Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Medicina Experimental y Salud Publica
versión impresa ISSN 1726-4634versión On-line ISSN 1726-4642
Rev. perú. med. exp. salud publica vol.40 no.4 Lima oct./dic. 2023 Epub 18-Dic-2023
http://dx.doi.org/10.17843/rpmesp.2023.404.12629
Original article
Characteristics and factors associated with mortality in tracheostomized patients with COVID-19: a retrospective cohort study in a hospital in Tacna, Peru
1 Daniel Alcides Carrión Hospital III, Tacna, Peru.
2 Postgraduate School, Private University of Tacna, Tacna, Peru.
3 Diagnosis, treatment and research of infectious and tropical diseases, Private University of Tacna, Tacna, Peru.
4 Roe Clinical Laboratory, Lima, Peru.
5 San Ignacio de Loyola University, Research Unit for Health Evidence Generation and Synthesis, Lima, Peru.
Objective:
We aimed to describe the main demographic, clinical, laboratory and therapeutic characteristics and to identify whether they are associated with mortality in tracheostomized patients.
Material and methods.
Retrospective cohort study in adult patients diagnosed with COVID-19, admitted to ICU (Intensive Care Unit) and requiring tracheostomy. Demographic, clinical, laboratory and treatment data were obtained from the medical records of patients admitted to Hospital III Daniel Alcides Carrión in Tacna. The Cox proportional hazards model was used for survival analysis and hazard ratios (HR) with their 95% confidence intervals (95%CI) were calculated.
Results.
We evaluated 73 patients, 72.6% were men, the most common comorbidities were obesity (68.5%), type 2 diabetes mellitus (35.6%), and arterial hypertension (34.2%). Thirty-seven percent of the participants died during their stay at the ICU. The median time from intubation to tracheostomy and the duration of tracheostomy was 17 (RIC: 15-21) and 21 (RIC: 3-39) days, respectively. Multivariate analysis showed that the factors associated with mortality were procalcitonin > 0.50 ng/dL at the time of tracheostomy (HRa: 2.40 95%CI: 1.03-5.59) and a PaO2/FiO2 ratio less than or equal to 150 mmHg (HRa: 4.44 95%CI: 1.56-12.60).
Conclusions.
The factors associated with mortality at the time of tracheostomy were procalcitonin > 0.50 ng/dL and a PaO2/FiO2 ratio less than or equal to 150 mmHg.
Keywords: Tracheostomy; Tracheotomy; Survival; SARS-CoV-2; COVID-19; Mortality; Peru; Risk Factors
Motivation for the study. Patients diagnosed with COVID-19 who are candidates for tracheostomy are complex cases with long hospital stay and high risk of death; the performance of this medical procedure in the best possible conditions is essential for their survival.
Main findings. The risk factors for death at the time of tracheostomy were low PaO2/FiO2 ratio (less than or equal to 150) and a procalcitonin level >0.5 ng/mL.
Implications. Early recognition of these risk factors may help to find the best time for the patient to undergo tracheostomy in a safer manner and with fewer complications.
Keywords: Tracheostomy; Tracheotomy; Survival; SARS-CoV-2; COVID-19; Mortality; Peru; Risk Factors
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has caused more than 5.4 million deaths worldwide and is the most important health crisis since the influenza pandemic of 1918 1. As of July 24, 2023, nearly 4.5 million cases of COVID-19 have been diagnosed in Peru, with 221,203 deaths and a case fatality rate of 4.9% 2. Peru is the seventh country with the highest number of deaths in the world due to COVID and the third in Latin America 3.
Patients with acute respiratory distress syndrome (ARDS) and COVID-19 usually remain intubated for prolonged periods 4. Tracheostomy plays a fundamental role in the management of these patients when the intubation and mechanical ventilation time exceeds 14 days (prolonged mechanical ventilation) by allowing better airway management and facilitating the withdrawal of invasive ventilatory support. Tracheostomy has been associated with less need for deep sedation, shorter weaning time from the mechanical ventilator, shorter stay in the intensive care unit (ICU) and shorter hospital stay 5.
However, most ICU patients who are tracheostomy candidates have a higher risk of death due to the following characteristics: (a) ICU stay increases the risk of nosocomial infections 6; (b) prolonged mechanical ventilation, which requires longer sedation, analgesia and muscle relaxation, causes polyneuropathy and myopathy associated with immobility 7, (c) delirium upon withdrawal of sedation and analgesia; (d) the severe inflammatory process (cytokine storm) caused by COVID-19 directly affects gas exchange 8 and (e) the advanced age and presence of comorbidities that patients admitted to the ICU usually present 9.
Likewise, tracheostomy is a procedure that is not free of risks and potential complications such as hemorrhage, surgical site infection, pneumothorax and tracheal stenosis. So, due to all these conditions, tracheostomy is not always safe 4. Therefore, in order to improve clinical decision making and optimize results, the factors associated with higher mortality should be identified even if a patient with severe COVID-19 has the indication for tracheostomy, taking into account the high complexity and the accumulated mortality due to the baseline conditions of these patients.
