Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634On-line version ISSN 1726-4642
Rev. perú. med. exp. salud publica vol.38 no.2 Lima Apr./Jun. 2021 Epub July 01, 2021
http://dx.doi.org/10.17843/rpmesp.2021.382.7768
Special section
Procedure for infusion of autologous mitochondria through the carotid artery in porcine brain
1Laboratorio de Inmunopatología en Neurocisticercosis, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia, Lima, Perú.
2 Universidad de Washington, Escuela de Medicina, Departamento de Cirugía Neurológica y Centro de Neurociencia Aplicada, Seattle, Washington, Estados Unidos.
3 Unidad de Cisticercosis, Instituto Nacional de Ciencias Neurológicas, Lima, Perú.
Mitochondria are complex organelles that play a critical role within the cell; mitochondrial dysfunction can result in significant cell damage or death. Previous studies have demonstrated the promising therapeutic effects of autologous mitochondria transplantation into ischemic cardiac tissue; however, few studies have examined the in vivo effects of mitochondria infusion into the brain. The aim of this study is to report a procedure for carotid infusion of autologous mitochondria into porcine brains. By using this infusion technique, we propose that a selective and minimally invasive administration is feasible and may provide benefits in the treatment of various central nervous system disorders.
Keywords: Mitocondria; Microcatheter; Infusion; Carotid; Brain; Porcine
INTRODUCTION
Mitochondrial transplantation has been performed as a therapeutic measure for cardiac ischemia and reperfusion injury in both animals 1 and humans 2. These studies demonstrate that the infusion of autologous mitochondria, isolated from the patient’s tissue, compensates for the decrease or loss of mitochondrial function in local cells damaged during ischemia and improves post-ischemic recovery. Preclinical studies 1 and biosafety trials 3 have shown that the procedure is well-tolerated, without immunoreactivity 4, local injury, or systemic adverse effects reported. Studies are currently underway to understand the mechanism(s) involved in the internalization of extracellular mitochondria, their longevity once transplanted, and measurement of their effects, among other aspects.
In vitro 5 , 6 and murine studies of direct infusion of autologous mitochondria into the spinal cord and brain tissue have also shown positive effects in different neurological conditions such as trauma 7 , 8, schizophrenia 9, Parkinson’s disease 10 and ischemic stroke 11 , 12. Xenogeneic transplantation has also shown promising results after local intra-cerebral or intra-arterial injection in ischemic rat brains 13 and an experimental mouse model of Parkinson’s disease 14.
Unlike cardiac tissue, the central nervous system (CNS) presents a challenge for the treatment of many pathologies, which is to achieve the passage of therapeutic agents through the blood-brain barrier (BBB) that may limit the entry and absorption of various molecules 15; however, several neuropathological conditions are associated with a disruption of the BBB and tissue damage 16. Increasing the permeability of the BBB can also be achieved using hyperosmotic solutions such as mannitol 17, which is a 6-carbon polyol with diuretic activity, widely used in clinical trials and approved by the U.S. Food and Drug Administration (FDA).
Here, the authors propose a methodology for mitochondrial infusion via the carotid artery into the porcine brain. Additionally, the permeability of the BBB was increased to ensure mitochondrial entry. The authors conclude that microcatheter infusion may be a feasible option to study the potential therapeutic use of mitochondrial infusion in several neurologic pathologies.
DESCRIPTION OF THE PROCEDURE
Three female pigs aged two to six months were used. Two pigs were used to evaluate mitochondria infusion and one as a control. Accessed was obtained bilaterally to the carotid artery in all animals; the left artery for mannitol injection to induce a disruption in the BBB, and the contralateral artery without mannitol, thus, each brain had its control hemisphere without disruption of the BBB. This approach was taken to minimize the number of animals required for the study.
Ethics statement
The animals were fed and maintained with free access to water until one day before the intervention. The treatment of animals adhered to the Council for International Organizations of Medical Sciences (CIOMS) International Guiding Principles for Biomedical Research Involving Animals. The protocol for the use of animals was reviewed and approved by the Institutional Ethics Committee for the Use of Animals of the Universidad Peruana Cayetano Heredia (SIDISI 201478). The animals were sedated using an intramuscular injection of ketamine (10 mg/kg) and xylazine (2 mg/kg). The intervention sites were shaved, followed by soap scrubs. Endotracheal intubation was achieved in all animals and kept sedated with intravenous doses of ketamine (5 mg/kg) every 20 minutes. The entire cerebrovascular surgical procedure was performed with microcatheters in a sterile environment.
