Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Horizonte Médico (Lima)
versión impresa ISSN 1727-558X
Horiz. Med. vol.23 no.2 Lima abr./jun. 2023 Epub 30-Mayo-2023
http://dx.doi.org/10.24265/horizmed.2023.v23n2.12
Review article
Free radicals and antioxidant system
1 Universidad de San Martín de Porres, School of Human Medicine, Centro de Investigación de Bioquímica y Nutrición (Biochemistry and Nutrition Research Center). Lima, Peru.
Free radicals are compounds characterized by having an unpaired electron in their outer orbit, a condition that makes them highly reactive, i.e., they interact through diffusion-controlled reactions with proteins, lipids and nucleic acids. They have also been referred to as reactive oxygen species (ROS), reactive nitrogen species (RNS) or reactive sulfur species (RSS). In the human organism, they are mainly produced in the mitochondrial electron transport chain, where respiratory complexes I and III specifically participate and reduce oxygen by converting it into superoxide anion. Likewise, they can be formed through a wide variety of enzymatic and non-enzymatic reactions involving substances that are synthesized by cells or are ingested with food and some medicines. Human beings have an antioxidant system which is both enzymatic and non-enzymatic in nature and whose function is to protect the organism from the harmful action of free radicals. This system includes enzymes—such as catalase, superoxide dismutase, thioredoxin, etc.—and non-enzymatic compounds— such as glutathione, ferritin, myoglobin, etc. However, they are not efficient enough to protect it, so it is necessary to eat foods that contain substances with antioxidant properties whose protective action will depend on their chemical reactivity and their concentration. These antioxidant compounds are mainly found in fruits and vegetables, where polyphenols, flavonoids, carotenoids, vitamin C, vitamin E, etc. have been identified. A significant amount of evidence suggests that the intake of antioxidant substances protects the body from the damaging effect of free radicals, but when the oxidative action prevails over the antioxidant action, it can lead to oxidative stress, a condition that is closely linked to a wide variety of chronic non-communicable diseases including cancer, diabetes mellitus, obesity, psoriasis, atherosclerosis, among others. All this seems to indicate that the term “cellular redox steady state” more appropriately describes the constant adaptation to a situation of rapid chemical turnover and suggests that the substances involved in this process be designated as “biologically reactive species” due to the existence of harmful compounds such as hydrogen peroxide, peroxynitrite, etc., which are not—strictly speaking—free radicals but have toxic effects on cells.
Keywords: Free Radicals; Antioxidants; Oxidative Stress; Reactive Oxygen Species
Introduction
The existence of free radicals in a chemical reaction was first mentioned by Moses Gomberg 1 when he was trying to synthesize tetraphenylmethane. A free radical is a molecule characterized by having an unpaired electron in its outer orbit. Studies concerning the effect of oxygen on enzymes such as pepsin, catalase, cholinesterase and carbonic anhydrase showed that they were affected by oxygen 2. Likewise, the exposure of rats to a pure oxygen environment 3 caused their death three days later, and this effect could not be attributed to free radicals due to the lack of appropriate scientific support. Later, the possible transfer of only one electron to oxygen 4 and the formation of superoxide anion in the mitochondrial respiratory chain were suggested. One article about the theory of aging claimed that the damaging effect of X-rays on animal tissues 5 would occur by the production of hydroxyl radicals. Subsequently, there was evidence of the existence of the hydroxyl radical (OH•), superoxide anion radical (O₂-•), peroxyl radical (ROO•), etc., referred to as reactive oxygen species 6; nitric oxide (NO•) and nitrogen dioxide (NO₂•), identified as reactive nitrogen species; and the thiyl radical (RS•), identified as reactive sulfur species, etc.
Several epidemiological studies show a close relationship between oxidative stress and chronic non-communicable diseases such as cancer, diabetes mellitus, psoriasis, atherosclerosis, etc. Oxidative stress is an imbalance between the production of free radicals and the antioxidant defense system, where the former prevails; this situation occurs notwithstanding human beings have a partially efficient antioxidant defense system 6-8. Therefore, it is necessary to eat food containing substances with antioxidant properties. This narrative review aims at providing knowledge about processes leading to the production of free radicals as well as antioxidant defense systems.
