]: 4-15 days). Positive molecular tests were obtained from 15/33 cases.
Of the total number of children with COVID-19, 31/33 patients were symptomatic. We found that 78.8% presented fever, the median number of days with fever was 2 (range: 1-7) and 57.6% had a cough. Digestive, neurological, and dermatologic symptoms were less frequent (Table 1). We also found that 16.7% had tachypnea, 23% tachycardia, the median saturation was 97% (IQR: 94- 98%), and only 18.2% of patients had abnormal findings on chest examination.
Table 1 Clinical and epidemiological characteristics of children with COVID-19 (n = 33)
| Variables | n (%) |
|---|---|
| Sex | |
| Male | 19 (57.6) |
| Female | 14 (42.4) |
| Age in months* | 58 (2-204) |
| Type of patient | |
| Inpatient | 21 (63.6) |
| Outpatient | 12 (36.4) |
| Comorbidities | 20 (60.6) |
| Neurological | 8 (40) |
| Congenital heart disease | 4 (20) |
| Digestive | 2 (10) |
| Others | 6 (30) |
| Contact with COVID-19 patient | |
| Unknown | 6 (18.2) |
| Household | 13 (39.4) |
| Community | 3 (9.1) |
| Hospital | 11 (33.3) |
| Incubation period (days)** | 7 (4-15) |
| Symptoms at presentation | |
| Fever | 26 (78.8) |
| Cough | 19 (57.6) |
| Diarrhea | 10 (30.3) |
| Rhinorrhea | 9 (27.3) |
| Nausea or vomiting | 8 (24.2) |
| Throat pain | 6 (21.2) |
| Headache | 5 (15.2) |
| Myalgia | 3 (9.1) |
| Neurological symptoms | 3 (9.1) |
| Skin lesions | 2 (6.1) |
*Median (range)
**Median (interquartile range)
Laboratory tests were only performed on hospitalized patients, the blood count and acute phase reactants were normal in most patients (Table 2). We found that 3 out of 8 patients had positive IgM results for Mycoplasma pneumoniae, 1 out of 13 had positive blood culture for S. aureus, 2 out of 3 had positive culture results for respiratory secretions, one for Stenotrophomona maltophila and one for Pseudomona aeruginosa; 60.7% of patients received antibiotics for co-infections.
Table 2 Laboratory test results for children hospitalized with COVID-19 (n = 21)
| Laboratory test | Results Median (IQR) | Normal values |
|---|---|---|
| Leukocytes (103/µL) | 8,870 (6,530-14,790) | 5-15.5 |
| Neutrophiles (103/µL) | 4,380 (2,420-6,652) | 1.5-8.5 |
| Lymphocytes (103/µL) | 2,380 (1,400-4,040) | 2-8 |
| Hemoglobin (g/dL) | 11.3 (10.4-12.7) | 11.5-13.5 |
| C-reactive protein (mg/L) | 2.3 (0.75-26.65) | <5 |
| Procalcitonin (mg/dL) | 0.03 (0.02-0.13) | <0.5 |
| Lactate Dehydrogenase (U/L) | 650 (403-943) | 240-849 |
| Ferritin (ng/mL) | 93 (55-1,327) | 4-67 |
| D-dimer (FEU/mL) | 0.58 (0.27-1.37) | <0.5 |
| CPK (U/L) | 55 (40-55) | 7-25 |
IQR: interquartile range, CPK: creatine phosphokinase.
Out of the 21 hospitalized patients, 16 had a chest X-ray. Abnormal images were found in 68.8% (11/16); the most frequent was limited lung involvement, in less than 25% (63.6%, 7/11), of this group the most frequent finding was peribronchial cuffing (Figure 1A). Only three patients had extensive involvement with consolidation and involvement of more than 75% of both lung fields (Figure 1B). None of the outpatients had chest X-rays.