Skeletal Muscle Biopsy Collection
In the two pigs chosen for mitochondria infusion, the area selected for biopsy collection was the hind leg. The zone was desinfected with a povidone-iodine solution (10%), and a small incision was made using a #11 scalpel blade (USA Medical, St. Louis, MO; USA). Three samples of the gracilis muscle were collected for each pig using a #6 biopsy punch (Fisher Scientific, Waltham, MA, USA) and transported in sterile 50 ml tubes with 20 ml of phosphate-buffered saline (PBS pH 7.4) inside a container with ice. Finally, manual pressure and sutures were applied to the biopsy site.
Isolation and purification of mitocondria
Isolation of mitochondria was performed in a laminar flow cabinet following the protocol of Preble 18. Sample were transfered to a 15 ml tube containing 10 ml of Homogenization Buffer (300 mM sucrose; 10 mM HEPES; 1 mM EDTA; all Sigma-Aldrich, ST. Louis, USA) and disintegrated using a homogenizer (Omni International, Kennesaw, GA, USA) in 4 cycles of 60 sec. 250 µl of a trypsin solution (4 mg of trypsin dissolved in 1 ml of Homogenization Buffer) was added and incubated on ice for 10 minutes. Each homogenate was filtered with a 40 µm filter (Falcon) pre-wet with Homogenization Buffer into a new 15 ml tube, then 250 µl of a BSA solution (20 mg BSA dissolved in 1 ml Homogenization Buffer) was added. The samples were filtered with 40 and 10 µm filter pre-wet with homogenization buffer. They were centrifuged at 9,000 G (9000 x g) for 10 min at 4°C, the supernatant was discarded, and the pellet was resuspended in 1 ml of Respiration Buffer (sucrose 250 mM; KH2PO4 2 mM; MgCl2 10 mM; HEPES 20 mM, EDTA 0.5 mM; all Sigma-Aldrich brand, ST. Louis, USA).
Viability and mitochondrial labeling
A 1 ml aliquot of the mitochondria suspension was taken and incubated with MitoView Green (Biotium, Fremont, CA, USA) (200 nM) for 30 min to determine the total number of mitochondria obtained, then incubated with Mitotracker Red CM-H2Xros (Thermo Fisher Scientific, Waltham, MA, USA) (500 mM) for 30 min to determine the number of viable mitochondria. Counting was performed using a hemocytometer, and images were captured by confocal microscopy (Zeiss, LSM880, Oberkochen, Germany). The mean ± standard deviation of total and viable mitochondria count was obtained from the 3 muscle samples collected for each pig. Before proceeding with the infusion, the remaining reconstituted mitochondria were incubated with a mouse anti-mitochondrial monoclonal antibody (Abcam ab14730, Cambridge, UK) at a concentration of 1 µg/ml for 30 min at 4°C.
Vascular access and BBB disruption
Using the manubrium as an anatomical landmark and assisted by ultrasound guidance, a sterile #11 blade was used to incise the tissues of the ventral neck to expose the carotid artery. A Braun micro-introducer kit (Bethlehem, Pennsylvania USA), including a 21-gauge X 7 mm echogenic introducer needle was used to access the left common carotid artery. The needle was advanced until pulsatile flow was observed, followed by an 0.018 in X 40 cm stainless steel mandrel guidewire. Using the Seldinger technique 19, the needle was removed and replaced with a microcatheter and dilator. After microcatheter aspiration, an 0.9% intra-arterial saline drip line was attached. Through the intra-arterial saline drip line, osmotic blood-brain barrier (BBB) disruption 17 was performed using 0.25 mL/s per-kg infusion of mannitol over 20 seconds.