Search strategy
The strategy to carry out this review consisted in searching in review and research articles in Google Scholar, PubMed and SciELO databases, without time limit, the following English and Spanish terms: free radicals, reactive oxygen species, antioxidants, oxidative stress, radicales libres, especies reactivas de oxígeno, antioxidantes and estrés oxidativo.
Production of free radicals
In human beings, free radicals are produced in the mitochondrial electron transport chain with the participation of respiratory complexes I and III 9, which transfer an electron to oxygen by converting it into superoxide anion (Figure 1).
Free radicals are mildly produced during moderate physical activity and are increased during vigorous physical activity 10-12. Fructose is a carbohydrate mainly metabolized in the liver and, since it lacks an adequate metabolic regulation process, it exerts high pressure of electrons at the mitochondrial level, thus increasing the production of free radicals. This effect is closely related to pathologies such as obesity, diabetes mellitus, hypertension, etc. 13-17
Superoxide anion is a free radical produced in the mitochondrion 18 and transformed by the superoxide dismutase (SOD) enzyme 19 into hydrogen peroxide and oxygen (Equation 1):
Nitric oxide (NO•) 20 is synthesized by nitric oxide synthase, reacts with superoxide anion and produces peroxynitrite, a compound with low activity in aqueous medium, which can be transformed into a more reactive form when entering the lipid membrane. Peroxynitrite is not a free radical per se (Equation 2):
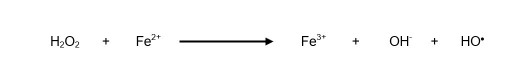
The following reaction occurs between superoxide anion and hydrogen peroxide in the presence of ferric ion (Fe3+) as a catalyst 21, thus forming the hydroxyl radical (Equation 3):
In the ischemia/reperfusion injury process 22, characterized by oxygen deficiency at the cellular level, ATP is degraded until forming hypoxanthine. Then, xanthine dehydrogenase is converted into xanthine oxidase. Subsequently, when reperfusion occurs, this enzyme reacts with hypoxanthine and oxygen, forming xanthine and superoxide anion. Thereafter, it reacts with xanthine and another oxygen molecule, thus producing uric acid and superoxide anion.
NADPH oxidase (NOX) 23 is an enzyme that oxidizes the NADPH coenzyme, thus producing superoxide anion, contributes to regulate the vascular tone and participates in the “respiratory burst,” etc.: reactions that use phagocytic cells to destroy pathogens. Therefore, a defect in the expression of one of the subunits may lead to the development of chronic granulomatous disease and atherosclerosis. (Equation 4)
The hydroxyl radical is the most harmful to human beings. It is produced in quite different ways, the most important being the Fenton and Haber-Weiss reactions 24, which are developed in two stages: the first one was previously described, where Fe3+ interacts with O₂•and forms Fe2+, and the second one is shown below. (Equation 5)
Vitamin C is known for its antioxidant properties, but it may act as a prooxidant 25 in the presence of transition metals such as Fe3+ or Cu2+ (Equation 6):
It was observed that ferrous sulfate syrup used for the treatment of iron deficiency anemia, in the presence of vitamin C, increases the formation of thiobarbituric acid reactive substances (TBARS) 26, i.e., it increases the production of free radicals. Moreover, when ferrous sulfate and vitamin C were administered to albino rats 27, all the animals presented hepatocellular damage, which allows inferring that people who are prescribed ferrous sulfate and orange juice or lemonade because of its content of vitamin C to improve iron absorption may develop liver damage. It was also observed that the administration of ferrous sulfate to pregnant women 28 caused oxidative damage, an effect that was probably a consequence of the production of free radicals.
Myeloperoxidase 29 is an enzyme found in neutrophil granules that forms hypochlorous acid by the following reaction (Equation 7):
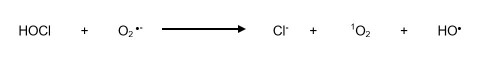
Hypochlorous acid may react with superoxide anion and produce the hydroxyl radical (Equation 8):
It must also be taken into account that some medication, cigarette smoke and environmental pollution 30 produce free radicals and harmful substances. Hence, we suggest that it is more appropriate to designate them as “biologically reactive species” (BRS) since there are substances which are not—strictly speaking—free radicals and cause damage.
Antioxidant system
Human beings have an antioxidant system composed of substances that they synthetize and antioxidant compounds that they eat in their diet (Figure 2); therefore, compounds synthetized by cells can be referred to as endogenous antioxidants and substances included in food as exogenous antioxidants.
