Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634On-line version ISSN 1726-4642
Rev. perú. med. exp. salud publica vol.38 no.2 Lima Apr./Jun. 2021 Epub July 08, 2021
http://dx.doi.org/10.17843/rpmesp.2021.382.6764
Original articles
Situation of the tuberculosis-diabetes comorbidity in adults in Peru: 2016-2018
1 Instituto de Medicina Tropical Alexander von Humboldt, Facultad de Medicina, Universidad Peruana Cayetano Heredia, Lima, Perú.
2 TB Centre, London School of Hygiene and Tropical Medicine, Londres, Reino Unido.
3 Centro Nacional de Salud Pública, Instituto Nacional de Salud, Lima, Perú
4 Servicio de Neumología, Hospital Nacional Arzobispo Loayza, Lima, Perú.
Objective:
To describe the characteristics of adult patients with tuberculosis (TB) and diabetes mellitus (DM) in Peru, and to explore the association of DM and mortality in people with TB.
Materials and methods:
We carried out a secondary analysis of the database of the Management Information System of Tuberculosis of the Tuberculosis Prevention and Control Directorate of the Ministry of Health of Peru. Adult patients who started treatment with the scheme for drug-sensitive TB in 2016, 2017 and 2018 were included. We carried out a descriptive analysis of patients with TB and DM, and an exploratory analysis to assess the association of DM with mortality using a Poisson regression to determine the relative risk (RR).
Results:
We registered 67,524 adults with drug-sensitive TB, of which 6,529 (9.7%) people were reported as having TB and DM; and 4,048 (6.0%) had HIV infection. Of the patients reported with TB and DM, most were men (60.2%) with a median age of 53 years. Regarding mortality, people with TB and DM had a higher frequency of death compared to those with TB without DM (7.2% vs 5.4%). In the exploratory analysis of factors associated with mortality, DM had a crude RR of 1.32 (95% CI: 1.20-1.50); however, this association varied in the adjusted model with a RR of 0.93 (95% CI: 0.84-1.04).
Conclusions:
DM is the most frequent comorbidity in patients with TB in Peru, although no association with higher mortality was found.
Keywords: Tuberculosis; Diabetes Mellitus; Comorbidity; Mortality; Survival
INTRODUCTION
In recent years, there has been an increase in non-communicable diseases such as diabetes mellitus (DM) in populations of middle and low-income countries such as Peru 1. The fact that Peru is one of the countries with the highest burden of tuberculosis (TB) in the Americas puts it on alert for a possible increase in cases of TB/DM comorbidity 2. A systematic review of 200 studies found a combined prevalence of DM in TB patients of 15.3%, where the estimate for Latin America was 7.7% 3.
This comorbidity may jeopardize ongoing efforts to control TB. A mathematical model determined how the increase in DM prevalence could jeopardize the reduction in TB incidence observed over the past few years. With an overall DM prevalence of 10% (as estimated by the International Diabetes Federation in 2013), TB incidence would increase by 3% in 2035, compared with the estimated incidence without an increase in DM prevalence 4. Similarly, considering that DM is a multifactorial disease and that factors, such as obesity, are increasing, the prevalence for DM would be 12.5%, with a change in TB incidence of 8% in 2035. This model demonstrates that the increase in DM prevalence may directly affect TB incidence in the coming years, which would negatively impact the targets set to eliminate TB 4.
There is evidence that TB/DM comorbidity increases the risk of poor TB treatment outcomes. A recent systemic review found that DM increases the risk of death (OR: 1.88; 95% CI: 1.59-2.21) and relapse (OR: 1.64; 95% CI: 1.29-2.08) 5. Another systematic review found that patients with DM are two to four times more at risk of having active TB 6. Likewise, in the United Kingdom it was found that having a history of TB increases the risk of DM by almost six times, so that this comorbidity increases the clinical impact on the patient even after the TB episode 7. Finally, the presence of DM in TB patients increases the risk of cardiovascular death five years after the end of TB treatment (HR: 1.70; 95% CI: 1.23-2.35) 8.
