Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Medicina Experimental y Salud Publica
Print version ISSN 1726-4634
Rev. perú. med. exp. salud publica vol.39 no.1 Lima Jan./Mar. 2022 Epub Mar 24, 2022
http://dx.doi.org/10.17843/rpmesp.2022.391.8580
Originales breves
Extended-spectrum β-lactamases and virulence factors in uropathogenic Escherichia coli in nursing homes in Lima, Peru
1 Facultad de Medicina Humana, Universidad de Piura, Lima, Peru.
2 Instituto de Investigaciones en Bacteriología y Virología Molecular (IBaViM), Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina.
Nursing homes are institutions with high prevalence of urinary tract infections caused by ESBL-producing E. coli with several virulence factors. The aim of this study was to determine the frequency of the bla CTX-M gene and eight virulence genes in 35 ESBL-producing uropathogenic E. coli from six nursing homes in Peru during 2018. Of the E. coli samples, 57.1% (20/35) were carriers of the bla CTX-M gene. Furthermore, we obtained frequencies of 46% (15/35) and 37% (13/35) for hly-alpha and cnf-1, respectively; we also found high presence of the iucC (63%, 22/35), aer (94%, 33/35) and chuA genes (94%, 33/34) as well as a frequency of 46% (16/35) and 91% (32/34) for the pap GII and nanA genes, respectively. The bla CTX-M gene is predominant and a high frequency of exotoxins gives it a competitive advantage for spreading into the bloodstream.
Keywords: Urinary infections; Antibacterials; Beta-Lactam Resistance; Enterobacteriaceae Infections; Nursing Homes; Uropathogenic Escherichia coli ; Virulence Factors; Urinary Tract Infections, ß-lactamases
INTRODUCTION
Nursing homes are long-term care institutions with a high prevalence of infectious diseases 1; urinary tract infection (UTI), whose main etiological agent is Escherichia coli, is the most frequent infection in this type of institution 1. Likewise, between 20 to 40% of bacteremias in the elderly population are caused by bacteria that ascend through the urinary tract; E. coli and Proteus mirabilis are the most common microbial agents 2.
Nursing home residents have a high probability of colonization and infections by multidrug-resistant E. coli 3. In addition, the high recurrence of UTI and inappropriate treatment of asymptomatic bacteriuria increases the use of antibiotics, which leads to the selection of resistant bacteria 1, with an increase in the mortality rate of residents 2.
ß-lactam antibiotics represent approximately 50% of the antibiotics prescribed worldwide 4; however, their therapeutic efficiency has decreased due to the rapid selection of resistance mechanisms. Extended-spectrum ß-lactamases (ESBLs) represent the most important mechanism for resistance to third-generation cephalosporins in E. coli. CTX-M enzymes, due to their high propagation efficiency, have displaced SHV and TEM type ß-lactamases as the most prevalent worldwide 4. CTX-M has a heterogeneous lineage that includes six groups (CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, CTX-M-25 and KLUC) that are different from each other by 10% or more amino acid residues 4. ESBL-producing E. coli. are highly frequent in Peru; in 2011, a multinational surveillance program that evaluated eleven Latin American countries found that 54.0% of E. coli. were ESBL-producing 5.
On the other hand, although the dissemination of CTX-M type ESBL in uropathogenic E. coli (UPEC) is a global problem that decreases therapeutic options in patients, the severity of infection depends on the pathogenic capacity of UPEC 6. UPEC have adherence factors (e.g. Pap G II) that allow them to successfully initiate infection and migrate to the upper regions of the urinary tract, aggravating the patient’s condition 6. Likewise, UPEC produce siderophore systems (e.g. ChuA, Aer) and exotoxins (e.g. α-Hly, TcpC, Cnf-1) that together constitute a protein system that allow them to evade and/or affect the immune system to the detriment of the patient’s health 6. In bacteremias caused by an UTI, UPEC require the expression of proteins that allow them to adapt to the environmental conditions of the blood tissue (e.g. nanA) 7.
To our knowledge, no studies that describe the frequency of CTX-M type ESBL-producing UPEC in nursing homes have been published in Peru. The population of these nursing homes is susceptible to UTI and systemic complications; therefore, the aim of this study was to determine the frequency of virulence genes and the presence of the bla CTX-M gene in UPEC isolated from residents of nursing homes located in Metropolitan Lima.
