Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.37 no.4 Lima Oct-Dec 2020 Epub Nov 09, 2020
http://dx.doi.org/10.17843/rpmesp.2020.374.5167
Originales breves
Characteristics of oncological clinical trials submitted to the Instituto Nacional de Salud del Peru, 1995-2019
1 Instituto Nacional de Salud, Lima, Perú.
2 Departamento de Estadística, Demografía, Humanidades y Ciencias Sociales, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Perú.
INTRODUCTION
Clinical trials (CT) that address oncological issues have found a favorable scenario to evaluate effective therapeutic and/or palliative treatment alternatives, taking into account the different stages of the disease 1. This is because, cancer is currently considered a global public health problem 2.
Some CT regulatory agencies, beyond their well-known functions of providing the legal framework and safeguarding the integrity of research subjects, have implemented open databases with information on all the CTs they register and authorize. In this regard, the World Health Organization (WHO) manages the International Clinical Trials Registry Platform (ICTRP) https://apps.who.int/trialsearch/Default.aspx), in order to ensure free access to information 3.
This and other databases that consolidate information on CTs around the world have been the subject of research that seeks to identify how the frequency of CTs around the world has varied. Viergever et al. identified a steady increase in the number of reported CTs, from 3,294 in 2004 to 23,384 in 2013, although these figures include all types of CTs (oncological and non-oncological) ( 4.
In Peru, the Instituto Nacional de Salud (INS), as the regulatory authority for clinical trials in the country, has been operating the Peruvian Registry of Clinical Trials (REPEC) since 2007. This database has been used by previous studies to characterize CTs. According to the general report by Minaya et al. for the period 1995-2012 5 and by Alarcón Ruiz et al. for 1995-2017 6, it is known that cancer-related CTs are the most frequent in the country.
Although these are public databases and are available for all researchers, it was not possible to identify published studies on relevant aspects of the CTs that are oriented to oncological pathologies in Peru. Therefore, the aim of this research was to identify the characteristics of oncological clinical trials (OCT) reported in a 25-year period (1995 to 2019).
KEY MESSAGES
Motivation for the study: In Peru, clinical trials are presented for the evaluation of pharmaceutical products and medical devices in different specialties. The increase in the number of oncological trials is caused by an increase in the global prevalence of cancer. Therefore it, is necessary to determine the characteristics that these trials have had during the last 25 years.
Main findings: There is a variation in the number of clinical trials presented. We observed an increase in oncological trials, most of them sponsored by the pharmaceutical industry and mainly focused on the most prevalent types of neoplasms such as breast cancer.
Implications: Knowledge of the characteristics of oncological clinical trials will allow health professionals to identify the direction in which therapeutic development is headed.
THE STUDY
We carried out an observational and descriptive study to evaluate the characteristics of oncology-related clinical trials that were submitted to the General Office of Research and Technology Transfer (OGITT) of the INS for evaluation and authorization between 1995 and December 31, 2019. The public information found in the REPEC was reviewed and freely accessed through the portal https://ensayosclinicos-repec.ins.gob.pe; the last review was carried out on January 3, 2020. The records found were collected in a database for analysis.
The variables evaluated in the OCTs were grouped into general, design, and studied product characteristics. The general characteristics included type of sponsor, trial status, number of Research Ethics Committees (RECs) that approved the OCT, and number of research centers where the study was conducted.
Sponsors were grouped into five categories: cooperative groups (research networks, scientific societies, civil associations, foundations and research organizations), pharmaceutical industry (industries, laboratories and companies), national health institutes (both in Peru and abroad) and universities (public and private, national and foreign). The status of the trials were grouped into three categories: authorized (active, partial suspension, early termination, finished, suspended after authorization, canceled), unauthorized and others (process without effect, suspended before obtaining authorization, declared in abandonment, declared as withdrawal, declared unsuitable, under evaluation).
Regarding design characteristics of the OCTs, we considered phase of the trial, the specific design, type of blinding, type of randomization and the main endpoint. Regarding the studied product’s characteristics, we considered the type of product and the medical indication.