Some of these results can be explained by the fact that DM, due to hyperglycemia, creates inflammatory and vascular changes that can increase the risk of TB, in addition to changes in the immune response 9. Likewise, there are studies that provide evidence that glycemia control can decrease this risk 10, this evidence is important for the treatment of TB/DM comorbidity.
In this context, it is important to evaluate the epidemiological characteristics of patients with TB and DM in Peru, in order to have a better overview for the planning of potential interventions for their comprehensive management. This study aims to describe the demographic and treatment characteristics of TB in the adult population with TB/DM comorbidity, in addition to exploring the association between DM and mortality in TB patients.
KEY MESSAGES
Motivation for the study: The prevalence of diabetes mellitus (DM) is increasing worldwide, especially in countries such as Peru, which may affect the control of tuberculosis (TB).
Main findings: DM is the most frequent comorbidity in TB patients, with an older median age compared to TB patients without DM. Despite a higher frequency of mortality in TB patients with DM, no evidence of association of DM with mortality in TB patients has been found.
Implications: Comorbidity of TB with DM is frequent in Peruvian patients, interventions that help in the management of this comorbidity should be implemented.
MATERIALS AND METHODS
Study design
This is an observational retrospective cohort study based on a secondary analysis of the Tuberculosis Management Information System (SIGTB) database in Peru.
Data Source
The SIGTB database 11 of the Tuberculosis Prevention and Control Directorate (DPCTB) of the Peruvian Ministry of Health (MINSA) registers patients with TB treatment throughout the country. This database includes patients from MINSA, Social Insurance (EsSalud), the Military and Police Forces and the National Penitentiary Institute (INPE). Upon request, the DPCTB provided this database, in an Excel file and without personal identifiers, for the analysis of this study.
Selection criteria
We included those patients who started treatment during the years 2016, 2017 and 2018, and who were older than 18 years and included in the treatment scheme for drug-sensitive TB. No patients who met the inclusion criteria were excluded.
Variables
A case of TB with DM was considered to be a patient who had a record of previous diagnosis of DM and/or diagnosis of DM with glycemia test according to the SIGTB report, which is included in the clinical record of the TB patient. According to the DPCTB Technical Standard, all patients with a diagnosis of TB (with or without a history of DM) should undergo a fasting glucose test, in addition to other laboratory tests such as complete blood count, liver profile or HIV test 12.
Statistical analysis
We used frequencies to describe the categorical variables (sex, age group, location of the disease, institution providing care, geographic area, treatment outcome) as well as medians and interquartile range for continuous variables (age). In the case of treatment outcome, we used the categories established in the Technical Standard (cured, complete treatment, death, treatment failure, treatment loss), which are consistent with the definitions of TB treatment outcome suggested by the World Health Organization (WHO) 12 , 13. Other variables evaluated were HIV infection, history of TB treatment, adverse drug reaction and report of smoking, alcoholism and drug addiction according to the definition used in the SIGTB based on the requirements of the DPCTB Technical Standard 12.
To evaluate the differences between TB patients with and without DM, we used the Chi-square test (categorical variables) and the Mann-Whitney test (numerical variables). An exploratory analysis was carried out to evaluate factors associated with mortality, using a Poisson regression with robust variance to determine the relative risk (RR) with 95% confidence intervals (95% CI); we also evaluated crude and adjusted models for HIV, sex, age group, TB location, and TB history. These variables were selected because they were reported as confounding variables, according to the available epidemiological evidence 14 - 17. The analysis was carried out using the statistical package STATA 16.1® (StataCorp, USA). Due to the fact that we analyzed an operational database of the entire country with quality control, several of the variables had missing data, although less than 10%; however, the DM information had 14.5% missing data, but not randomly (there were regions that had different filling ratios), so multiple imputation could not be performed and we only described the information.