Microcatheter-based brain infusion of autologous mitochondria
At the five-minute peak of osmotic BBB disruption 21, the saline drip was disconnected from the microcatheter, and a sterile 10 ml syringe was used to deliver 5 mL of labeled mitochondria followed by a 10 mL flush of respiration buffer. The catheter was removed from the left carotid artery, and hemostasis was obtained by applying manual pressure for 15 minutes. This process was replicated in the contralateral control, minus the osmotic BBB disruption. The deep and superficial tissues of the ventral neck were closed with sutures. In the case of the control pig, it was infused only with respiration buffer.
Immunofluorescence in brain biopsies
After 4 hours, the animals were euthanized with a lethal dose of pentobarbital (120 mg/kg). The brains were removed and cut into 10-mm slices. Samples were fixed with 10% neutral buffered formalin for 24 h then transferred to 70% ethanol and embedded in paraffin. The formalin-fixed paraffin-embedded pig brain samples were cut in sections of 4 µm and placed on poly-L-lysine coated slides. The sections were deparaffinized at 56°C, immersed in xylene and then rehydrated in solutions with decreasing proportion of ethanol (100% to 70%), then permeabilized by heating in citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) for 30 min at 95°C. To eliminate nonspecific staining, slides were blocked for 30 min in a humid chamber at room temperature with a blocking solution (PBS pH 7.2, 0.05% Tween 20, 0.1 % Triton X-100, 2% goat serum, 2% BSA). Sections were incubated overnight at 4°C with the fluorescein-labeling goat anti-mouse IgG polyclonal antibody (Abcam, Cambridge, UK) diluted 1/500 in PBS. The sections were washed three times for 2 min with washing solution (PBS pH 7.2, 0.05% Tween 20) and mounted with VectaShield mounting medium with DAPI (Vector, Laboratories, Burlingame, Ca). The images were captured by confocal microscopy using the 10X objective (Zeiss, LSM880, Oberkochen, Germany).
RESULTS OF THE PROCEDURE APPLICATION
Mitochondrial viability and quantification
The quantification of total mitochondria isolated from muscle tissue using a hemocytometer and the green signal from Mitoview Green staining (Figure 1A) determined an average total concentration of 1.05 X 107 ± 7.20 X 105 mitochondria per ml. However, quantification based on the Mitotracker red signal (Figure 1B) corresponding to mitochondria with an intact membrane potential determined an approximate concentration of 63% of viable mitochondria (6.61 X 106 ± 9.90 X 105 mitochondria per ml).
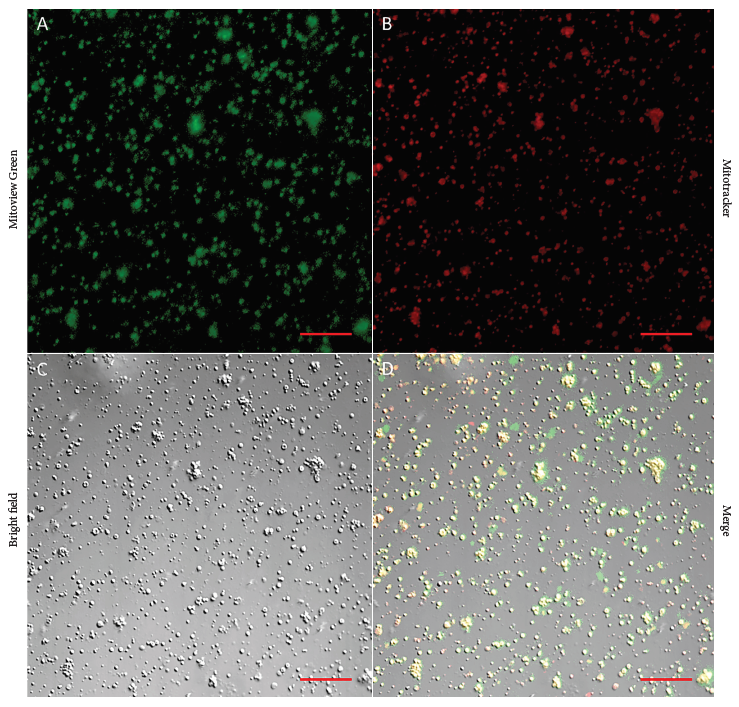
Figure 1 Mitochondrial viability and yield. Representative images of double staining in mitochondria isolated from skeletal muscle biopsies. Total mitochondria were count using the green signal from Mitoview Green (1A), the percentage of viable mitochondria was obtained using the red signal from Mitotracker (1B), mitochondria were also observed with brightfield (1C), and the merge of green and red signals is shown in (1D). Images were captured with a confocal microscope using a 20X objective. Scale bar: 20 μm.