KEY MESSAGES
Motivation for the study: Nursing homes deserve special epidemiological attention because of the high prevalence of urinary tract infections due to multidrug-resistant Escherichia coli and the high risk of urosepsis.
Main findings: The bla CTX-M gene was carried by 57.1% of the ESBL-producing E. coli and 70% belonged to the bla CTX-M-group 2 gene. Additionally, we obtained a frequency of 46% and 37% for hly-alpha and cnf-1, respectively; as well as 46% and 91% for the pap GII and nanA gene, respectively.
Implications: This is the first report of resistant genes associated with ESBL in uropathogenic E. coli identified in nursing homes in Peru, which is of great relevance because it represents a threat to the health of the elderly population.
THE STUDY
Study design and population
Observational descriptive study conducted in 2018, of 35 random non-duplicate isolates of ESBL-producing E. coli obtained from urine samples of older adult residents of six private nursing homes in Metropolitan Lima in Peru.
Microbiological study
Bacteria were identified by using conventional biochemical tests (triple sugar iron agar, lysine iron agar, citrate agar, mobility-indol-orinithine medium and methyl red/Voges-Proskauer) and confirmed molecularly, by polymerase chain reaction (PCR), through the amplification of the uspA gene.
Susceptibility testing
Presence of ESBL-producing E. coli was confirmed by the diffusion disc method specified in Clinical & Laboratory Standards Institute (CLSI) document M02-13 8; in addition, antimicrobial susceptibility was determined by the diffusion disc method according to CLSI guidelines 8, using E. coli ATCC 25922 as a quality control. Antibiotic discs with the following antibiotics were included: piperacillin/tazobactam 100/10 µg (PTZ); amoxicillin/clavulanic acid 20/10 µg (AMC); cefotaxime 30 µg (CTX); ceftazidime 30 µg (CAZ); cefepime 30 µg (FEP); cefoxitin 30 µg (FOX); aztreonam 30 µg (AZM); meropenem 10 µg (MEM); imipenem 10 µg (IMP); amikacin 30 µg (AK); gentamicin 10 µg (GM); ciprofloxacin 5 µg (CIP); nitrofurantoin 300 µg (NIT) and trimethoprim/sulfamethoxazole 1.25/23.75 µg (SXT).
Detection of virulence and resistance genes
Bacterial DNA was extracted using the DNA Purification kit Gene-JetGenomic (ThermoScientific), following the manufacturer’s recommendations. Eight virulence genes were identified: aer, hly, cnf-1, chuA, TcpC, nanA, pap GII, iucC 9 , 10, as well as three ESBL-associated resistance genes: bla CTX-M, bla SHV and bla TEM 11. In addition, different groups of isolates carrying bla CTX-M (1, 2, 8 and 9) were detected 12. PCR conditions were carried out in a final volume of 30 μl containing 1X of MaximoTaq DNA (GeneON) as well as 0.5 uM of each of the primers and 1.5 mM MgCl2. The volume of incorporated DNA was 2.0 μL. The Labnet, Multigene optimax thermal cycler was programmed with the following parameters: initial denaturation at 94 °C for three minutes; denaturation at 94 °C for 30 seconds; extension at 72 °C for 30 seconds; in 30 reaction cycles and final extension at 72 °C for three minutes. Table 1 shows the sequence of the primers used, the size of the amplification products, the hybridization temperature and the reference of the study. The amplified DNA fragments were separated by 1% agarose gel electrophoresis for 50 min at 110 volts. Finally, Runsafe loading buffer (GeneON) was used for UV development.
Table 1 Virulence and resistance genes, amplification characteristics.