RESULTS
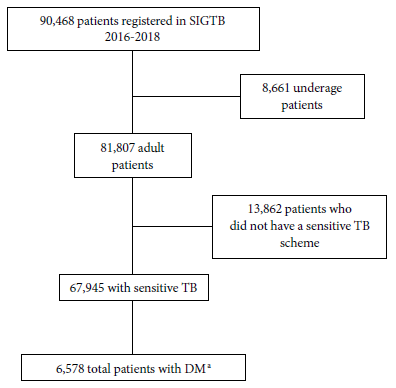
A total of 90,468 TB patients were registered in the SIGTB (Figure 1), of which 81,807 were adults. We excluded those who were not on the treatment schedule for drug-sensitive TB and who did not have a favorable treatment outcome. Resulting in 67,524 people with TB, whose sociodemographic characteristics are shown in Table 1. We found that 9.7% of patients had DM (6,529/67,524) and 6.0% of patients had HIV (4,048/67,524). The description of TB patients with DM is presented in Table 2.
Table 1 General characteristics of the population with drug-sensitive tuberculosis in Peru, 2016-2018.
| Characteristics | People with TB (n=67,524) |
|---|---|
| Men (%) | 43,486 (64.4) |
| Age median (IQR) | 35 (25-52) |
| Age groups (n=67,355) (%) | |
| 18-24 | 16,476 (24.5) |
| 25-34 | 17,128 (25.4) |
| 35-44 | 10,680 (15.9) |
| 45-54 | 7,774 (11.6) |
| 55-64 | 6,297 (9.4) |
| > 65 | 9,000 (13.4) |
| No history of TB (%) | 59,280 (87.8) |
| HIV infection (n = 62 988) (%) | 4,048 (6.0) |
| Diabetes (n = 57 736) (%) | 6,529 (9.7) |
| Institution (%) | |
| MINSA | 49,430 (73.2) |
| EsSalud | 12,018 (17.8) |
| INPE | 5,879 (8.7) |
| Military and Police Forces | 197 (0.3) |
| TB location (%) | |
| Pulmonary | 53,893 (79.8) |
| Extrapulmonary | 13,631 (20.2) |
| Smoking | 6,218 (9.2) |
| Alcoholism | 7,810 (11.6) |
| Drug addiction | 6,916 (10.2) |
| Irregularity in 1 a phase (%) | 3,652 (5.4) |
| Irregularity in 2 a phase (%) | 9,132 (13.5) |
| Discharge condition (%) | |
| Healed | 44,196 (65.5) |
| Complete treatment | 13,626 (20.2) |
| Lost in treatment | 5,670 (8.4) |
| Deceased | 3,831 (5.7) |
| Treatment failure | 201 (0.3) |
| Adverse drug reaction | 716 (1.1) |
IQR: interquartile range; TB: tuberculosis; HIV: human immunodeficiency virus; MINSA: Ministry of Health; EsSalud: Social Health Insurance; INPE: National Penitentiary Institute.
Table 2 General characteristics of patients with diagnosed tuberculosis with or without the comorbidity of diabetes mellitus.
| Characteristics | TB without DM | TB with DM | p value |
|---|---|---|---|
| (n=52,357) | (n=6,577) | ||
| Men (%) | 31,904 (62.3) | 3,932 (60.2) | 0.001 |
| Median age (IQR) | 33 (24-51) | 53 (41-63) | <0.001 |
| Age groups (n = 67 773) (%) | |||
| 18-24 | 13,483 (26.3) | 396 (6.1) | |
| 25-34 | 13,274 (26.0 | 604 (9.3) | |
| 35-44 | 8,055 (15.8) | 1,003 (15.4) | <0.001 |
| 45-54 | 5,323 (10.4) | 1,526 (23.4) | |
| 55-64 | 4,200 (8.2) | 1,520 (23.3) | |
| > 65 | 6,749 (13.2) | 1,478 (22.6) | |
| HIV (%) | 3,284 (6.7) | 256 (4.2) | <0.001 |
| Extrapulmonary TB (%) | 11,366 (22.2) | 790 (12.1) | <0.001 |
| Smoking (%) | 3,002 (5.7) | 280 (4.3) | <0.001 |
| Discharge condition (%) | |||
| Healed | 33,241 (64.9) | 4,500 (68.9) | |
| Complete treatment | 10,886 (21.3) | 1,170 (17.9) | |
| Lost in treatment | 4,165 (8.1) | 368 (5.6) | <0.001 |
| Deceased | 2,775 (5.4) | 467 (7.2) | |
| Treatment failure | 140 (0.3) | 24 (0.4) | |
| Adverse drug reaction (%) | 582 (1.2) | 81 (1.2) | 0.297 |
DM: diabetes mellitus; IQR, interquartile range; TB, tuberculosis; HIV, human immunodeficiency virus.