Arterial access and infusion
Successful arterial access and subsequent carotid microcatheterization was performed in all three pigs. A total of 5.24 x 107 ± 3.59 x 106 mitochondria were reconstituted into the respiration buffer and injected into the carotid artery as a 5 mL infusate. Procedural duration, including tissue harvest, vascular access, and mitochondrial infusion was 48 and 42 minutes; approximately 40 minutes dedicated to mitochondrial isolation, quantification, and labeling. The time from sedation onset to extubation was about 90 minutes. No difficulty in microcatheterization was encountered. No other complications, including hemodynamic instability, pneumothorax, vascular perforation, stroke, or incision site hematoma were seen.
Post-infusion evaluations
Samples from all cortical regions were collected and evaluated in triplicate by immunofluorescence. Representative images of frontal lobe sections with and without BBB disruption are shown (Figure 2). In the absence of osmotic BBB disruption, few labeled mitochondria were observed only within the lumen of capillaries (Figure 2B-C). There were no clusters or focal areas of aggregation appreciated, and they were completely absent in the brain parenchyma. After BBB disruption using a hyperosmotic mannitol solution, an increased concentration of labeled mitochondria was observed in all regions of the cortex and throughout the parenchyma in small clusters in a diffuse and linear pattern (Figure 2D-F). The control sample infused with respiration buffer gave no signal with or without BBB disruption (Figure 2G-I).
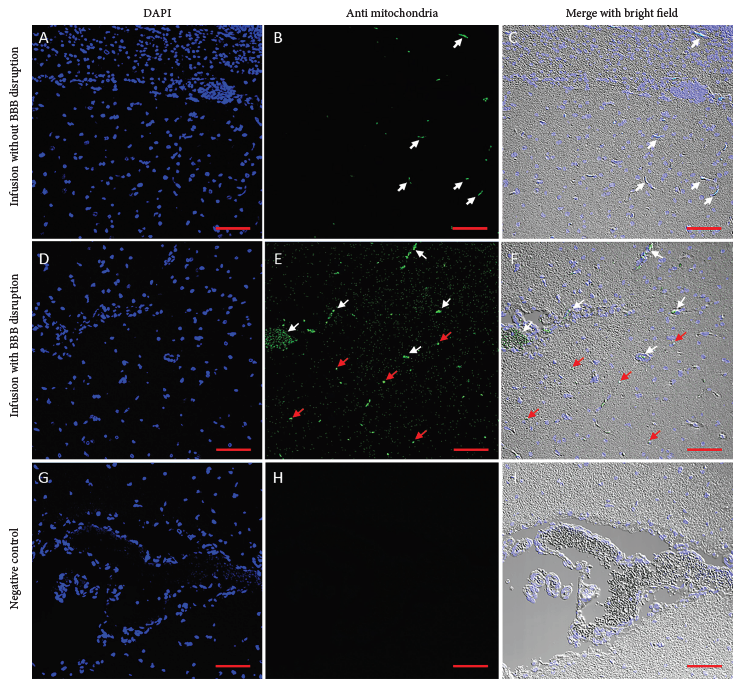
Figure 2 Autologous mitochondria infused in pig brain. Representative images of brain tissue after mitochondria infusion. In brain samples with BBB integrity (2A-C), few mitochondria are observed in the lumen of the capillaries (white arrows). The brain parenchyma did not show a positive signal for mitochondria. In samples with osmotic BBB disruption (2D-F), a large number of mitochondria are present in capillaries (white arrowheads) and the form of clusters within the parenchyma (red arrowheads). In the control sample infused with respiration buffer (2G-I), no positive signal for mitochondria was observed. Histological sections of 4 μm were stained with DAPI (2A, D, G), treated with an anti-IgG antibody (2B, E, H). The merge of both signals with the brightfield is observed in (2C, F, I). Images were captured with confocal microscopy using a 10X objective. Scale bar: 100 μm.