| Gene | Primer sequence (5’ - 3’) | Product (bp) | Hybridization temperature (°C) |
|---|---|---|---|
| Virulence genes | |||
| α-hemolysin (α-hly) | AACAAGGATAAGCACTGTTCTGGCT | 1177 | 63 |
| ACCATATAAGCGGTCATTCCCGTCA | |||
| chuA | GACGAACCAACGGTCAGGAT | 279 | 55 |
| TGCCGCCAGTACCAAAGACA | |||
| Aerobactin synthesis (aer) | TACCGGATTGTCATATGCAGACCGT | 602 | 63 |
| AATATCTTCCTCCAGTCCGGAGA AG | |||
| Aerobactin synthesis (iucC) | CTCGAATTCACTGGGATTTGGTCAACC | 1701 | 62 |
| CTCTCTAGAATTCCTGAGTTACCAGCC | |||
| Cytotoxic necrotizing factor (cnf1) | AAGATGGAGTTTCCTATGCAGGAG | 498 | 61 |
| CATTCAGAGTCCTGCC CTCATTATT | |||
| P - fimbriae (pap)- alelo II | GGGATGAGCGGGCCTTTGAT | 190 | 65 |
| CGGGCCCCCAAGTAACTCG | |||
| nanA | ACCGGTGAGGGGAAATAAAC | 216 | 59 |
| GGTGAGTACCAGGGCGATTA | |||
| tcpC | GGCAACAATATGTATAATATCCT | 386 | 51 |
| GCCCAGTCTATTTCTGCTAAAGA | |||
| Resistance genes | |||
| blaSHV | ATGCGTTATATTCGCCTGTG | 544 | 58 |
| GTTAGCGTTGCCAGTGCTCG | |||
| blaTEM | ATAAAATTCTTGAAGACGAAA | 1080 | 54 |
| GACAGTTACCAATGCTTAATC | |||
| blaCTX-M | TTTGCGATGTGCAGTACCACTAA | 865 | 60 |
| CGATATCGTTGGTGGTGCCAT | |||
| blaCTX-M-group 1 | ATGGTTAAAAAATCACTG C | 900 | 55 |
| GGTGACGATTTTAGCCGC | |||
| blaCTX-M-group 2 | CGTTAACGGCACGATGAC | 404 | 59 |
| CGATATCGTTGGTGGTGCCAT | |||
| blaCTX-M-group 8 | ACGCTCAACACCGCGATC | 490 | 63,3 |
| CGTGGGTTCTCGGGGATAA | |||
| blaCTX-M-group 9 | GATTGACCGTATTGGGAGTTT | 831 | 58 |
| CGGCTGGGTAAAATAGGTCA |
FINDINGS
Antimicrobial Susceptibility
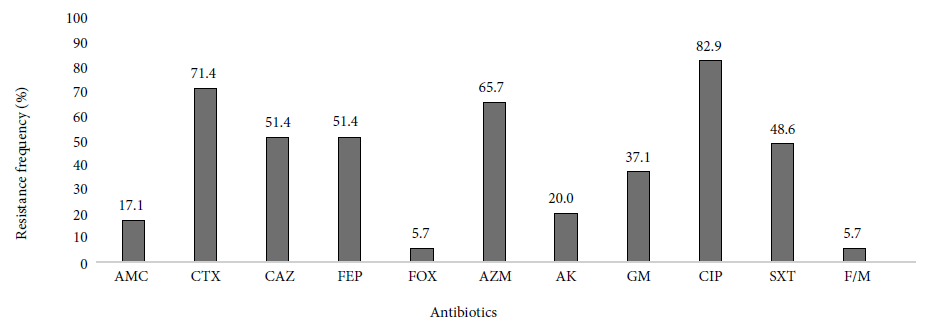
Thirty-five ESBL-producing UPEC from nursing homes were analyzed. Most isolates were resistant to cefotaxime (25/35), there were no carbapenem-resistant isolates, and only two (2/35) isolates were resistant to cefoxitin. In addition to resistance to ß-lactams, we observed that UPEC strains were highly resistant to ciprofloxacin, 82.9% (29/35) of the cases. UPEC resistant to PTZ, MEM and IMP were not found. Figure 1 shows the frequencies of resistance by antibiotic.
Genotyping of UPEC carrying bla CTX-M
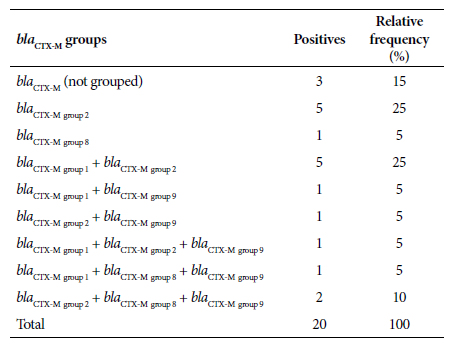
Of the 35 isolates, 57.1% (20/35) carried the bla CTX-M gene, of which 70% (14/20) corresponded to bla CTX-M-group 2, 40% (8/20) to bla CTX-M-group 1, 30% (6/20) to bla CTX-M-group 9 and 20% (4/20) to bla CTX-M-group 8. On the other hand, 60% (12/20) of the UPEC carrying the bla CTX-M gene had two or more allelic variants of the bla CTX-M gene in the same bacterium. In addition, there was a higher level of confluence between the genes from bla CTX-M-group 1 and bla CTX-M-group 2 (Table 2). The presence of other ß-lactamase-producing genes (bla SHV and bla TEM) was also evaluated in bla CTX-M producers and non-producers. Of a substratum of the analyzed UPEC, 48.1% (13/27) did not carry the bla CTX-M gene, but did carry the bla SHV and/or bla TEM genes. Likewise, 51.9% (14/27) of the UPEC presented the bla CTX-M gene, in addition to the bla SHV and/or bla TEM genes. The nucleotide sequences of the allelic variants that would confirm belonging to the ESBL group were not analyzed.