The regions with the highest proportion of patients with TB and DM were Tumbes, Moquegua, Piura, Ucayali, San Martin and Cajamarca. Regarding the frequency of TB by institutions, in EsSalud we found 13.1% (1,570/12,018), in the Peruvian National Police Health Service we found 12.4% (10/81) and in MINSA we found 9.8% (4860/49,430). The INPE reported 1.4% of TB with DM, but had 66.8% of its TB population with no result and/or report of DM (3,929/5,879). The Air Force Health institution (1 patient) and the Army Health institution (14 patients) did not report patients with TB and DM.
Regarding TB treatment outcomes, we found that TB patients with DM had similar proportion of successful outcome (defined as cured and treatment completed): 86.8% of people with TB and DM successfully completed treatment, compared to people with TB without DM (86.2%); regarding mortality during TB treatment, TB patients with DM have a higher frequency of death compared to those with TB without DM (7.1% vs. 5.4%).
In the exploratory analysis of the factors associated with mortality, DM presented, in the crude analysis, a RR of 1.32 (95% CI: 1.20-1.50); however, this association varied when adjusted for HIV, sex, age group, extrapulmonary tuberculosis and history of TB (RR: 0.93; 95% CI: 0.84- 1.04) (Table 3).
Table 3 Poisson regression model for the association between diabetes mellitus and mortality in patients with tuberculosis (n = 55,322).
| Factors | Crude model | Adjusted model a | ||
|---|---|---|---|---|
| RR (95% CI) | p value | RR (95% CI) | p value | |
| DM | ||||
| No | 1 (Ref.) | <0.001 | 1 (Ref.) | |
| Yes | 1.32 (1.20-1.50) | 0.9 (0.8-1.0) | 0.185 | |
| HIV | ||||
| No | 1 (Ref.) | 1 (Ref.) | ||
| Yes | 5.48 (5.08-5.91) | <0.001 | 6.8 (6.2-7.4) | <0.001 |
| Sex | ||||
| Female | 1 (Ref.) | 1 (Ref.) | ||
| Male | 1.31 (1.23- 1.41) | <0.001 | 1.3 (1.2-1.4) | <0.001 |
| Age group (years) | ||||
| 18-24 | 1 (Ref.) | 1 (Ref.) | ||
| 25-34 | 2.1 (1.8-2.5) | <0.001 | 1.7 (1.4-2.0) | <0.001 |
| 35-44 | 3.2 (2.7-3.7) | <0.001 | 2.3 (2.0-2.8) | <0.001 |
| 45-54 | 4.4 (3.8-5.2) | <0.001 | 3.7 (3.1-4.4) | <0.001 |
| 55-64 | 6.1 (5.2-7.1) | <0.001 | 6.1 (5.2-7.2) | <0.001 |
| > 65 | 11.8 (10.2-13.5) | <0.001 | 11.4 (9.8-13.3) | <0.001 |
| Extrapulmonary TB | ||||
| No | 1 (Ref.) | 1 (Ref.) | ||
| Yes | 1.6 (1.4-1.7) | <0.001 | 1.5 (1.4-1.7) | <0.001 |
| History of TB | ||||
| No | 1 (Ref.) | 0.278 | 1 (Ref.) | 0.331 |
| Yes | 1.1 (1.0-1.2) | 1.1 (0.9-1.2) | ||
DM: diabetes mellitus; TB: tuberculosis; HIV: human immunodeficiency virus; RR: relative risk.
a Adjusted for HIV, sex and age.