Endogenous enzymatic antioxidant system
The components of this system comprise the following enzymes:
Superoxide dismutase (SOD) 31. This enzyme converts superoxide anion into hydrogen peroxide. There are three isoforms: SOD1 (Cu/Zn) is the main isoform, which reacts with O₂•in cytosol and the mitochondrial intermembrane space; SOD2 (Mn), which reacts with O₂•in the mitochondrial matrix; and SOD3 (Cu/Zn), which is found in extracellular fluid.
Glutathione peroxidase (GPx) 32. It reacts with H₂O₂ and removes hydroperoxides, compounds that are formed during lipid oxidation reactions. This enzyme requires glutathione (GSH) and selenium for its activity. Several isoforms have been described, and GPx1 is the one that prevails.
Thioredoxin (Trx) 33. It is a thiol-disulfide oxidoreductase, which reduces other proteins—such as ribonucleotide reductase—and different peroxidases. Trx1 is found in cytosol and outside the cells, while Trx2 is found in the mitochondrion.
Peroxiredoxin (Prx) 30. It is another antioxidant enzyme that catalyzes the reduction of hydrogen peroxide and hydroperoxides, thus forming water and alcohol, respectively. Moreover, it regulates the concentration of hydrogen peroxide, and this characteristic allows it to modulate the cell signaling pathways, where TNF-α and growth factors participate. There are three different types: Prx1, Prx2 and Prx3.
Catalase (CAT) 34. This enzyme, which is widely distributed in the human body and found particularly in the liver and kidneys, has a very high catalytic activity since it breaks down hydrogen peroxide into water and oxygen. Catalase may oxidize phenolic compounds at low concentrations of hydrogen peroxide, this type of catalysis being its peroxidase activity.
Glutathione reductase 35. It reduces oxidized glutathione (GSSG) and forms two molecules of reduced glutathione (GSH) with the active participation of the NADPH cofactor. This enzyme is very important because it reconstitutes GSH since an increased GSSG/GSH ratio is toxic to cells. GSH levels are very important so that glutathione peroxidase may efficiently remove hydrogen peroxide.
Glutaredoxin (Glrx) 36. This enzyme separates glutathione from the protein to which it is bonded, thus allowing proteins to have their free sulfhydryl groups available. This enzyme and glutathione S-transferase are key to maintaining the cellular redox signaling cycle, and its action overlaps the activity of thioredoxins. It participates in the control of apoptosis, inhibiting Fas and activating Akt and NF-kB.
Thioredoxin reductase (TrxR) 37. It is one of the antioxidant enzymes that reduce thioredoxin (Trx) and protein disulfide isomerase (PDI) using NADPH as coenzyme. It also has Se and FAD cofactors at its active site. There are several thioredoxin reductase isoforms: TrxR1, which is found in cytosol; TrxR3, which is found in the mitochondrion; and thioredoxin glutathione reductase (TGR), which is found specifically in the testicles.
Endogenous non-enzymatic antioxidant system
This system consists of multiple antioxidants such as glutathione (GSH), a substance found in high concentrations in liver and plasma, i.e., 5 to 10 mM and approximately 20 µM, respectively 38. It is used by glutathione peroxidase to remove hydroperoxides, a reaction that converts it into oxidized glutathione (GSSG), a compound that must necessarily be reduced to regenerate GSH, for which glutathione reductase and NADPH are required. Myoglobin is a protein whose main function is to store oxygen in muscle tissue and neutralize the action of nitric oxide free radical 39. One of the most important functions of ferritin is to maintain iron intracellular balance, thus preventing this metal from producing free radicals 40. Metallothioneins are low molecular weight proteins characterized by having several sulfhydryl groups that allow them to react with superoxide anion and hydroxyl radicals 41. Coenzyme Q (CoQ) participates in the mitochondrial transport of electrons and reacts with molecular oxygen and different free radicals 42. Polyamines comprise compounds such as spermine, spermidine and putrescine 43, which protect cellular membranes against the peroxyl radical, hydrogen peroxide, superoxide radical, etc. Melatonin 44 blocks the harmful action of the hydroxyl radical, nitric oxide and peroxynitrite.