Design and studied product’s characteristics were analyzed in all OCTs submitted and in those authorized. Data not found were considered as “not registered” (NR).
Data collected was evaluated with the statistical program Stata V16.1 (Stata Corporation, College Station, Texas, USA) and qualitative variables were described with descriptive summary measures of proportions (%). No statistical imputation processes or inferential tests were carried out.
This study did not require the approval of an ethics committee, since it was a secondary analysis of a free access-database, and because it did not contain data that could identify the subjects.
FINDINGS
We identified 1,996 CTs from the period 1995-2019, of which 470 were oncological (23.5%); the first records were submitted in 1999 (n = 8; 1.7%). The number of CTs varies per year; it was evident that between 1999 and 2002 there were less than 20 per year. Then we observed a constant increase until 2008, when the maximum peak occurred (n = 50; 10.6%) and after this year the number of CTs progressively decreased in the following years. In spite of this, the proportion of OCTs submitted, with respect to the total number of CTs, has increased during the evaluated period (Figure 1).
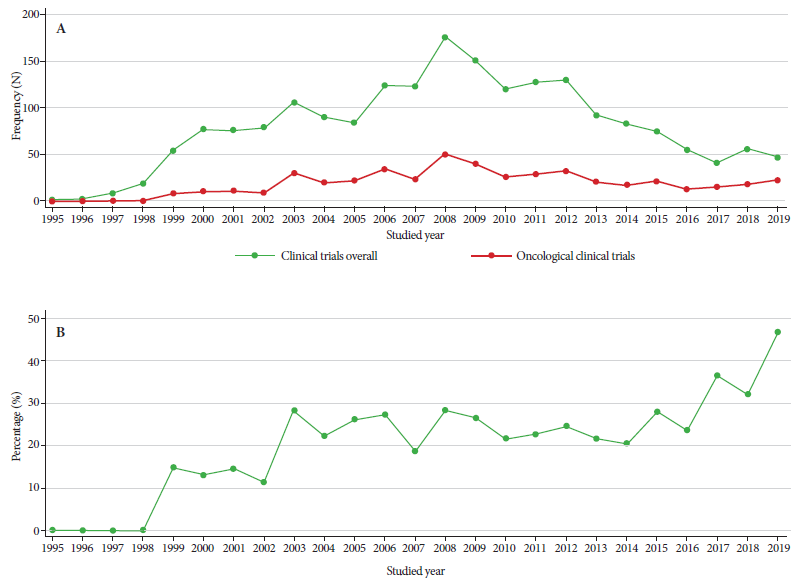
Figure 1 A. Frequency of clinical trials overall and oncological trials presented according to the year studied. B. Proportion of oncological clinical trials according to year during the period 1995-2019.
Regarding the general characteristics of the OCTs, we found that the most frequent type of sponsor was the pharmaceutical industry (74.9%). Most OCTs were authorized (n = 405; 86.2%), of which 61.2% were finished (n = 248). Nearly half (49.6%) of the OCTs were approved by only one REC and the most frequent (n = 249; 26.7%) was the one from the Instituto Nacional de Enfermedades Neoplásicas (INEN). Most of the OCTs (38.1%) were conducted in only one research center. The highest number of research locations for a single study was 14 (Table 1). The proportion of authorized OCTs varied over the years, with a tendency towards an irregular decrease (Supplementary material).
Table 1 General characteristics of oncological clinical trials presented during the period 1995-2019.