DISCUSSION
We found that during the 2016-2018 period, adult Peruvians who had sensitive TB presented a higher prevalence of DM compared to HIV, and even compared to addictions such as alcoholism, smoking and drug addiction, placing it as the most common comorbidity among those registered in the SIGTB. However, although a higher frequency of mortality was found in patients with TB and DM, this mortality was not associated with DM status. This result is different from that found in other studies 5 , 18, which could be explained by the presence of unmeasured confounding variables (treatment for DM, body mass index at TB diagnosis) that would have allowed us to better isolate the effect of DM on mortality in patients with TB.
These results on the frequency of DM are similar to those found in Peruvian studies that evaluated the association of TB and DM, where prevalence values of 12.5% 19 and 14.0% 20, respectively, have been found; however, in these studies glycosylated hemoglobin tests were performed to confirm DM status. It is likely that there is an underreporting of cases with DM in this study because glycosylated hemoglobin was not used for diagnosis. These findings of high prevalence of DM in TB patients have also been found in other countries 21 , 22 where prevalence values of around 10% have been described.
It has been reported that in most of these studies one of the most important limitations is the lack of data on non-infectious comorbidities (arterial hypertension, obesity, depression, cardiovascular disease), which are usually associated with DM and a greater degree of severity 23. Within the person-centered approach, it should be recognized that a person with TB should be evaluated in a comprehensive manner, especially if cardiovascular risk factors are identified. There is evidence that shows that even after TB treatment, there is an increased risk of mortality. A study in Brazil found that having DM was associated with mortality from cardiovascular causes five years after TB diagnosis (HR: 1.70; 95% CI: 1.23-2.34) 8. Likewise, it should be considered that in addition to DM, there are other cardiovascular conditions that affect people with TB. Evidence has been found of the association between Mycobacterium tuberculosis infection and myocardial infarction 24, so in a context where our populations are facing an increase in the prevalence of non-communicable diseases and the still high prevalence of TB, the patient should be evaluated in a comprehensive manner.
As mentioned, the lack of association between DM and mortality may be due to unmeasured confounding variables. One example is treatment for DM; there is evidence that shows that patients with controlled DM are successfully treated, similar to those patients with TB who do not have DM 25; likewise, there is evidence that shows that the use of metformin could be associated with a better TB treatment outcome 26 , 27, even reducing the risk of TB relapses three years after the end of treatment 28.
One of the problems we encountered in this study was the lack of data related to DM, which were not included in the SIGTB, such as type of DM treatment, glucose results, glycosylated hemoglobin, lipid profile, adherence to DM treatment, body mass index, among other variables. Since they are not considered as important information for the evaluation of the TB patient, there is a tendency to see the diseases as “silos” (independent of one another) and this does not allow for an adequate comprehensive evaluation, in addition to not being able to adequately isolate the real effect of DM on TB due to confounding variables that were not measured 13. However, in the new versions of the SIGTB, several of these variables are being included, which will allow better surveillance of TB/DM comorbidity.
Also, because the SIGTB does not record specific causes of death, in patients with TB, it is not known whether the cause of death was TB or a cardiovascular or metabolic cause. In addition, 14% of the database does not report the DM status (in some regions more than others), this could underestimate the association found. In the case of DM, it is important to have an adequate diagnostic evaluation, as a transient hyperglycemic state is common in TB patients (as in other infections). A study in Tanzania found that hyperglycemia was reduced after treatment for TB, but despite this, an association was found between hyperglycemia at the beginning of treatment with adverse treatment outcomes 29. Because of this, evaluation of glycemia in patients with DM and even hyperglycemia should be followed during TB treatment to confirm whether it is a case of transient hyperglycemia. Finally, treatment of DM is not regularly recorded in the TB treatment record, as there is not much evidence on the best treatment in these cases. Experts suggest that the use of insulin is the best option because it allows adequate glucose management, but it implies a greater involvement of health personnel to train the patient and his or her environment. On the other hand, metformin has shown, in some studies, activity against Mycobacterium tuberculosis 26 , 27 , 30, but there are still no randomized clinical trials that confirm this effect, in addition to presenting adverse events such as lactic acidosis, nausea or vomiting that can affect adherence to TB treatment.