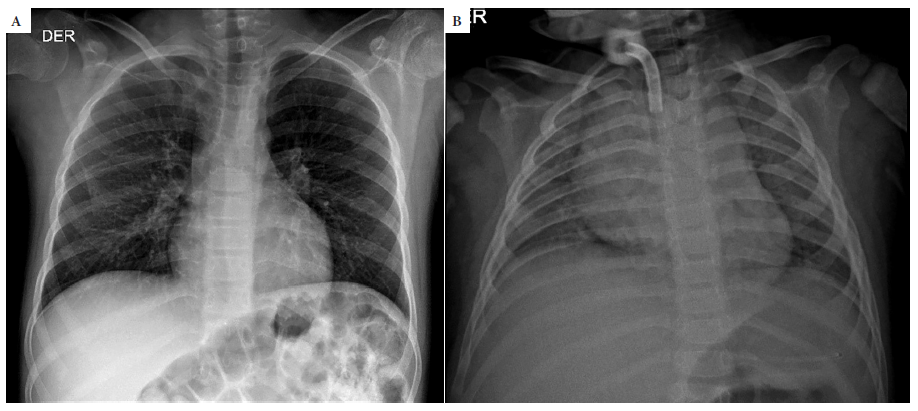
Figure 1 A. 12-year-old child with a history of intracerebral hemorrhage, parents with positive COVID-19 results. Chest x-ray shows bilateral peribronchial cuffing. B. 2-year-old child. Chest x-ray shows consolidation in both lungs with presence of air bronchograms.
All outpatients had mild symptomatology, and none presented complications during follow-up. During the study, no patients with COVID-19 were admitted to the intensive care unit. One patient died from complications of endocranial hypertension due to advanced brain tumor.
The treatment was mainly supportive, in case of fever or pain, paracetamol was used orally at doses of 10 to 15 mg/kg up to every 4 hours. One patient received corticoids (intravenous dexamethasone, 0.3 mg/kg/dose every 12 hours) as part of the intracranial tumor management, and other received subcutaneous enoxaparin 1 mg/kg/dose every 12 hours during his hospitalization for vena cava thrombosis secondary to central venous catheter placement. The parents of two outpatients had previously administered ivermectin but did not specify the dose. Only two patients needed supplemental oxygen by nasal cannula.
DISCUSSION
The cases of COVID-19 in children that we described in this study correspond to the first cases treated in a national referral institute during the first months of the pandemic in Peru. Most patients were of preschool age as described in other series 8; however, infection may occur in children of all ages 9. Epidemiologic contact was found in 82% of cases and mostly within the household; similar information has been reported in other studies 10, probably because children had to stay at home during the mandatory quarantine since going to school was not an option. The median incubation period was seven days, similar to the incubation period mentioned by Shen et al. ( 11.
As reported in other series 12, fever was the most frequent symptom in children, temperature under 39 °C and lasted less than three days. Cough was the second most frequent symptom. Headache and pharyngeal pain were less frequent in children than in adults because these symptoms are not expressed by young children. Tachypnea and abnormal findings on chest examination were uncommon even in hospitalized patients with abnormal radiological findings. Gastrointestinal symptoms were less frequent, similar to the symptoms found by Tian et al. 13, who found that diarrhea is the most common gastrointestinal symptom in children, and that vomiting occurs more frequently in children than in adults; these findings may be due to the binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) receptors on upper esophageal epithelial cells and intestinal epithelial cells in the ileum and colon 14. During the assessment period, there were no cases of multisystem inflammatory syndrome, which has been reported in later stages of the pandemic and may be related to immune mechanisms triggered by previous exposure to the virus.
Half of the hospitalized patients had complex comorbidities and mostly were of surgical nature; however, none of these patients were admitted to intensive care because of complications inherent to the infection. There is not enough evidence about the factors associated with complications of COVID-19 in children 15.
Most patients presented mild symptoms, as described by Dong et al. 9, who reported that 90% of children diagnosed with COVID-19 had mild or moderate asymptomatic disease. The low pediatric sensitivity to SARS-CoV-2 infection may be attributed to the low expression of the cellular receptors ECA2 and transmembrane protease, serine 2 (TMPRSS2) in children 14. Other hypotheses include immunity training by the use of live vaccines and cross-media immunity with other coronavirus infections 16. In addition, the fact that children have less endothelial damage may be a protective factor because there is a direct relationship between endothelial damage and the inflammatory response to SARS-CoV-2 14.