So far, the factors related to mortality in critically ill patients tracheostomized due to COVID-19 are the following: high values of C-reactive protein (CRP) at the time of tracheostomy 10, higher inspired oxygen fraction (FiO2) requirement 11 and early timing of tracheostomy 12. Unfortunately, most of this information comes from developed countries and does not reflect the reality of Peru, a country with a fragile health system. In this context, critical illness due to COVID-19 was more frequent, aggressive and fatal 13, since after the three waves of this disease, the number of respiratory critical patients and tracheostomized patients increased, with a mortality rate of 32.9% in our ICU 14.
This study aimed to describe the demographic, clinical, laboratory and therapeutic characteristics as well as to identify predictors that allow timely identification of patients with COVID-19 who are at higher risk of death after percutaneous tracheostomy, since this group of patients have prolonged length of stay and high mortality rates 15.
MATERIALS AND METHODS
Study design
This was a retrospective cohort study. Data were collected between September and October 2022 and included patients with critical COVID-19, defined by the presence of ARDS, sepsis or septic shock 16, who were hospitalized at the Daniel Alcides Carrion Hospital in Tacna, from March 28, 2020 to March 1, 2022.
Population
We analyzed 329 medical records from the Intelligent Health Service (ESSI), which is a software that contains digital medical records 17, including all patients hospitalized in the COVID-19 ICU (COVID-ICU) of the level III Daniel Alcides Carrión Hospital - EsSalud, located in Tacna, Peru, which has more than 120 beds for patients with COVID-19 and 14 beds in the ICU-COVID 14. Critical patients were treated according to the institutional protocols developed for patients with severe COVID-19; these protocols are constantly updated according to new scientific evidence.
We included patients over 18 years of age, admitted to the COVID-ICU, with a confirmed diagnosis of SARS-CoV-2 infection, severe respiratory failure due to COVID-19 and the need for invasive mechanical ventilation who, during their hospital stay, required percutaneous tracheostomy placement. All tracheostomies were performed percutaneously, by two ICU physicians, one in charge of the airway and the other in charge of the procedure itself. Patients admitted to the ICU for reasons other than COVID-19 pneumonia, patients with incomplete clinical/laboratory data and patients whose outcome was not death or decannulation were excluded. Patient death was corroborated by ESSI.
Although we analyzed 329 medical records, only 73 records of tracheostomized patients were included. Statistical power was calculated based on the study by Tang et al. 26, in which 73.3% of patients with early tracheostomy (≤14 days) died during follow-up, compared to a 42% mortality rate in those with late tracheostomy (>14 days). We considered an unexposed/exposed ratio of 1.30 (73/56). With these parameters, a confidence level of 95% and 73 participants in our sample, we obtained a statistical power of 76.5%.
Study variables
The dependent variable was death during hospitalization (yes/no), with survivors being patients who were decannulated and subsequently discharged; this information was collected directly from the ESSI. The independent variables were age (years), sex (male/female). We considered comorbidities as dichotomous variables (yes/no), these were obesity, diabetes mellitus, arterial hypertension, and chronic kidney disease. The infection period was considered by years: 2020, 2021, and 2022. Vaccination (2 doses/3 doses). The time variables were: hospital stay (days), ICU stay (days), days with mechanical ventilation, days from intubation to tracheostomy, days of tracheostomy, and late tracheostomy was categorized as >14days (yes/no).
We obtained the following laboratory data: leukocytes (>12,000 cells/mm3), C-reactive protein (CRP>6 mg/dL), procalcitonin (procalcitonin> 0.5 ng/dL), PaO2/FiO2 (arterial oxygen pressure / inspired oxygen fraction) at the time of tracheostomy (≤150 mmHg), creatinine (>1.3 mg/dL). The ventilatory variables were: FiO2 (>35%), PEEP (positive end-expiratory pressure) (>8 cm H2O), tidal volume (>460 mL) and inspiratory pressure (15 cm H2O), peak pressure (>25 cm H2O), respiratory rate (>25 rpm). We included the following complications during hospitalization: superinfections, defined as clinical deterioration recorded and associated with a positive culture (yes/no), these were: bacteremia (yes/no), ventilator-associated pneumonia (yes/no), urinary tract infection (yes/no), septic shock (yes/no) and barotrauma (yes/no).
Procedures
All patients hospitalized during the COVID-19 pandemic from March 28, 2020 to March 1, 2022 were included. Once identified, medical records were reviewed through the ESSI, information was collected on comorbidities, diagnoses, laboratory tests, procedures and outcome.
Data was collected by the researchers (RFP, MHZ and AGA) in a spreadsheet created in Microsoft Excel 2019; double data entry was performed to control inconsistencies. Demographic, clinical and laboratory characteristics were detailed at the time of the tracheostomy and additionally the treatment and its outcome during hospitalization (decannulation or death) were also collected.
The cases were followed during their stay in the critical care unit. The time of tracheostomy was considered to be time zero and the final time as the occurrence of death or discharge from the intensive care unit in decannulated patients.