The strength of this study is to have evaluated all patients registered in Peru during 2016-2018, with treatment results of almost all patients (>99%), thus making it representative of that period. The SIGTB platform allows a better evaluation and follow-up of this comorbidity, as it supports updates of important data such as glucose or body mass index. It also makes it possible to identify areas in the country where 100% screening for DM has not yet been achieved (something almost achieved for HIV), in order to strengthen the health system and achieve the objective of identifying patients with this comorbidity.
In conclusion, DM has been identified as the most common comorbidity in TB patients in Peru. Studies that evaluate the healthcare process of patients with TB and DM are needed to assess the barriers and difficulties presented by these patients, as well as to propose a multimorbidity approach within person-centered care.
REFERENCES
. International Diabetes Federation. IDF Diabetes atlas. Eighth edition. 2017. Disponible en: https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf. [ Links ]
. World Health Organization. Global tuberculosis report 2020: executive summary. Disponible en: https://apps.who.int/iris/bitstream/handle/10665/337538/9789240016095-eng.pdf. [ Links ]
. Noubiap JJ, Nansseu JR, Nyaga UF, Nkeck JR, Endomba FT, Kaze AD, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Health. 2019;7(4):e448-e60. doi: 10.1016/s2214-109x(18)30487-x. [ Links ]
. Odone A, Houben RM, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol. 2014;2(9):754-64. doi: 10.1016/s22138587(14)70164-0. [ Links ]
. Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(7):783-96. doi: 10.5588/ijtld.18.0433. [ Links ]
. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. doi: 10.1371/journal.pone.0187967. [ Links ]
. Pearson F, Huangfu P, McNally R, Pearce M, Unwin N, Critchley JA. Tuberculosis and diabetes: bidirectional association in a UK primary care data set. J Epidemiol Community Health. 2019;73(2):142-7. doi: 10.1136/jech-2018-211231. [ Links ]
. Ranzani OT, Rodrigues LC, Bombarda S, Minto CM, Waldman EA, Carvalho CRR. Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010-15: a population-based, longitudinal study. Lancet Infect Dis. 2020;20(1):123-32. doi: 10.1016/s1473-3099(19)30518-3. [ Links ]
. Ronacher K, van Crevel R, Critchley JA, Bremer AA, Schlesinger LS, Kapur A, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest. 2017;152(1):174-80. doi: 10.1016/j.chest.2017.02.032. [ Links ]
. Viney K, Mills T, Harley D. Tuberculosis and diabetes mellitus: a do-se-response relationship between the odds of tuberculosis and HbA1c. Int J Tuberc Lung Dis. 2019;23(10):1055-9. doi: 10.5588/ijtld.18.0657. [ Links ]
. Dirección de Prevención y Control de Tuberculosis. Sistema de Información Gerencial de Tuberculosis Data. Lima: MINSA; 2020. Disponible en: https://appsalud.minsa.gob.pe/sigtbdata/wflogin.aspx. [ Links ]
. Ministerio de Salud. Norma técnica de salud para la atención integral de las personas afectadas por tuberculosis. Lima: MINSA; 2013. [ Links ]
. Huangfu P, Pearson F, Ugarte-Gil C, Critchley J. Diabetes and poor tuberculosis treatment outcomes: issues and implications in data interpretation and analysis. Int J Tuberc Lung Dis. 2017;21(12):1214-9. doi: 10.5588/ijtld.17.0211. [ Links ]
. Jacob S, George LS, Joy A, Mathew MM, Vijayakumar K, Kumar A, et al. Prevalence of diabetes mellitus and HIV/AIDS among tuberculosis patients in Kerala. J Family Med Prim Care. 2020;9(12):6209-12. DOI: 10.4103/jfmpc.jfmpc_1583_20. [ Links ]