DISCUSSION
This study constitutes the first report of successful intra-carotid infusion of autologous mitochondria into the porcine brain, observing the entry of extracellular mitochondria into the brain parenchyma after altering the permeability of the BBB with mannitol (Figure 2). In healthy mammals, the movement of ions, molecules, and cells is limited by the BBB, the structural and chemical interface between the brain and systemic circulation 15. The permeability of the BBB can be increased during certain pathological conditions 15 , 16 , 21 but can also be temporarily modulated by osmotic agents 19. In addition, the dose and time for disruption using mannitol have been established, and its temporal effects have been observed so that proper synchronization between BBB disruption and mitochondria infusion is required to take advantage of osmotic alterations.
An additional challenge in translating experimental methods using pigs is their particular anatomy. A detailed appreciation of porcine vasculature structures is necessary for this procedure 26 , 27, requiring mindful microcatheter positioning and infusion technique in order for infusate to reach the brain instead of extracranial tissue (Figure 3). The rete mirabile is often considered an additional structural barrier for porcine cerebrovascular procedures; however, the diameter of the micro arteries of the rete mirabile range from 50 to 250 µm 28 allow egress of mitochondria which are typically less than 1 µm 29. Particles up to 30 µm in size have been shown to enter and localize in the brain after injection via the carotid artery 16 suggesting that passage beyond the rete mirabile is possible. Given the extensive collateral network, some infused mitochondria may have been diverted to other tissues; however, evaluation in this study was focused on their entry into the brain and extracranial tissues were not investigated.
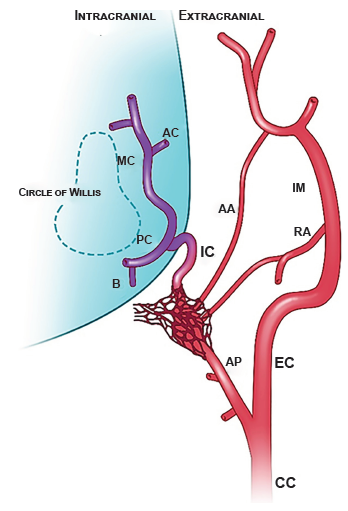
Figure 3 Porcine cervical and intracranial vasculature. The carotid rete mirabile is a mesh-like structure located at the terminus of the ascending pharyngeal artery. In pigs, the internal carotid artery serves to connect the rete to the circle of Willis. AA = arteria anastomotica; AC = anterior cerebral artery; AP = ascending pharyngeal artery; B = basilar artery; CC = common carotid artery; EC = external carotid artery; IC = internal carotid artery; IM = internal maxillary artery; MC = middle cerebral artery; PC = posterior communicating artery; RA = ramus anastomoticus supplied by the middle meningeal artery. Illustration by Emma C. Vought commissioned by Dr Melanie Walker.
Using validated methodologies 1 , 3 , 17 , 22 and standard cerebrovascular arteriographic administration techniques 18 , 23 , 24, this procedure can be completed within 45 minutes. While dosing of mitochondria has not been specifically established for the brain, cardiac ischemia studies demostrate that a dose of 1 X 109 mitochondria may be suitable 3 , 17. Cardiac studies are more advanced, and few, mostly murine models exist for cerebral infusion. Across the spectrum of murine studies, a range of doses and delivery methods were used. In this evaluation, the yield from 0.1 grams of skeletal muscle up to a dose of 1 X 109 mitochondria was used. However, taking into consideration the selective vascular delivery technique, porcine cerebral blood flow, cerebral blood volume 30, and the possible deviation by rete mirabile anatomy of the carotid artery in pigs 26, the dose for experimental mitochondria infusion was translated to be 5 X 107. The samples provided a total of 1.1 X 107 - 2.4 X 1010 mitochondria (Figure 1), ensuring quantification and delivery at the established dose. These findings on the yield and viability of mitochondria isolated from skeletal muscle tissue are consistent with experiments performed in other animals 1 , 3 , 17 , 22 , 25 and humans 2.