Statistical analysis
Data were imported into Stata® v17 (StataCorp., College Station, TX, USA). Comparisons were made between critically ill patients with tracheostomy who were successfully decannulated and those who died during their stay in the COVID-ICU. Categorical variables were expressed as frequencies and percentages, and numerical categorical variables as medians and interquartile ranges, as appropriate. All hypothesis tests were two-tailed, with a significance level of 0.05. Regarding the comparison of variables, the chi-square test or Fisher’s exact test (as appropriate) and the U-Mann-Whitney test were used for numerical variables, due to their non-normal distribution. In order to determine the factors associated with mortality at the time of tracheostomy, we used the Cox proportional hazards model, with the time variable being the days elapsed from hospitalization to death or discharge (in decannulated patients), to identify the crude and adjusted hazard ratios (HR) and their respective 95% confidence intervals (CI).
The variables that showed a statistically significant association in the bivariate analysis of mortality (p<0.05) were included in the crude analysis. The variables with a statistically significant association with mortality in the crude analysis (p<0.05) were included in the adjusted analysis. Proportionality assumptions were corroborated using Schoenfeld residuals. The collinearity of the variables included in the Cox regression was calculated using the variance inflation factor (VIF) with a cut-off point of 6.0. All the values of the variables included in the regression were acceptable [infection period (VIF=3.06), procalcitonin>0.5ng/dL (VIF=1.73), PaO2/FiO2 <150 mmHg (VIF=2.21), FiO2 >35% (VIF=1.98), and peak pressure >25 (VIF=3.33) [.
Finally, we described the survival of patients undergoing tracheostomy admitted due to COVID-19 using the Kaplan-Meier method. The log-rank test was used to evaluate differences between survival functions.
Ethical Aspects
This research complies with the Helsinki norms for research on human beings. The research protocol was approved by the ethics committee of the Hospital III Daniel Alcides Carrión (CIEI-Tacna-N.º 036). This study was registered in the Health Research Projects Platform (PRISA) of the National Institute of Health with code EI00000003060. Due to the retrospective and observational nature of the study, informed consent was not requested and the data collected were confidential.
RESULTS
Of the 329 critically ill patients hospitalized in the COVID-ICU, 83 (25.2%) underwent tracheostomy as part of their evolution, but 10 did not meet the inclusion criteria (one patient was admitted with a diagnosis other than COVID-19 pneumonia, 2 patients did not have the outcome of death or decannulation and 7 patients had incomplete clinical and laboratory data). We included 73 medical records of patients admitted to COVID-ICU with a diagnosis of SARS-CoV-2 infection, COVID-19 pneumonia and percutaneous tracheostomy carriers.
The median age was 59 (IQR: 52-66) years, 72.6% of the patients were male and only 6.8% were vaccinated against SARS-CoV-2. The most frequent comorbidities were obesity (68.4%), type 2 diabetes mellitus (35.6%) and arterial hypertension (34.2%).
The median ICU and mechanical ventilation (MV) length of stay was 32 (IQR: 24-41) and 31 (IQR: 24-43) days, respectively, the median tracheostomy time was 18 (IQR: 10-25) days (decannulation or death) and the time at which tracheostomy was performed was 17 (IQR: 15-20) days counted from the time of intubation.
Regarding laboratory tests, the median PaO2/FiO2 at the time of tracheostomy was 213 (IQR: 175-270), procalcitonin was 0.22 ng/mL (IQR: 0.13-0.40), CRP of 6.35 mg/dL (IQR: 3.2-12.1), and leukocytes of 11,950 cells/mL (8.810-15.510). Concerning mechanical ventilation variables, FiO2 at the time of tracheostomy was 30% (IQR: 28-35), PEEP (positive end-expiratory pressure) was 7 cm H2O (IQR: 6-8), inspiratory pressure was 15 cm H2O (IQR: 14-18) and the tidal volume was 466 mL (IQR: 406-509).
Regarding the treatment, 71 (97.3%) patients received antibiotics, 20 (27.4%) received antifungals and 9 (12.3%) received vasopressors at the time of tracheostomy. MV-associated pneumonia was found in 66 patients (90.4%), bacteremia in 14 (19.2%) and urinary tract infection in 9 (12.3%) patients. We found that 27 (37%) tracheostomized patients died during ICU stay and 46 (63%) were successfully decannulated and discharged (Table 1).
Table 1 Clinical, laboratory characteristics and ventilatory parameters of the study population and comparison between surviving and deceased decannulated patients.