| Characteristics | n (%) |
|---|---|
| Type of sponsor | |
| Cooperative groups | 54 (11.5) |
| Pharmaceutical Industry | 352 (74.9) |
| National Institutes of Health | 5 (1.1) |
| Universities | 2 (0.4) |
| Other | 3 (0.6) |
| NR | 54 (11.5) |
| Status of the trial | |
| Authorized | 405 (86.2) |
| Active | 88 (18.7) |
| Partial suspension | 1 (0.2) |
| Early termination | 13 (2.8) |
| Finished | 248 (52.8) |
| Suspended after authorization | 48 (10.2) |
| Canceled | 7 (1.5) |
| Unauthorized | 18 (3.8) |
| Other | 47 (10.0) |
| Process without effect | 13 (2.8) |
| Suspended before | 4 (0.9) |
| obtaining authorization | |
| Declared in abandonment | 10 (2.1) |
| Declared as withdrawal | 13 (2.8) |
| Declared unsuitable | 1 (0.2) |
| Under evaluation | 6 (1.3) |
| Number of RECs that approved the protocol | |
| 1 | 233 (49.6) |
| 2 | 0 (0.0) |
| 3 | 101 (21.5) |
| 4 | 78 (16.6) |
| 5 | 37 (7.9) |
| 6 | 15 (3.2) |
| 7 | 3 (0.6) |
| 8 | 2 (0.4) |
| 9 | 1 (0.2) |
| Number of research centers | |
| 1 | 179 (38.1) |
| 2 | 81 (17.2) |
| 3 | 70 (14.9) |
| 4 | 49 (10.4) |
| 5 | 36 (7.7) |
| 6 | 18 (3.8) |
| 7 | 14 (3.0) |
| 8 | 10 (2.1) |
| 9 | 5 (1.1) |
| 10 | 3 (0.6) |
| 11 | 2 (0.4) |
| 12 | 0 (0.0) |
| 13 | 1 (0.2) |
| 14 | 2 (0.4) |
| Total | 470 (100.0) |
NR: Not registered
REC: Research Ethics Committee.
Regarding the design characteristics, most of the OCTs were in phase III, followed by phase II; the most used specific design was parallel groups (84.5%), adaptive CTs were the least frequent ones (basket = 0.4%, umbrella = 0.2). Among the authorized OCTs, open-label and double-blind CTs were found in similar frequency (47.9 and 46.4%, respectively); 85.2% were randomized (Table 2).
Table 2 Characteristics of the design of oncological clinical trials presented and authorized during the period 1995-2019.
| Characteristics | Presented n (%) | Authorized n (%) |
|---|---|---|
| Trial phase | ||
| I | 18 (3.8) | 14 (3.5) |
| I-II | 1 (0.2) | 1 (0.2) |
| II | 135 (28.7) | 119 (29.4) |
| II-III | 1 (0.2) | 1 (0.2) |
| III | 289 (61.5) | 246 (60.7) |
| IV | 22 (4.7) | 20 (4.9) |
| NR | 4 (0.9) | 4 (1.0) |
| Specific design | ||
| Single arm | 59 (12.6) | 51 (12.6) |
| Parallel groups | 397 (84.5) | 343 (84.7) |
| Cross-sectional | 7 (1.5) | 6 (1.5) |
| Factorial | 4 (0.9) | 2 (0.5) |
| Adaptative-Basket | 2 (0.4) | 2 (0.5) |
| Adaptative-Umbrella | 1 (0.2) | 1 (0.2) |
| Type of blinding | ||
| Open-label | 222 (47.2) | 194 (47.9) |
| Simple | 13 (2.8) | 10 (2.5) |
| Doble | 222 (47.2) | 188 (46.4) |
| Triple | 5 (1.1) | 5 (1.2) |
| NR | 8 (1.7) | 8 (2.0) |
| Type of randomization | ||
| Not randomized | 9 (1.9) | 7 (1.7) |
| Randomized | 400 (85.1) | 345 (85.2) |
| Does not apply | 61 (13.0) | 53 (13.1) |
| Main endpoint | ||
| Quality of life | 1 (0.2) | 1 (0.2) |
| Cancer incidence | 2 (0.4) | 2 (0.5) |
| Report of symptoms | 10 (2.1) | 10 (2.5) |
| Safety | 23 (4.9) | 22 (5.4) |
| Sensitivity and Specificity | 2 (0.4) | 2 (0.5) |
| Overall survival | 63 (13.4) | 54 (13.3) |
| Disease-free survival | 20 (4.3) | 18 (4.4) |
| Event-free survival | 5 (1.1) | 5 (1.2) |
| Progression-free survival | 106 (22.6) | 89 (22.0) |
| Complete response rate | 8 (1.7) | 5 (1.2) |
| Objective response rate | 221 (47.0) | 189 (46.7) |
| Time to Progression | 9 (1.9) | 8 (2.0) |
| Total | 470 (100) | 405 (100) |
NR: Not registered.