Genotyping of virulence factors in ESBL-producing UPEC
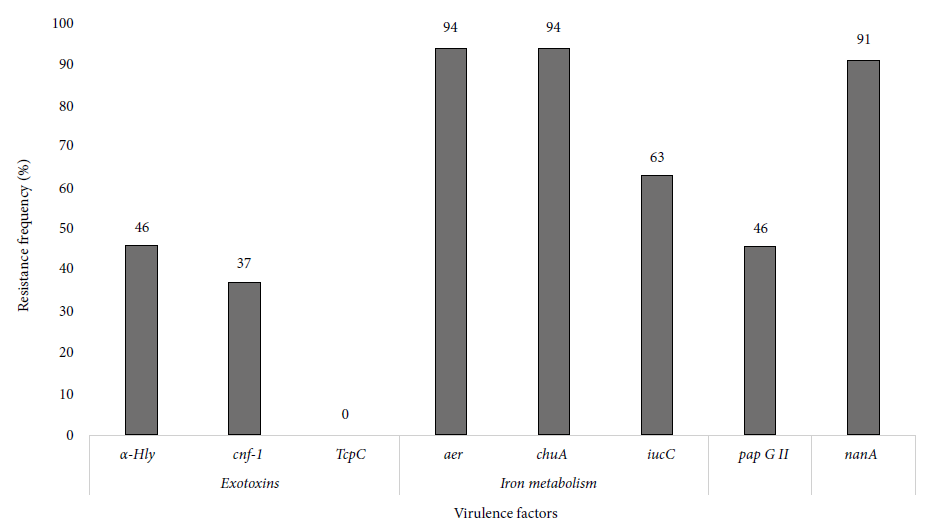
After analyzing genes related to exotoxins in the 35 ESBL-producing UPEC, we obtained a frequency of 46% (15/35) and 37% (13/35) of hly-alpha and cnf-1, respectively. aer (94%, 33/35) and chuA (94%, 33/35) were the most frequent genes associated with iron metabolism. Furthermore, adhesin pap GII was found in 46% (16/34) of the isolates and the nanA gene in 91% (32/35) (Figure 2).
DISCUSSION
The results of this study show that 57.1% of the ESBL-producing Escherichia coli carried the bla CTX-M gene, 70% of which belonged to bla CTX-M-group 2. On the other hand, the genes: hly-alpha and cnf-1, that code for exotoxins, were detected with a frequency of 46 and 37%, respectively.
Nursing homes are long-stay institutions where the prevalence of UTI caused by multidrug-resistant bacteria is high 3. In this study, 57.1% of the UPEC carried the bla CTX-M gene. This result is similar to those described by Galván et al.13 and Arce-Gil et al.11, who reported a frequency of 54.7% and 51.4%, respectively. However, they differ from those described by Ramirez et al. 14 and Chavez 15 in various hospitals in Lima (70% and 91.7%). The latter demonstrates the great variability in the presence of this gene in health institutions.
Results regarding the bla CTX-M subgroups showed that bla CTX-M-group 2 (14/20) followed by bla CTX-M-group 1 (8/20) were the most frequent. This differs from what was reported by Chavez 15, where bla CTX-M-group 2 was not detected and bla CTX-M-group 1 (26/33) had the highest frequency; and by Palma et al. 16, where bla CTX-M-group 1 predominated (13/27). However, recent studies indicate that bla CTX-M-group 2 is still significant in South America 17 and a recent review in Brazil identifies bla CTX-M-group 2 and bla CTX-M-group 1 as the most prevalent variants in the region 18.