Transferrin 45 is a glycoprotein that bonds trivalent iron with a high binding constant, thus preventing iron from participating in reactions that form the hydroxyl radical. Likewise, ceruloplasmin 46, another circulating plasma protein, transports copper, a metal that produces the hydroxyl radical when reacting with hydrogen peroxide. Uric acid 47 is the final product of the metabolism of purines and behaves as an efficient antioxidant; therefore, it can block the hydroxyl radical, peroxynitrite and lipid peroxides. Albumin 48 is a component of plasma proteins that fulfills multiple functions, one of which is related to the antioxidant activity due to its sulfhydryl groups. Furthermore, it has been described that bilirubin 49 has a high antioxidant action, similar to that of lactoferrin 50.
Exogenous antioxidants
Human beings may increase their antioxidant defense by eating fruits and vegetables, which are antioxidant-rich foods. These compounds (Figure 3) are abundant, and their properties have not been totally studied yet but have demonstrated beneficial effects for health 51.
This group includes polyphenols, carotenoids, some vitamins, trace elements, etc. Polyphenols 52 are organic compounds characterized by having aromatic rings with one or several phenolic groups naturally found in vegetables. Their effect is closely related to the prevention of chronic non-communicable diseases such as cancer, psoriasis, atherosclerosis, diabetes mellitus, hypertension, obesity, etc. More than 4,000 types of polyphenols have been identified, and molecules such as phenolic acids have been chemically recognized in this group. Phenolic acids are composed of benzoic acid and cinnamic acid derivatives. Furthermore, polyphenols with slightly different structures such as stilbenes, lignans and flavonoids have been recognized.
Fruits and vegetables are important sources of polyphenols, particularly hydroxybenzoic acid derivatives such as gallic acid 53, found in blackcurrant, and protocatechuic acid, found in mulberries/blackberries and especially tea leaves, which can contain up to 4.5 g/kg of fresh leaves, etc. Hydroxycinnamic acid derivatives such as coumaric acid are found in plums, kiwis and coffee; chlorogenic acid in cherries; sinapic acid in apples, pears, potatoes, coffee, etc.; and ferulic acid 54 especially in cereals such as wheat, rice, oats, corn, etc.
Stilbenes 55 are a small group of polyphenols whose main representative is resveratrol, a very efficient antioxidant mainly found in black grapes, mulberries/blackberries and blueberries/cranberries. Another important group of polyphenols are lignans 56; one of its best-known compounds, secoisolarisirecinol, is found in linseed and also in cereals, fruits and vegetables but in very low amounts.
Flavonoids are a particularly important group of naturally occurring compounds with antioxidant properties, the study of which has received special attention. These chemical compounds can be divided into six big subgroups: flavonols, flavanones, flavones, flavanols, isoflavones and anthocyanidins.
Flavonols such as rutin, isorhamnetin, myricetin, quercetin, etc. are an important subgroup of flavonoids found in many fruits and vegetables, including cabbages, onions, broccoli, parsley, tomatoes, grapes, mulberries/blackberries, berries, apples, raspberries, etc., as well as red wine, black tea and green tea 57. Another subgroup of flavonoids are flavanones 58, compounds that confer important antioxidant properties to grapefruits, tangerines, tangelos, lemons, sweet oranges, etc.
Flavones 59 are another important subgroup of flavonoids found in different food products such as oranges, apples, parsley, tea, chamomile, celeries, broccoli, rice, carrots, onions, cabbages, corn, etc. Flavanols 60 are also a subgroup having important antioxidant effects and are found in cereals and fruits such as kiwis, mulberries/ blackberries, raspberries, as well as black tea, green tea, cacao, red wine, chocolate, etc.
Another important flavonoid are anthocyanidins 61, which are characterized by coloring the food that contain them, such as mulberries/blackberries, cranberries/blueberries, nuts, cacao, cherries, strawberries, red cabbages, black grapes, etc. It is also important to mention isoflavones 62, which are found in peas, sunflower seeds, chickpeas, fava beans, soya beans, black beans, etc.
Carotenoids 63 are chemical compounds characterized by being liposoluble because of their eminently hydrophobic long hydrocarbon chain structure, except for xanthophylls, which have one oxygen atom or more. These compounds consist of two subgroups: carotenes and xanthophylls. Carotenes include α-carotene, β-carotene, γ-carotene and lycopene, and are found in food products such as pumpkins, carrots, tomatoes, spinach, broccoli, lettuces, mangoes, watermelons, etc., while xanthophylls, which comprise compounds such as zeaxanthin, lutein, β-cryptoxanthin, violaxanthin and zeinoxanthin, are found in spinach, corn, oranges, tomatoes, lettuces, etc.