| Variable | Total n=73 (%) | Decannulated survivors n=46 (%) | Deceased n=27 (%) | p-value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age a | 59 (52-66) | 58.5 (49-65) | 61 (52-66) | 0.212b |
| Sex | ||||
| Male | 53 (72.6) | 34 (64.2) | 19 (35.8) | 0.743c |
| Female | 20 (27.4) | 12 (60.0) | 8 (40.0) | |
| Clinical characteristics | ||||
| Comorbidities | ||||
| Yes | 65 (89.0) | 39 (60.0) | 26 (40.0) | 0.247c |
| No | 8 (11.0) | 7 (87.5) | 1 (12.5) | |
| Obesity | ||||
| Yes | 50 (68.5) | 20 (58.0) | 21 (42.0) | 0.191c |
| No | 23 (31.5) | 17 (73.9) | 6 (26.1) | |
| Diabetes mellitus | ||||
| Yes | 26 (35.6) | 15 (57.7) | 11 (42.3) | 0.484c |
| No | 47 (64.4) | 31 (65.9) | 16 (34.1) | |
| Hypertension | ||||
| Yes | 25 (34.3) | 15 (60.0) | 10 (40.0) | 0.700c |
| No | 48 (65.7) | 31 (64.6) | 17 (35.4) | |
| Chronic kidney disease | ||||
| Yes | 3 (4.1) | 2 (66.7) | 1 (33.3) | 0.693d |
| No | 70 (95.9) | 44 (62.8) | 26 (37.2) | |
| Infection period | ||||
| 2020 | 22 (30.1) | 10 (21.7) | 12 (44.4) | |
| 2021 | 42 (57.5) | 32 (69.6) | 10 (37.0) | 0.025c |
| 2022 | 9 (12.4) | 4 (8.7) | 5 (18.6) | 0.261d |
| SARS-CoV-2 Vaccination | 5 (6.9) | 2 (40.0) | 3 (60.0) | |
| 2 doses | 2 (2.7) | 0 (0.0) | 2 (100.0) | |
| 3 doses | 3 (4.1) | 2 (66.7) | 1 (33.3) | |
| Days of hospital stay a | 41 (34-51) | 47,5 (39-57) | 34 (29-40) | <0.001b |
| Days of stay in ICU a | 32 (24-41) | 32,5 (24-46) | 27 (22-36) | 0.172b |
| Days on mechanical ventilation a | 31 (24-43) | 31,5 (23-45) | 31 (27-38) | 0.899b |
| Days from intubation to tracheostomy a | 17 (15-20) | 16 (13-21) | 17 (15-20) | 0.791b |
| Tracheostomy days a | 18 (10-25) | 19 (10.5-27.5) | 13 (8-20) | 0.087b |
| Late tracheostomy >14 days | ||||
| Yes | 56 (76.7) | 33 (58.9) | 23 (41.1) | 0.189c |
| No | 17 (23.3) | 13 (76.5) | 4 (23.5) | |
| Laboratorial characteristics at the time of Tracheostomy | ||||
| Leucocytes >12,000 cel/mm3 | ||||
| Yes | 36 (49.3) | 19 (52.8) | 17 (47.2) | 0.074c |
| No | 37 (50.7) | 27 (73.0) | 10 (27.0) | |
| C-reactive protein >6 mg/dL | ||||
| Yes | 33 (45.2) | 17 (51.5) | 16 (48.5) | 0.065c |
| No | 40 (54.8) | 29 (72.5) | 11 (27.5) | |
| Procalcitonin >0,5 ng/dL | ||||
| Yes | 14 (19.2) | 2 (14.3) | 12 (85.7) | <0.001c |
| No | 59 (80.8) | 44 (74.6) | 15 (25.4) | |
| PaO2/FiO2 <150 mmHg | ||||
| Yes | 9 (12.3) | 0 (0.0) | 9 (100.0) | <0.001c |
| No | 64 (87.7) | 46 (71.8) | 18 (28.2) | |
| Creatinine >1,3 mg/dL | ||||
| Yes | 9 (12.3) | 5 (55.6) | 4 (44.4) | 0.621c |
| No | 64 (87.7) | 41 (64.0) | 23 (36.0) | |
| Ventilatory parameters at the time of tracheostomy | ||||
| FiO2 >35% | ||||
| Yes | 27 (37.0) | 11 (40.7) | 16 (59.3) | 0.003c |
| No | 46 (63.0) | 35 (76.1) | 11 (23.9) | 0.003c |
| PEEP >8 cm H2O | ||||
| Yes | 28 (38.4) | 16 (57.1) | 12 (42.9) | 0.412c |
| No | 45 (61.6) | 30 (66.7) | 15 (33.3) | |
| Tidal volume >460 mL | ||||
| Yes | 41 (56.2) | 27 (65.9) | 14 (34.1) | 0.569c |
| No | 32 (43.8) | 19 (59.3) | 13 (40.7) | |
| Inspiratory pressure >15 cm H2O | ||||
| Yes | 48 (65.8) | 28 (58.3) | 20 (41.7) | 0.251c |
| No | 25 (34.2) | 18 (72.0) | 7 (28.0) | |
| Peak pressure >25 cm H2O | ||||
| Yes | 28 (38.4) | 12 (42.9) | 16 (57.1) | 0.005b |
| No | 45 (61.6) | 34 (75.5) | 11 (24.5) | |
| Respiratory frequency >25rpm | ||||
| Yes | 22 (30.1) | 13 (59.1) | 9 (40.9) | 0.648c |
| No | 51 (69.9) | 33 (64.7) | 18 (35.3) | |
| Complications | ||||
| Nosocomial infection | ||||
| Yes | 64 (87.7) | 39 (60.9) | 25 (39.1) | 0.701d |
| No | 9 (12.3) | 7 (77.7) | 2 (33.3) | |
| Ventilator-associated pneumonia | ||||
| Yes | 66 (90.4) | 41 (62.1) | 25 (37.9) | 0.483d |
| No | 7 (9.6) | 5 (71.4) | 2 (28.6) | |
| Bacteriemia | ||||
| Yes | 14 (19.2) | 10 (71.4) | 4 (28.6) | 0.344d |
| No | 59 (80.8) | 36 (61.0) | 23 (39.0) | |
| UTI | ||||
| Yes | 9 (12.3) | 5 (55.6) | 4 (44.4) | 0.440d |
| No | 64 (87.7) | 41 (64.0) | 23 (36.0) | |
| Septic shock | ||||
| Yes | 63 (86.3) | 42 (66.7) | 21 (33.3) | 0.098d |
| No | 10 (13.7) | 4 (40.0) | 6 (60.0) | |
| Barotrauma | ||||
| Yes | 14 (19.2) | 7 (50.0) | 7 (50.0) | 0.262c |
| No | 59 (80.8) | 39 (66.1) | 20 (33.9) | 0.262c |
a Median and interquartile range, b Mann-Whitney U test, c Chi-square test, d Fisher exact test.