We also detected bla TEM (54.3%) and bla SHV (51.4%) genes, which were found among UPEC carrying the bla CTX-M gene. It is important to note that in 37% of the isolates, only the genes bla TEM and/or bla SHV were identified, despite the fact that its allelic variant and its belonging to the ESBL group was not determined, this is a strong indicator of its membership. None of the evaluated resistance genes were detected in 5.7% of the isolates, which indicates the presence of other ESBL-type resistance genes that were not analyzed in this study.
Regarding virulence factors, the pap GII gene was present in 47.1% of UPEC. These findings differ from the results obtained by Paniagua-Contreras et al. 19, who reported a 21.1% frequency of the pap GII gene in community UPEC in Mexico. We also found a high frequency of genes associated with iron transport, similar to what was reported by Dadi et al. 20, with 54.5% for the iucC gene.
Regarding exotoxins, we obtained a frequency of 44.2% and 38.2% of hly-alpha and cnf-1, respectively, these results are comparable to those reported by Dadi et al. 20 who found 50.4% and 29% of hly-alpha and cnf-1, respectively. On the other hand, in this study we did not find UPEC carrying the tcpC gene, in contrast to other studies that have reported prevalence of up to 25% 10.
We also found a frequency of 88.2% for the nanA gene, which is important for energy production. It has been proposed that the presence of the nanA gene creates high competitiveness in UPEC to cause bacteremias, although its role in the pathogenesis of UTI is less important 6, the results point to the existence of high risk in residents with bacteremia that develops into sepsis.
This study has some limitations. Although it was carried out in several nursing homes, no clinical information was obtained from the patients, in addition to having a limited number of samples. Moreover, we did not analyze further virulence and resistance genes (other types of ESBL) relevant to the epidemiology of UPEC.
In conclusion, this is the first report of ESBL-producing UPEC in nursing homes in Peru, which shows predominance of the bla CTX-M gene, 70% of which belong to bla CTX-M-group 2. Also, we can point out that ESBL-producing UPEC in nursing homes present a high frequency of exotoxins and the nanA gene, which gives them an advantage to disseminate into the bloodstream. We hope that these findings will allow strengthening the epidemiological surveillance of multidrug-resistant bacteria and prevent their dissemination.
Acknowledgments:
To César Gutiérrez for his contributions in reviewing the statistical analysis and Brenda Moy for her technical support.
REFERENCES
. Nicolle LE; SHEA Long-Term-Care-Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001; 22(3):167-175. doi: 10.1086/501886. [ Links ]
. Leibovici-Weissman Y, Tau N, Yahav D. Bloodstream infections in the elderly: what is the real goal?. Aging Clin Exp Res. 2021; 33(4):1101-1112. doi: 10.1007/s40520-019-01337-w. [ Links ]
. Van der Donk CF, Schols JM, Driessen CJ, Hagenouw RG, Meulendijks A, Stobberingh EE. Prevalence and spread of multi drug resistant Escherichia coli isolates among nursing home residents in the southern part of The Netherlands. J Am Med Dir Assoc. 2013;14(3): 199-203. doi: 10.1016/j.jamda.2012.09.026. [ Links ]
. D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type ß-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol. 2013; 303(6-7):305-17. doi: 10.1016/j.ijmm.2013.02.008. [ Links ]
. Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, et al. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis. 2013; 17(6):672-81. doi: 10.1016/j.bjid.2013.07.002. [ Links ]
. Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Micro Rev. 1991; 4(1), 80-128. doi: 10.1128/cmr.4.1.80. [ Links ]
. Smith SN, Hagan EC, Lane MC, Mobley HLT. Dissemination and Systemic Colonization of Uropathogenic Escherichia coli in a Murine Model of Bacteremia. MBio. 2010;1(5):1-9. doi: 10.1128/mBio.00262-10. [ Links ]
. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 29a ed. CLSI suplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019. ISBN: 978-1-68440-032-4. [ Links ]