During the analysis of the studied product’s characteristics, we found that chemical products were the most frequent among the authorized OCTs (55.6%). Regarding the indication of the product, we found that most were therapeutic (91.4%) and those aimed at breast cancer (35.9%) stand out. Among those with palliative indications, products for emesis (45.7%) and pain (34.3%) were the most frequent. The most used main endpoint was the objective response rate, which was used in 47.0% of all the trials and 46.7% among those authorized (Table 3).
Table 3 Characteristics of the studied product of oncological clinical trials presented and authorized during the period 1995-2019.
| Characteristics | Presented n (%) | Authorized n (%) |
|---|---|---|
| Type of product | ||
| Medical device | 3 (0.6) | 2 (0.5) |
| Chemical product | 263 (56.0) | 225 (55.6) |
| Biological product | 196 (41.7) | 172 (42.5) |
| Other | 8 (1.7) | 6 (1.5) |
| Product indication | ||
| Palliative use | 38 (8.1) | 35 (8.6) |
| Anemia | 3 (7.9) | 2 (5.7) |
| Liver dysfunction | 1 (2.6) | 1 (2.9) |
| Intestinal Dysfunction | 2 (5.3) | 2 (5.7) |
| Pain | 12 (31.6) | 12 (34.3) |
| Emesis | 18 (47.4) | 16 (45.7) |
| Hypercalcemia | 1 (2.6) | 1 (2.9) |
| Pneumonia | 1 (2.6) | 1 (2.9) |
| Therapeutic use | 432 (91.9) | 370 (91.4) |
| Anal | 1 (0.2) | 0 (0.0) |
| Head and neck | 10 (2.3) | 7 (1.9) |
| Brain | 1 (0.2) | 1 (0.3) |
| Colorectal | 12 (2.8) | 9 (2.4) |
| Cervix | 7 (1.6) | 4 (1.1) |
| Esophageal | 3 (0.7) | 3 (0.8) |
| Gastrointestinal | 24 (5.6) | 22 (5.9) |
| Hematological | 32 (7.4) | 29 (7.8) |
| Liver | 7 (1.6) | 6 (1.6) |
| Lymphatic | 36 (8.3) | 28 (7.6) |
| Breast | 148 (34.3) | 133 (35.9) |
| Multiple | 13 (3.0) | 10 (2.7) |
| Nasopharyngeal | 1 (0.2) | 1 (0.3) |
| Osteosarcoma | 1 (0.2) | 1 (0.3) |
| Ovary | 10 (2.3) | 7 (1.9) |
| Pancreas | 8 (1.9) | 6 (1.6) |
| Skin | 10 (2.3) | 8 (2.2) |
| Prostate | 28 (6.5) | 22 (5.9) |
| Lung | 66 (15.3) | 59 (15.9) |
| Kidney | 4 (0.9) | 4 (1.1) |
| Myeloid Sarcoma | 2 (0.5) | 2 (0.5) |
| Thyroid | 2 (0.5) | 2 (0.5) |
| Uterus | 1 (0.2) | 1 (0.3) |
| Vagina and vulva | 1 (0.2) | 1 (0.3) |
| Bladder | 4 (0.9) | 4 (1.1) |
| Total | 470 (100) | 405 (100) |
DISCUSSION
We identified that the number of OCTs submitted has varied annually. However, considering the proportion of OCTs with respect to the total number of registered CTs, an upward trend was evident, by 2019 these represented almost 50%. This behavior is not specific to Peru, according to the data registered in ClinicalTrials.gov and EudraCT, we found similar distributions in other countries 7.