Initially, co-infusion of the vital dye Evans blue was considered for macroscopic interpretation of BBB permeability; however, the decision was made to proceed without Evans blue because there are no studies that confirm or exclude direct toxicity on isolated mitochondria in this setting when using this dye 20.
In conclusion, this experimental work details the procedures employed for the isolation and infusion of autologous mitochondria into the porcine brain in a safe manner using standard equipment and validated protocols. This study demonstrates the entry of mitochondria into brain tissue following infusion via the carotid artery in pigs whose BBB has been osmotically disrupted. Further studies are required to determine localization, region-specific distribution of infused mitochondria and the mechanisms involved in the internalization of autologous mitochondria into various tissues (neurons or glia).
While much work remains to be done, the potential benefit of mitochondria infusion can be applied to a spectrum of neurological diseases, and carotid injection using the porcine model may be a useful tool for its evaluation.
Acknowledgments:
The authors would like to thank the Drs. Ana Vargas-Calla and Juan F. Calcina, and Elton Sánchez for their veterinary expertise and support, Dr. James McCully for his mentorship, Dr. Yashar Kalani for his experimental suggestions, Emma Vought for her illustration of the porcine cerebral vasculature, and Kris Oshiro for her assistance creating a Good Manufacturing Practice (GMP) protocol for microcatheter-based mitochondrial infusion.
REFERENCES
. McCully JD, Cowan DB, Emani SM, Del Nido PJ. Mitochondrial transplantation: From animal models to clinical use in humans. Mitochondrion. 2017; 34: 127-34. doi: 10.1016/j.mito.2017.03.004. [ Links ]
. Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017; 154: 286-289. doi: 10.1016/j. jtcvs.2017.02.018. [ Links ]
. Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K, et al. A Novel Biological Strategy for Myocardial Protection by Intracoronary Delivery of Mitochondria: Safety and Efficacy. JACC Basic Transl Sci. 2019; 4: 871-888. doi: 10.1016/j.jacbts.2019.08.007. [ Links ]
. Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion. 2019; 46: 103-115. doi: 10.1016/j.mito.2018.03.002. [ Links ]
. Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535: 551-555. doi: 10.1038/nature18928 [ Links ]
. Liu K, Guo L, Zhou Z, Pan M, Yan C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res. 2019; 123: 74-80. doi: 10.1016/j.mvr.2019.01.001. [ Links ]
. Fang SY, Roan JN, Lee JS, Chiu MH, Lin MW, Liu CC, et al. Transplantation of viable mitochondria attenuates neurologic injury after spinal cord ischemia. J Thorac Cardiovasc Surg. 2019; 14: 32775-8. doi: 10.1016/j.jtcvs.2019.10.151. [ Links ]
. Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, et al. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma. 2018; 35: 1800-1818. doi: 10.1089/neu.2017.5605. [ Links ]
. Robicsek O, Ene HM, Karry R, Ytzhaki O, Asor E, McPhie D, et al. Isolated mitochondria transfer improves neuronal differentiation of schizophrenia-derived induced pluripotent stem cells and rescues de?cits in a rat model of the disorder. Schizophr Bull. 2018; 44: 432-442. doi: 10.1093/schbul/sbx077. [ Links ]
. Chang JC, Wu SL, Liu KH, Chen YH, Chuang CS, Cheng FC, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson's disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res. 2016; 170: 40-56. doi: 10.1016/j.trsi.2015.12.003. [ Links ]
. Heyck M, Bonsack B, Zhang H, Sadanandan N, Cozene B, Kingsbury C, et al. The brain and eye: treating cerebral and retinal ischemia through mitochondrial transfer. Exp Biol Med Exp Biol Med (Maywood). 2019; 244: 1485-1492. doi: 10.1177/1535370219881623. [ Links ]
. Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, et al. Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res. 2019; 356: 322-331. doi: 10.1016/j.bbr.2018.09.005. [ Links ]
. Huang PJ, Kuo CC, Lee HC, Shen CI, Cheng FC, Wu SF, et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transplant. 2016; 25: 913-27. doi: 10.3727/096368915X689785. [ Links ]
. Shi X, Zhao M, Fu C, Fu A. Intravenous administration of mitochondria for treating experimental Parkinson's disease. Mitochondrion. 2017; 34: 91-100. doi: 10.1016/j.mito.2017.02.005. [ Links ]
. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005; 57: 173-185. doi: 10.1124/pr.57.2.4. [ Links ]
. Alroy KA, Arroyo G, Gilman RH, Gonzales-Gustavson E, Gallegos L, Gavidia CM, et al. Carotid Taenia solium Oncosphere Infection: A Novel Porcine Neurocysticercosis Model. Am J Trop Med Hyg. 2018; 99: 380-387. doi: 10.4269/ajtmh.17-0912. [ Links ]
. Cosolo WC, Martinello P, Louis WJ, Christophidis N. Blood-brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol. 1989; 256: R443-R447. doi: 10.1152/ajpregu.1989.256.2.R443. [ Links ]
. Preble JM, Pacak CA, Kondo H, MacKay AA, Cowan DB, McCully JD. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. JoVE. 2014; 91: e51682. doi: 10.3791/51682. [ Links ]
. Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953; 39: 368-376. doi: 10.3109/00016925309136722. [ Links ]
. Saunders NR, Dziegielewska KM, Møllgård K, Habgood MD. Markers for blood-brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives?. Front Neurosci. 2015; 385: 1-16. doi: 10.3389/fnins.2015.00385. [ Links ]
. Bellavance MA, Blanchette M, Fortin D. Recent advances in bloodbrain barrier disruption as a CNS delivery strategy. AAPS J. 2008; 10: 166-177. doi: 10.1208/s12248-008-9018-7. [ Links ]
. Rasmussen HN, Andersen AJ, Rasmussen UF. Optimization of preparation of mitochondria from 25-100 mg skeletal muscle. Anal Biochem. 1997; 252: 153-9. doi: 10.1006/abio.1997.2304. [ Links ]
. Blanc R, Piotin M, Mounayer C, Spelle L, Moret J. Direct cervical arterial access for intracranial endovascular treatment. Neuroradiology. 2006; 48: 925-929. doi: 10.1007/s00234-006-0157-1. [ Links ]
. Dorfer C, Standhardt H, Gruber A, Ferraz-Leite H, Knosp E, Bavinzski G. Direct percutaneous puncture approach versus surgical cutdown technique for intracranial neuroendovascular procedures: technical aspects. World Neurosurg. 2012; 77: 192-200. doi: 10.1016/j.wneu.2010.11.007. [ Links ]
. Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle, and cultured fibroblasts. Nat Protoc. 2007; 2: 287-295. doi: 10.1038/nprot.2006.478. [ Links ]
. Dondelinger RF, Ghysels MP, Brisbois D, Donkers E, Snaps FR, Saunders J, et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur Radiol. 1998; 8: 1254-1273. doi: 10.1007/s003300050545. [ Links ]
. Kagetsu NJ, Berenstein A, Choi IS. Interventional radiology of the extracranial head and neck. Cardiovasc Intervent Radiol. 1991; 14: 325-333. doi: 10.1007/BF02577890. [ Links ]
. Massoud TF, Vinters HV, Chao KH, Viñuela F, Jahan R. Histopathologic characteristics of a chronic arteriovenous malformation in a swine model: preliminary study. AJNR Am J Neuroradiol. 2000; 21: 1268-1276. PMID: 10954279. [ Links ]
. Miettinen TP, Björklund M. Mitochondrial Function and Cell Size: An Allometric Relationship. Trends Cell Biol. 2017; 27: 393-402. doi: 10.1016/j.tcb.2017.02.006. [ Links ]
. Sakoh M, Røhl L, Gyldensted C, Gjedde A, Ostergaard L. Cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking after acute stroke in pigs: comparison with [(15)O] H(2)O positron emission tomography. Stroke. 2000; 31: 1958-64. doi: 10.1161/01.str.31.8.1958. [ Links ]
Funding: MAO, GA and LT were partially supported by the Fogarty-NIH International Center training grant (TW001140).
Received: March 30, 2021; Accepted: June 02, 2021











 text in
text in 