ICU: intensive care unit; PaO2/FiO2: arterial oxygen pressure to inspired oxygen fraction (FiO2) ratio; PEEP: positive end-expiratory pressure; VAP: ventilator-associated pneumonia; UTI: urinary tract infection; rpm: respirations per minute.
No significant differences were found regarding the mortality in patients according to age group, sex, vaccination status, comorbidities, length of stay in the ICU, time on mechanical ventilation, time of tracheostomy, or according to the treatment. However, the time of tracheostomy was longer in those who achieved decannulation than in those who died (21 vs. 15 days; p=0.043). Likewise, PaO2/FiO2 was lower in the group of patients who died (p<0.001), while a procalcitonin value >0.5mg/dL, higher FiO2, inspiratory pressure and peak pressure at the time of tracheostomy were associated with a higher risk of death.
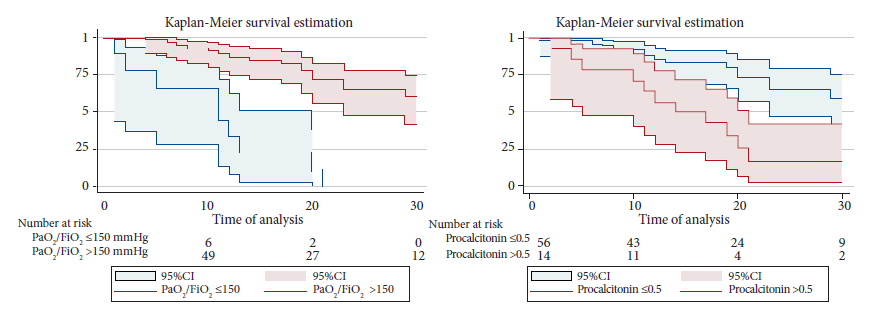
Factors associated with mortality were evaluated by Cox proportional hazards regression. In the adjusted analysis, procalcitonin >0.50 at the time of tracheostomy was shown to be a factor associated with mortality with a aHR of 2.40 (95%CI: 1.03-2.59). Likewise, a PaO2/FiO2 level ≤150 mmHg was independently associated with higher mortality, with an aHR of 4.40 (95%CI: 1.56-12.60) (Table 2). Figure 1 presents the Kaplan-Meier survival curves for the variables PaO2/FiO2 (p<0.001) and procalcitonin (p<0.001).
Table 2 Cox regression analysis to evaluate predictors of mortality in tracheostomized patients hospitalized due to COVID-19.
| Variable | cHR (95%CI) | p-value | aHR (95%CI) | p-value |
|---|---|---|---|---|
| Infection period | ||||
| 2020 | Ref. | - | ||
| 2021 | 0.52 (0.22-1.21) | 0.130 | - | |
| 2022 | 1.13 (0.39-3.26) | 0.816 | - | |
| Procalcitonin >0,5 ng/dL | ||||
| Yes | 3.76 (1.75-8.10) | 0.001 | 2.41 (1.04-2.60) | 0.005 |
| No | Ref. | Ref. | ||
| PaO2/FiO2 ≤150 mmHg | ||||
| Yes | 7.60 (3.21-17.99) | <0.001 | 4.41 (1.57-12.61) | 0.005 |
| No | Ref. | Ref. | ||
| FiO2 >35% | ||||
| Yes | 2.02 (0.93-4.36) | 0.075 | - | - |
| No | Ref. | - | ||
| Peak pressure >25 cmH20 | ||||
| Yes | 2.40 (1.09-5.29) | 0.030 | 1.31 (0.52-3.30) | 0.569 |
| No | Ref. | Ref. |
cHR: crude hazard ratio; aHR: adjusted hazard ratio; PaO2/FiO2: ratio of arterial oxygen pressure to inspired oxygen fraction (FiO2); PEEP: positive end-expiratory pressure. The variables included in the adjusted analysis were those that showed a statistically significant association with mortality in the crude analysis (p<0.05).
DISCUSSION
We found that 36.9% of all patients who were tracheostomized by COVID-19 died. Furthermore, we were able to establish two risk factors for mortality, which are the procalcitonin value >0.5 ng/dL, and a low PaO2/FiO2 ratio (less than or equal to 150) at the time of tracheostomy.