In the 25-year period that was analyzed, we found that 23.5% of the CTs were oncological; this figure is similar to the 22.4% reported by Minaya et al. 5 for the period 1995-2012. These results show the significant presence of this type of research in our country over time, slightly higher than the 21.8% found in ClinicalTrials.gov for the period 2007-2010 8.
On the other hand, the studied products were mostly for therapeutic use (91.9%), aimed at the treatment of breast and lung cancer. These findings are consistent with the analysis of Minaya et al. 5 ), and reflect the high incidence of these types of cancer in the world 9. The high morbimortality of cancer has encouraged the production of OCTs on diagnostic methods, like for example the study of the efficacy of the folate receptor-mediated staining solution as a tool for early detection of cervical cancer 10, or the use of new “digital tomosynthesis” equipment for the systematic identification of breast cancer 11.
Most Peruvian CTs were sponsored by the pharmaceutical industry. A similar pattern is evident in Western European countries, where for the period 2007-2015; 74% of the CTs had commercial sponsorship 12. Participation of the pharmaceutical industry in the research of new cancer drugs is not something new; this type of financing has already been identified and has shown favorable results in these studies 13.
During the last two years, two new OCTs designs have been registered; these adaptive designs or so-called “Master Protocols”, are classified as Basket (defined by cohorts with different types of tumors assigned to the same treatment), and Umbrella (defined by cohorts with the presence of the same type of tumor with or without associated biomarkers that receive different treatments) 14 , 15. These adaptive designs, together with the Platform type (not yet registered in our country), have been increasingly adopted worldwide since 2001 as an alternative for OCTs; and are more frequent in the United States 16.
Traditionally, the main endpoints used in the OCTs were clinical improvement measures, such as the improvement of signs and/or symptoms, and the patient’s quality of life 17. However, during the last decade, sponsors have used other criteria to achieve the accelerated approval of anti-cancer drugs, among these we have the tumor shrinkage evaluation and tumor growth retardation 18. Given this scenario, regulatory agencies have been in charge of evaluating the relevance, case by case, of the use of substitute endpoints and of providing guidelines for reasonable use 19. All of this has meant that currently the main endpoints used for these studies are the objective response rate, or progression-free survival, considering that the need for their use will always depend on the context of the disease and the magnitude of the effect, among other factors 20.
A limitation of this study was that the information comes from what was registered in the REPEC, a platform created in 2007, which has been collecting data retrospectively on CTs that had previously been evaluated and authorized by the Ministerio de Salud. This leads to a possible information bias for data from the period 1995-2006. Despite this limitation, this study characterizes the most frequent group of CTs presented in our country in the last 25 years.
In conclusion, the frequency of the OCTs in Peru shows variations during the evaluated period, although proportionally an ascending behavior has been evidenced over the years. It was also possible to identify that phase II and III OCTs are the most frequent in our country. We evidenced that most studied products were for therapeutic use and were aimed at breast cancer.
The identification of OCTs with adaptive designs represents a challenge for ethics committees, patients, researchers and regulatory authorities alike. For this reason, in compliance with its role as the governing body in the area of CTs, the INS is called upon to establish training activities that guarantee an adequate implementation in our country.