Vitamin C 64 is a water-soluble compound that human beings must necessarily eat in their diet; is absorbed in the intestinal tract with the help of the sodiumdependent vitamin C transporter (SVCT); participates in non-heme iron absorption processes in the bowel; facilitates glucose membrane transport; and is involved in the uptake of transferrin-bound iron, iron homeostasis, collagen hydroxylation, carnitine and norepinephrine biosynthesis, tyrosine metabolism, vitamin E recycling, tetrahydrobiopterin recycling, cholesterol metabolism, etc. It is a potent antioxidant agent that reduces the lipid peroxidation process and diminishes the oxidation of proteins and DNA by the action free radicals.
The most important sources 65 of vitamin C among vegetables are watercress, broccoli, wild cabbage, Chinese broccoli, kohlrabies, peppers, etc., while the sources among fruits are camu-camu, which has an extremely high content of this vitamin (2,780 mg/100 g), guavas, oranges, cashews, shiraca, banana passion fruits, strawberries, limes, lemons, grapefruits, etc.
Vitamin E 66 is a water-soluble naturally occurring antioxidant that chemically falls into compounds referred to as tocopherols, out of which α-tocopherol is the one with the highest antioxidant capacity. This vitamin interrupts the chain reaction caused by free radicals, which occurs in the mitochondrial membrane and endoplasmic reticulum, reacts preferably with lecithins and protects them against oxidative damage caused by free radicals. The mechanistic processes of the reactions in which vitamin E is involved are very complex. In such reactions, vitamin E converts into a α-tocopheroxyl radical, which can later become vitamin E again with the participation of vitamin C 67. The most important sources of vitamin E 68 are corn oil, sunflower oil, soybean oil, peanuts, hazelnuts, sunflower seeds, egg yolks, avocados, nuts, beans and fish, including bonito and mackerel.
Conclusions
Free radicals are compounds produced in the human body by multiple enzymatic and non-enzymatic reactions. These substances are characterized by having high reactivity. For this reason, they react with proteins, lipids and nucleic acids very quickly and, therefore, they damage cell structures. These effects are related to several chronic non-communicable diseases.
To avoid the production and spread of free radicals, cells and tissues have an antioxidant system that provides a protective effect, whose efficiency depends on its components’ characteristics and location in tissues. Hence, there is a special interest in studying the mechanistic processes of the antioxidant system. The efficacy of the antioxidant defense can improve with the intake of antioxidant substances found in fruits and vegetables.
REFERENCES
1. Gomberg M. An instance of trivalent carbon: Triphenylmethyl. J Am Chem Soc. 1900;22(11):757-71. [ Links ]
2. Stadie WC, Haugaard N. Oxygen poisoning; the effect of high oxygen pressure upon enzymes; succinicdehydrogenase and cytochrome oxidase. J Biol Chem. 1945;161:153-73. [ Links ]
3. Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and X-radiation: A mechanism in common. Science. 1954;119(3097):623-6. [ Links ]
4. Michaelis L. Fundamentals of oxidation and respiration. Am Sci. 1946;34(4):573-596. [ Links ]
5. Harmand D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298-300. [ Links ]
6. Janciauskiene S. The Beneficial Effects of Antioxidants in Health and Diseases. Chronic Obstr Pulm Dis. 2020;7(3):182-202. [ Links ]
7. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55-74. [ Links ]
8. Gui-Fang D, Xi L, Xiang-Rong X, Li-Li G, Jie-Feng X, Hua-Bin L. Antioxidant capacities and total phenolic contents of 56 vegetables. J Funct Foods. 2013;5(1):260-6. [ Links ]
9. Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45(7-8):466-72. [ Links ]
10. Margaritelis NV, Kyparos A, Paschalis V, Theodorou AA, Panayiotou G, Zafeiridis A, et al. Reductive stress after exercise: the issue of redox individuality. Redox Biol. 2014;2:520-8. [ Links ]