Reported mortality rates in patients with tracheostomy and COVID-19 ranges from 15 to 66% 18-21. A meta-analysis of 5268 tracheostomized patients reported a pooled mortality of 22%; however, there was great heterogeneity among the included studies 19. It has also been reported that critically ill patients who underwent tracheostomy had lower mortality than those who were not tracheostomized, even in severely ill patients with APACHE II >17 18,21. In Peru, the overall mortality due to ARDS associated with COVID-19 was 25% 21, which is lower than that in our cohort of tracheostomized patients, perhaps due to the low proportion of patients immunized against COVID-19 and longer mechanical ventilation and intensive care stay.
Although the fact that vaccines arrived in Peru in February 2021, vaccination in our region started some months later 2 and that the number of COVID-ICU beds was not constant throughout the pandemic 14, we could not find statistical differences in relation to deaths and decannulated patients according to the infection period (2020, 2021, 2022), probably because most of our patients were not vaccinated at the time of the study, and the management of these critical patients was always protocolized and homogeneous, since it was always in charge of highly trained human resources in intensive care 23.
The benefits of performing an early tracheostomy (≤7 days) in critically ill patients with pneumonia are the reduction of ventilator-associated pneumonia (VAP), more days free of mechanical ventilation, and shorter stay in the ICU 22. However, these benefits have not been demonstrated in critically ill patients diagnosed with COVID-19 23, where even higher mortality has been found in those patients with COVID-19 undergoing early tracheostomy (≤ 14 days from orotracheal intubation) compared to late tracheostomy 16,24,25. In our study the time from intubation to tracheostomy was not associated with mortality, and although most of our patients underwent late tracheostomy, we did not find differences in the incidence of VAP, days of mechanical ventilation, or ICU length of stay, between decannulated and deceased patients. We presume that, due to the complexity of these patients, achieving ventilatory stability was preferred to be able to perform the procedure in most cases.
Usually, tracheostomy is a safe procedure in which the most frequently reported complications are bleeding (7-20%), emphysema (4.4%), stenosis or obstruction (2%) and false airway, with a slightly lower number of complications in percutaneous tracheostomies 20,26,27. Another alternative is bronchoscopy-guided percutaneous tracheostomy, which allows direct visualization of the airway, increasing the safety of the procedure by allowing the location of the cannulation site, with a low incidence of late complications (<1%) 28. And although tracheostomy is a procedure that can cause aerosols and therefore a higher risk of SARS-CoV-2 infection, a study showed that no health worker involved in the tracheostomy procedure developed COVID-19 infection 9.
Peak pressure is the pressure reached during inspiration, when air is pushed into the lungs and is a measure of airway resistance 29. In the management of the patient with respiratory distress syndrome it is recommended that this value be <25 cm H2O to avoid pressure damage (barotrauma) 30. We found that this value was higher in patients who died, both in the bivariate analysis and in the crude regression model, although the adjusted regression model did not show a significant value.
Procalcitonin is an indicator of COVID-19 severity, and serial procalcitonin measurements may be useful in predicting disease prognosis 31,32. We found that 85% of patients with a high procalcitonin value died. These patients probably had a more intense hyperinflammatory state due to COVID-19, or bacterial superinfections 33) at the time of tracheostomy, which limited their recovery. Likewise, several studies have reported an inversely proportional association between PaO2/FiO2 and mortality in patients with COVID-19 13,22,33,34. In our study, the median PaO2/FiO2 at the time of tracheostomy was higher than that reported by other authors, who coincidentally observed higher mortality 18, a value less than or equal to 150 mmHg, was the most important factor associated with mortality in our cohort. Due to this finding, it is suggested to avoid tracheostomy when PaO2/FiO2 lower or equal to 150 mmHg.
Our study has some limitations. The main one was its retrospective nature, which did not allow us to fully evaluate other confounding variables. Another important limitation was the small number of patients, which reduced the possibility of controlling for some confounding factors, and this could also explain why the confidence intervals were so wide and why many variables did not present statistical association, such as the time of tracheostomy (early vs. late), period of infection or exposure to vaccines. Likewise, we cannot affirm whether the elevated procalcitonin level at the time of tracheostomy is due to persistence of COVID-19 or to an added infectious complication and therefore explain the lower survival of this group of patients as in other studies 35. Likewise, complications related to the percutaneous tracheostomy procedure in the short term (bleeding, pneumothorax, false airway, etc.) and long term (stenosis, tracheomalacia, fistulas, etc.) were not studied. Finally, it was impossible to measure the impact of changes in treatment strategies (corticosteroids, tocilizumab, etc.) and control methods implemented throughout the pandemic 20, which directly affect the practice of tracheostomy.
In conclusion, approximately one third of the patients in this cohort died during the study period. Among the factors associated with higher mortality in tracheostomy patients were a procalcitonin value >0.50ng/dL, and a PaO2/FiO2 value ≤150 at the time of performing the tracheostomy. Timely recognition of these factors should call for reflection of the personnel performing this procedure, to defer it until oxygen and infectious conditions improve, and thus decrease mortality in this group of critically ill patients.