Blood counts were mostly within normal limits, and only two children were found to have lymphopenia. Most children have been described as having normal white blood cell counts with neutrophilia and neutropenia found in less than 5% of cases 5. C-reactive protein and procalcitonin increased in 13.6% and 10.6% of cases, respectively, slightly higher percentages than those described by Wang et al. 12
Chest radiography is suggested for the initial study of cases with moderate and severe clinical presentation, while tomography is reserved for cases with clinical deterioration 17. The first published case series reported that most pediatric patients had normal X-rays with few findings 10 , 18. However, in a series where pediatric radiology experts evaluated 81 X-rays of children with COVID-19, they found abnormalities in 90% of the cases 6. In this study, we found that the percentage of patients with abnormal findings (63.8%) is much higher than earlier reports; this could be due to the fact that our institution now has a radiology service dedicated to pediatric pathology. However, the proportion of affected patients was lower than in the series presented by Caro et al. 6.
All children with COVID-19 received symptomatic and complementary treatment when necessary. No pharmacological treatment other than that administered was used to treat the underlying diagnosis or co-infection. Currently, there is no evidence of specific treatment for COVID-19 and randomized clinical trials with drugs have been developed in children over 12 years of age.
Only one patient died from causes other than COVID-19 (oncological disease with palliative care). Lower mortality rates are observed in children infected with COVID-19 than in adults affected by the disease. 19.
Since this is a retrospective study that reviews clinical records, the main limitation was the reliability of information registry. In addition, the sample size was small because it was conducted at a single center and represents the first months of the pandemic.
In conclusion, in this series, the clinical presentation of COVID-19 in children was similar to other viruses, mild disease was found in almost all cases and the epidemiological background was important for diagnosis.
REFERENCES
1. Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. doi: 10.1001/jama.2020.2648. [ Links ]
2. Ministerio de salud [Internet]. Lima: MINSA; 2020 [citado el 06 septiembre de 2020]. Disponible en: https://covid19.minsa.gob.pe/sala_situacional.asp. [ Links ]
3. Lu X, Zhang L, Li YY, Liu D, Shen K, Xu S, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663-5. doi: 10.1056/NEJMc2005073. [ Links ]
4. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-8. doi: 10.1016/S0140-6736(20)31103-X. [ Links ]
5. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58(7):1135-8. doi: 10.1515/cclm-2020-0272. [ Links ]
6. Caro-Dominguez P, Shelmerdine SC, Toso S, Secinaro A, Toma P, Damasio MB, et al. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol. 2020;50(10):1354-68. doi: 10.1007/s00247-020-04747-5. [ Links ]
7. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 -preliminary report. N Engl J Med. 2020. doi: 10.1056/NEJMoa2021436. [ Links ]
8. She J, Liu L, Liu W. COVID-19 epidemic: Disease characteristics in children. J Med Virol. 2020;92(7):747-54. doi: 10.1002/jmv.25807. [ Links ]
9. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [ Links ]
10. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689-96. doi: 10.1016/S1473-3099(20)30198-5. [ Links ]
11. Shen Q, Guo W, Guo T, Li J, He W, Ni S, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424-9. doi: 10.1002/ppul.24762. [ Links ]
12. Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58(4):269-74. doi: 10.3760/cma.j.cn112140-20200225-00138. [ Links ]
13. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843-51. doi: 10.1111/apt.15731. [ Links ]
14. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [ Links ]
15. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-95. doi: 10.1111/apa.15270. [ Links ]
16. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults?. Indian J Pediatr. 2020;87(7):537-46. doi: 10.1007/s12098-020-03322-y. [ Links ]
17. Foust AM, Phillips GS, Chu WC, Daltro P, Das KM, Garcia-Peña P, et al. International Expert Consensus Statement on Chest Imaging in Pediatric COVID-19 Patient Management: Imaging Findings, Imaging Study Reporting and Imaging Study Recommendations. Radiol Cardiothorac Imaging. 2020;2(2):e200214. doi: 10.1148/ryct.2020200214. [ Links ]
18. Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020:ciaa198. doi: 10.1093/cid/ciaa198. [ Links ]
19. Bai K, Liu W, Liu C, Fu Y, Hu J, Qin Y, et al. Clinical Analysis of 25 COVID-19 Infections in Children. Pediatr Infect Dis J. 2020;39(7):e100-3. doi: 10.1097/INF.0000000000002740. [ Links ]
Received: July 21, 2020; Accepted: September 16, 2020











 texto en
texto en 