REFERENCES
1. Sotelo-Rodríguez DC, Ruíz-Patiño A, Ricaurte L, Arrieta O, Zatarain-Barrón ZL, Cardona AF. Challenges and shifting paradigms in clinical trials in oncology: the case for immunological and targeted therapies. Ecancer. 2019;13:936. doi: 10.3332/ecancer.2019.936. [ Links ]
2. Unger JM, Cook E, Tai E, Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book. 2016;36:185-98. doi: 10.1200/EDBK_156686. [ Links ]
3. Ogino D, Takahashi K, Sato H. Characteristics of clinical trial websites: information distribution between ClinicalTrials.gov and 13 primary registries in the WHO registry network. Trials. 2014;15(1):428. doi: 10.1186/1745-6215-15-428. [ Links ]
4. Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015;5(9):e008932. doi: 10.1136/bmjopen-2015-008932. [ Links ]
5. Minaya G, Fuentes D, Obregón C, Ayala-Quintanilla B, Yagui M. Características de los ensayos clínicos autorizados en el Perú, 1995-2012. Rev Peru Med Exp Salud Publica. 2012;29(4):431-36. doi: 10.1590/s1726-46342012000400003. [ Links ]
6. Alarcon-Ruiz CA, Roque-Roque JS, Heredia P, Góme-Briceño AR, Quispe AM. Twenty-two years' experience registering trials in a low-middle income country: The Peruvian Clinical Trial Registry. J Evid Based Med. 2019;12(3):187-93. doi: 10.1111/jebm.12354. [ Links ]
7. The Lancet Oncology. Clinical trial registry reporting: a transparent solution needed. The Lancet Oncology. 2019;20(6):741. doi: 10.1016/S1470-2045(19)30350-X. [ Links ]
8. Hirsch BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K, et al. Characteristics of Oncology Clinical Trials: Insights From a Systematic Analysis of ClinicalTrials.gov. JAMA Intern Med. 2013;173(11):972. doi: 10.1001/jamainternmed.2013.627. [ Links ]
9. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [ Links ]
10. Zhao Y, Li M, Li Y, Lv Q, Chen F, Li B, et al. Evaluation of Folate Receptor-Mediated Cervical Dyeing as a Method for Detection of Cervical Lesions: Journal of Lower Genital Tract Disease. 2019;23(2):133-7. doi: 10.1097/LGT.0000000000000411. [ Links ]
11. SOS Study team, Chauvie S, De Maggi A, Baralis I, Dalmasso F, Berchialla P, et al. Artificial intelligence and radiomics enhance the positive predictive value of digital chest tomosynthesis for lung cancer detection within SOS clinical trial. Eur Radiol. 2020;30(7):4134-40. doi: 10.1007/s00330-020-06783-z. [ Links ]
12. Dombernowsky T, Hædersdal M, Lassen U, Thomsen SF. Development in the number of clinical trial applications in Western Europe from 2007 to 2015: retrospective study of data from national competent authorities. BMJ Open. 2017;7(7):e015579. doi: 10.1136/bmjopen-2016-015579. [ Links ]
13. Al-Badriyeh D, Alameri M, Al-Okka R. Cost-effectiveness research in cancer therapy: a systematic review of literature trends, methods and the influence of funding. BMJ Open. 2017;7(1):e012648. doi: 10.1136/bmjopen-2016-012648. [ Links ]
14. Goldberg RM, Wei L, Fernandez S. The Evolution of Clinical Trials in Oncology: Defining Who Benefits from New Drugs Using Innovative Study Designs. The Oncol. 2017;22(9):1015-9. doi: 10.1634/theoncologist.2017-0153. [ Links ]
15. Sudhop T, Brun NC, Riedel C, Rosso A, Broich K, Senderovitz T. Master protocols in clinical trials: a universal Swiss Army knife? The Lancet Oncology. 2019;20(6):e336-42. doi: 10.1016/S1470-2045(19)30271-2. [ Links ]
16. Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20(1):572. doi: 10.1186/s13063-019-3664-1. [ Links ]
17. Kilickap S, Demirci U, Karadurmus N, Dogan M, Akinci B, Sendur MAN. Endpoints in oncology clinical trials. JBUON. 2018; 23(s1):1-6. [ Links ]
18. Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. The Lancet Oncology. 2015;16(1):e32-42. doi: 10.1016/S1470-2045(14)70375-4. [ Links ]
19. Food and Drug Administration. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics [Internet]. Maryland: Estados Unidos. Departamento de Salud y Servicios Sociales; 2018. Disponible en: https://www.fda.gov/media/71195/download. [ Links ]
20. Kamat AM, Sylvester RJ, Böhle A, Palou J, Lamm DL, Brausi M, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. JCO. 2016;34(16):1935-44. doi: 10.1200/JCO.2015.64.4070. [ Links ]
Received: January 27, 2020; Accepted: August 05, 2020











 text in
text in 