REFERENCES
1. Aleem A, Akbar Samad AB, Slenker AK. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). En: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [citado 9 de octubre de 2022]. Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK570580/. [ Links ]
2. Ministerio de Salud. Sala Situacional Covid 19 en el Perú 2023 [Internet].Lima: MINSA; 2023 [citado 22 de mayo de 2023]. Disponible en: https://covid19.minsa.gob.pe/sala_situacional.asp. [ Links ]
3. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data [Internet]. Geneva: WHO; c2023 [citado 22 de mayo de 2023]. Disponible en: https://covid19.who.int/table. [ Links ]
4. Mecham JC, Thomas OJ, Pirgousis P, Janus JR. Utility of Tracheostomy in Patients With COVID-19 and Other Special Considerations. Laryngoscope. 2020;130(11):2546-9. doi: 10.1002/lary.28734. [ Links ]
5. Evrard D, Jurcisin I, Assadi M, Patrier J, Tafani V, Ullmann N, et al. Tracheostomy in COVID-19 acute respiratory distress syndrome patients and follow-up: A parisian bicentric retrospective cohort. PLoS ONE. 2021;16(12):e0261024. doi: 10.1371/journal.pone.0261024. [ Links ]
6. Asensio Martín MJ, Hernández Bernal M, Yus Teruel S, Minvielle A. Infecciones en el paciente crítico. Medicine (Baltimore). 2018;12(52):3085-96. doi: 10.1016/j.med.2018.03.014. [ Links ]
7. Vayas Valdivieso WA, Viteri Rodríguez JA, Viteri Villa MF, Wong Vázquez L. Principales secuelas neurológicas del COVID-19: una revisión exploratoria. Bol Malariol Salud Ambient. 2022;62(4):678-85. doi: 10.52808/bmsa.7e6.624.008. [ Links ]
8. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607-613. doi: 10.1016/j.jinf.2020.03.037. [ Links ]
9. Corona A, Santis V de, Vitale D, Nencini C, Potalivo A, Prete A, et al. Tracheostomy in critically ill patients with SARS 2 COVID-19 infection: a prospective observational multi-center study of short- and long-term outcomes. Can J Respir Ther. 2022;30;58:155-161. doi: 10.29390/cjrt-2022-018. [ Links ]
10. ALHumaid S, Elkrim MA, AlOqaili YA, AlSowailmi GA, AlObaid FA, AlSalem AA, et al. Outcomes of tracheostomy in COVID-19 patients in National Guard Health Affairs, Riyadh, Saudi Arabia. Saudi Med J. 2021;42(11):1217-22. doi: 10.15537/smj.2021.42.11.20210505. [ Links ]
11. Karna ST, Trivedi S, Singh P, Khurana A, Gouroumourty R, Dodda B, et al. Weaning Outcomes and 28-day Mortality after Tracheostomy in COVID-19 Patients in Central India: A Retrospective Observational Cohort Study. Indian J Crit Care Med. 2022;26(1):85-93. doi: 10.5005/jp-journals-10071-24080. [ Links ]
12. Flinspach AN, Booke H, Zacharowski K, Balaban Ü, Herrmann E, Adam EH. Association of mortality and early tracheostomy in patients with COVID-19: a retrospective analysis. Sci Rep. 2022;12(1):15406. doi: 10.1038/s41598-022-19567-w. [ Links ]
13. Diaz JV, Riviello ED, Papali A, Adhikari NKJ, Ferreira JC. Global Critical Care: Moving Forward in Resource-Limited Settings. Ann Glob Health. 2019;85(1):3. doi: 10.5334/aogh.2413. [ Links ]
14. Hueda-Zavaleta M, Copaja-Corzo C, Bardales-Silva F, Flores-Palacios R, Barreto-Rocchetti L, Benites-Zapata VA, et al. Factores asociados a la muerte por COVID-19 en pacientes admitidos en un hospital público en Tacna, Perú. Rev Perú Med Exp Salud Pública. 2021;38(2):214-23. doi: 10.17843/rpmesp.2021.382.7158. [ Links ]
15. Geiseler J, Westhoff M. [Weaning from invasive mechanical ventilation]. Med Klin Intensivmed Notfmed. 2021;116(8):715-26. doi: 10.1007/s00063-021-00858-5. [ Links ]
16. World Health Organization. Clinical management of COVID-19: Living guideline, 13 January 2023 [Internet]. Geneva: WHO; 2023 [cited 2023 Feb 8]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1. [ Links ]
17. Seguro Social del Perú, EsSalud [Internet]. Lima: EsSalud. 2019. [citado 31 de mayo de 2023]. Disponible en: http://www.essalud.gob.pe/essalud-implementa-historia-clinica-digital-para-atencion-de-asegurados/. [ Links ]
18. Vuu SKM, Soltani T, Liu H, DeMuro J, Albors LM, Crimi E, et al. Optimal timing and outcomes among COVID-19 patients undergoing tracheostomy. Surgery. 2023;173(4):927-935. doi: 10.1016/j.surg.2022.11.017. [ Links ]
19. Musso G, Managó M, Gomez C, Appendino G, Friscione L, González C, et al. Supervivencia y decanulación a los 90 días luego de traqueostomía por dilatación percutánea en unidad de cuidados intensivos COVID-19. Medicina (B Aires). 2022;82(6):836-44. [ Links ]