. Martins-Melo FR, Bezerra JMT, Barbosa DS, Carneiro M, Andrade KB, Ribeiro ALP, et al. The burden of tuberculosis and attributable risk factors in Brazil, 1990-2017: results from the Global Burden of Disease Study 2017. Popul Health Metr. 2020;18(Suppl 1):10. doi: 10.1186/s12963-020-00203-6. [ Links ]
. Menon S, Rossi R, Dusabimana A, Zdraveska N, Bhattacharyya S, Francis J. The epidemiology of tuberculosis-associated hyperglycemia in individuals newly screened for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):937. doi: 10.1186/s12879-020-05512-7. [ Links ]
. Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, van de Vijver S, Panduru NM, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014;2(9):740-53. doi: 10.1016/s2213-8587(14)70110-x. [ Links ]
. Mave V, Gaikwad S, Barthwal M, Chandanwale A, Lokhande R, Kadam D, et al. Diabetes Mellitus and Tuberculosis Treatment Outcomes in Pune, India. Open Forum Infect Dis. 2021;8(4):ofab097. doi: 10.1093/ofid/ofab097. [ Links ]
. Ugarte-Gil C, Alisjahbana B, Ronacher K, Riza AL, Koesoemadinata RC, Malherbe ST, et al. Diabetes Mellitus Among Pulmonary Tuberculosis Patients From 4 Tuberculosis-endemic Countries: The TANDEM Study. Clin Infect Dis. 2020;70(5):780-8. doi: 10.1093/cid/ciz284. [ Links ]
. Calderon RI, Arriaga MB, Lopez K, Barreda NN, Sanabria OM, Fróes Neto JF, et al. High prevalence and heterogeneity of Dysglycemia in patients with tuberculosis from Peru: a prospective cohort study. BMC Infect Dis. 2019;19(1):799. doi: 10.1186/s12879-019-4416-2. [ Links ]
. Alebel A, Wondemagegn AT, Tesema C, Kibret GD, Wagnew F, Petrucka P, et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2019;19(1):254. doi: 10.1186/s12879-019-3892-8. [ Links ]
. Liu Q, Lu P, Martinez L, Peng H, Zhu T, Zhu L, et al. Undiagnosed diabetes mellitus and tuberculosis infection: A population-based, observational study from eastern China. Diabetes Metab Res Rev. 2020;36(3):e3227. doi: 10.1002/dmrr.3227. [ Links ]
. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88-98. doi: 10.1038/nrendo.2017.151. [ Links ]
. Huaman MA, Ticona E, Miranda G, Kryscio RJ, Mugruza R, Aranda E, et al. The Relationship Between Latent Tuberculosis Infection and Acute Myocardial Infarction. Clin Infect Dis. 2018;66(6):886-92. doi: 10.1093/cid/cix910. [ Links ]
. Critchley JA, Restrepo BI, Ronacher K, Kapur A, Bremer AA, Schlesinger LS, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest. 2017;152(1):165-73. doi: 10.1016/j.chest.2017.04.155. [ Links ]
. Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6(263):263ra159. doi: 10.1126/scitranslmed.3009885. [ Links ]
. Yu X, Li L, Xia L, Feng X, Chen F, Cao S, et al. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: a systematic review. BMC Infect Dis. 2019;19(1):859. doi: 10.1186/s12879-019-4548-4. [ Links ]
. Ma Y, Pang Y, Shu W, Liu YH, Ge QP, Du J, et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis. 2018;37(7):1259-63. doi: 10.1007/s10096-018-3242-6. [ Links ]
. Boillat-Blanco N, Ramaiya KL, Mganga M, Minja LT, Bovet P, Schindler C, et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. J Infect Dis. 2016;213(7):1163-72. doi: 10.1093/infdis/jiv568. [ Links ]
. Rodriguez-Carlos A, Valdez-Miramontes C, Marin-Luevano P, González-Curiel I, Enciso-Moreno JA, Rivas-Santiago B. Metformin promotes Mycobacterium tuberculosis killing and increases the production of human ß-defensins in lung epithelial cells and macrophages. Microbes Infect. 2020;22(3):111-8. doi: 10.1016/j.micinf.2019.10.002. [ Links ]
Received: November 17, 2020; Accepted: June 02, 2021











 text in
text in