9. . Micenková L, Beňová A, Frankovičová L, Bosák J, Vrba M, Ševčíková A, et al. Human Escherichia coli isolates from hemocultures: Septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes tan bacteraemia linked to other conditions. Int J Med Microbiol. 2017;307(3):182-9. doi: 10.1016/j.ijmm.2017.02.003. [ Links ]
. Erjavec MS, Jesenko B, Petkovšek Ž, Žgur-Bertok D. Prevalence and associations of tcpC, a gene encoding a Toll/interleukin-1 receptor domain-containing protein, among Escherichia coli urinary tract infection, skin and soft tissue infection, and comensal isolates. J Clin Microbiol. 2010;48(3):966-8. doi: 10.1128/JCM.01267-10. [ Links ]
. Arce-Gil Z, Llontop-Nuñez J, Flores-Calvo R, Fernandez-Valverde D. Detección del gen CTX-M en cepas de Escherichia coli productoras de B-lactamasas de espectro extendido procedentes del Hospital Regional de Lambayeque; Chiclayo-Perú: Noviembre 2012-Julio 2013. Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo, 2013. 2014; 6(4):12-5. [ Links ]
. Bartoloni A, Pallecchi L, Riccobono E, Mantella A, Magnelli D, Maggio T Di, et al. Relentless increase of resistance to fluoroquinolones and expanded-spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin Microbiol Infect. 2013; 19(4), 356-361, doi: 10.1111/j.1469-0691.2012.03807.x. [ Links ]
. Galván F, Agapito J, Bravo N, Lagos J, Tamariz J. Caracterización fenotípica y molecular de Escherichia coli productoras de ß -Lactamasas de espectro extendido en pacientes ambulatorios de Lima, Perú. Rev Medica Hered. 2016;27(1): 22. doi: 10.20453/rmh.v27i1.2780. [ Links ]
. Ramirez MR, Marcelo M, Alcedo K, Alejos S. Detection of ESBL-producing E. coli isolates from urinary tract infections in Peru. Poster presentado en: ECCMID 2017 27th, Congreso Europeo de Microbiología Clínica; 2017 Apr 22-25; Vienna, Austria. Disponible en: https://www.escmid.org/escmid_publications/escmid_elibrary/q=peru&i-d=2173&L=0&x=0&y=0&tx_solr%5Bsort%5D=relevance%2Basc&tx_solr%5Bfilter%5%5B0%5D=main_filter_eccmid%253Atrue&tx_solr%-5Bfilter%5%5B1%5D=pub_date%253A201701010000-201712312359. [ Links ]
. Chávez D. Frecuencia y subtipos del gen blaCTX-M en enterobacterias productoras de BLEE aisladas de urocultivos en el Instituto Nacional de Enfermedades Neoplásicas de enero a diciembre del 2017 [Tesis de bachiller]. Lima: Universidad Nacional Mayor de San Marcos, Facultad de Medicina, Escuela Profesional de Tecnología Médica; 2019. Disponible en: http://cybertesis.unmsm.edu.pe/handle/20.500.12672/11389. [ Links ]
. Palma N, Pons MJ, Gomes C, Mateu J, Riveros M, García W, et al. Resistance to quinolones, cephalosporins and macrolides in Escherichia coli causing bacteremia in Peruvian children. J Glo Anti Res. 2017, 11:28-33. doi: 10.1016/j.jgar.2017.06.011.17. [ Links ]
. Bevan ER, Jones AM, Hawkey PM. Global epidemiologyof CTX-M B-lactamases: temporal and geographicalshifts in genotype. J of Ant Chem. 2017;2145-55. doi: 10.1093/jac/dkaa161. [ Links ]
. Ruligle F, Paulo V, Pinto T, Cesar F, Barbosa B. The spread of CTX-M-type extended-spectrum ß-lactamases in Brazil: a systematic review. Microbial Drug Resistance. 2015;2(4):301-311. doi: 10.1089/mdr.2015.0180. [ Links ]
. Paniagua-Contreras GL, Monroy-Perez E, Rodríguez-Moctezuma JR, Dominguez-Trejo P, Vaca-Paniagua F, Vaca S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community- acquired urinary tract infection patients in Mexico. J Microbiol Immunol Infect. 2015, 50(4):478-485, doi: 10.1016/j.jmii.2015.08.005. [ Links ]
. Dadi BR, Abebe T, Zhang L, Mihret A, Abebe W, Amogne W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect Dis. 2020; 20(1):108. doi: 10.1186/s12879-020-4844-z. [ Links ]
Cite as: Gonzales-Rodriguez AO, Infante Varillas SF, Reyes Farias CI, Ladines Fajardo CE, Gonzales Escalante E. Extended-spectrum β-lactamases and virulence factors in uropathogenic Escherichia coli in nursing homes in Lima, Peru. Rev Peru Med Exp Salud Publica. 2022;39(1):98-103. doi: https://doi.org/10.17843/rpmesp.2022.391.8580.
Received: June 15, 2021; Accepted: February 09, 2022











 text in
text in