20. Battaglini D, Premraj L, White N, Sutt AL, Robba C, Cho SM, et al. Tracheostomy outcomes in critically ill patients with COVID-19: a systematic review, meta-analysis, and meta-regression. Br J Anaesth. 2022;129(5):679-692. doi: 10.1016/j.bja.2022.07.032. [ Links ]
21. Breik O, Nankivell P, Sharma N, Bangash MN, Dawson C, Idle M, et al. Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth. 2020;125(6):872-879. doi: 10.1016/j.bja.2020.08.023. [ Links ]
22. Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of Early vs Late Tracheostomy Placement With Pneumonia and Ventilator Days in Critically Ill Patients: A Meta-analysis. JAMA Otolaryngol-Head Neck Surg. 2021;147(5):450-9. doi: 10.1001/jamaoto.2021.0025. [ Links ]
23. Hueda-Zavaleta M, Copaja-Corzo C, Miranda-Chávez B, Flores-Palacios R, Huanacuni-Ramos J, Mendoza-Laredo J, et al. Determination of PaO2/FiO2 after 24 h of invasive mechanical ventilation and 1PaO2/FiO2 at 24 h as predictors of survival in patients diagnosed with ARDS due to COVID-19. Peer J. 2022;10:e14290. doi: 10.7717/peerj.14290. [ Links ]
24. Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Base de datos Cochrane de Revisiones Sistemáticas 2015, Número 1. Art. Nº: CD007271. doi: 10.1002/14651858.CD007271. [ Links ]
25. Harrell Shreckengost CS, Foianini JE, Moron Encinas KM, Tola Guarachi H, Abril K, Amin D, et al. Outcomes of Early Versus Late Tracheostomy in Patients With COVID-19: A Multinational Cohort Study. Crit Care Explor. 2022;4(11):e0796. doi: 10.1097/CCE.0000000000000796. [ Links ]
26. Tang Y, Wu Y, Zhu F, Yang X, Huang C, Hou G, et al. Tracheostomy in 80 COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front Med (Lausanne). 2020;7:615845. doi: 10.3389/fmed.2020.615845. [ Links ]
27. Singh Y, Soni KD, Singh A, Choudhary N, Perveen F, Aggarwal R, et al. Clinical characteristics of COVID-19 patients who underwent tracheostomy and its effect on outcome: A retrospective observational study. World J Virol. 2022 Nov 25;11(6):477-484. doi: 10.5501/wjv.v11.i6.477. [ Links ]
28. Bisso IC, Ruiz V, Huespe IA, Rosciani F, Cantos J, Lockhart C, et al. Bronchoscopy-guided percutaneous tracheostomy during the COVID-19 pandemic. Surgery. 2023;173(4):944-949. doi: 10.1016/j.surg.2022.12.010. [ Links ]
29. Mora Carpio AL, Mora JI. Ventilator Management [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [citado 6 de junio de 2023]. Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK448186/. [ Links ]
30. Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319(7):698-710. doi: 10.1001/jama.2017.21907. [ Links ]
31. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2):106051. doi: 10.1016/j.ijantimicag.2020.106051. [ Links ]
32. Heidari-Beni F, Vahedian-Azimi A, Shojaei S, Rahimi-Bashar F, Shahriary A, Johnston TP, et al. The Level of Procalcitonin in Severe COVID-19 Patients: A Systematic Review and Meta-Analysis. Adv Exp Med Biol. 2021;1321:277-86. doi: 10.1007/978-3-030-59261-5_25. [ Links ]
33. Copaja-Corzo C, Hueda-Zavaleta M, Benites-Zapata VA, Rodriguez-Morales AJ. Antibiotic use and fatal outcomes among critically ill patients with covid-19 in Tacna, Peru. Antibiotics (Basel). 2021;10(8):959. doi: 10.3390/antibiotics10080959. [ Links ]
34. Zinellu A, De Vito A, Scano V, Paliogiannis P, Fiore V, Madeddu G, et al. The PaO2/FiO2 ratio on admission is independently associated with prolonged hospitalization in COVID-19 patients. J Infect Dev Ctries. 31 de marzo de 2021;15(3):353-9. doi: 10.3855/jidc.13288. [ Links ]
35. Pink I, Raupach D, Fuge J, Vonberg RP, Hoeper MM, Welte T, et al. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection. 2021 Oct;49(5):935-943. doi:10.1007/s15010-021-01615-8. [ Links ]
Cite as: Flores Palacios RJ, Hueda Zavaleta M, Gutiérrez Avila AG, Gómez de la Torre JC, Benites Zapata VA. Characteristics and factors associated with mortality in tracheostomized patients with COVID-19: a retrospective cohort study in a hospital in Tacna, Peru. Rev Peru Med Exp Salud Publica. 2023;40(4):441-50. doi: 10.17843/rpmesp.2023.404.12629.
Received: February 09, 2023; Accepted: October 18, 2023











 texto en
texto en