11. Margaritelis NV, Theodorou AA, Paschalis V, Veskoukis AS, Dipla K, Zafeiridis A, et al. Experimental verification of regression to the mean in redox biology: differential responses to exercise. Free Radic Res. 2016;50(11):1237-44. [ Links ]
12. Yavari A, Javadi M, Mirmiran P, Bahadoran Z. Exercise-Induced Oxidative Stress and Dietary Antioxidants. Asian J Sports Med. 2015;6(1):e24898. [ Links ]
13. Hannou SA, Haslam DE, McKeown NM. Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018;128(2):54555. [ Links ]
14. Yan-Bo Z, Yan-Hai M, Chang S, Rong-Yuan Z, Shi C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am J Transl Res. 2016;8(11):4869-80. [ Links ]
15. Loza-Medrano SS, Baiza-Gutman LA, Ibanez-Hernandez MA, CruzLopez M, Diaz-Flores M. Alteraciones moleculares inducidas por fructosa y su impacto en las enfermedades metabolicas. Rev Med Inst Mex Seguro Soc. 2019;56(5):491-504. [ Links ]
16. DiNicolantonio JJ, Oï¿1/2Keefe JH, Lucan SC. Added Fructose: A Principal Driver of Type 2 Diabetes Mellitus and Its Consequences. Mayo Clin Proc. 2015;90(3):372-81. [ Links ]
17. Jaiswal N, Maurya CK, Arha D, Avisetti DR, Prathapan A, Raj PS et al. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis. 2015;20(7):930-47. [ Links ]
18. Drose S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145-69. [ Links ]
19. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):604955. [ Links ]
20. Lancaster JR. Nitric oxide: a brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci OA. 2015;1(1):FS059. [ Links ]
21. Ferradini C, Foos J, Houee C, Pucheault J. The reaction between Superoxide Anion and Hydrogen-Peroxide. Photochem Photobiol. 1978;28(4-5):697-700. [ Links ]
22. Zhou T, Prather ER, Garrison DE, Zuo L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int J Mol Sci. 2018;19(2):417. [ Links ]
23. Schroder K. NADPH oxidases: Current aspects and tools. Redox Biol. 2020;34:101512. [ Links ]
24. Cascales M. Bioquimica y Fisiopatologia del estres oxidativo. 1997. Madrid, Espana: Fundacion Jose Casares Gil. [ Links ]
25. Inoue H, Hirobe M. Disulfide cleavage and insulin denaturation by active oxygen in the copper(II)/ascorbic acid system. Chem Pharm Bull (Tokyo). 1986;34(3):1075-9. [ Links ]
26. Guija-Guerra H, Guija-Poma E, Ponce-Pardo J, Inocente-Camones M, Camarena-Chaviguri L. Generacion de radicales libres por efecto de vitamina C sobre un jarabe antianemico de sulfato ferroso. Horiz Med. 2018;18(4):35-41. [ Links ]
27. Guija-Poma E, Troncoso-Corzo L, Palomino-Paz F, Guija-Guerra H, Oliveira-Bardales G, Ponce-Pardo J, et al. Estudio histopatologico de los efectos de la administracion de hierro hemo y sulfato ferroso con vitamina C en cerebro e higado de rata. Horiz Med. 2019;19(2):12-8. [ Links ]
28. Calderon-Velez JC. La suplementacion con hierro y el aumento del estres oxidativo en el embarazo: una paradoja poco discutida. Rev Colomb Obstet Ginecol. 2007;58(4):304-8. [ Links ]
29. Nussbaum C, Klinke A, Adam M, Baldus S, Sperandio M. Myeloperoxidase: A leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid Redox Signal. 2013;18:692-713. [ Links ]
30. Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defense and redox signalling. Biochem J. 2010;425(2):313-25. [ Links ]
31. Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem. 2011;11(4):341-6. [ Links ]
32. Borchert A, Kalms J, Roth SR, Rademacher M, Schmidt A, Holzhutter H et al. Crystal structure and functional characterization of selenocysteine-containing glutathione peroxidase 4 suggests and alternative mechanism of peroxide reduction. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(9):1095-107. [ Links ]
33. Collet J, Messens J. Structure, Function, and mechanism of thioredoxin Proteins. Antioxid Redox Signal. 2010;13:1205-16. [ Links ]
34. Goyal MM, Basak A. Human catalase: looking for complete identity. Protein Cell. 2010:1(10):888-97. [ Links ]
35. Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27-42. [ Links ]
36. Murdoch CE, Bachschmid MM, Matsui R. Regulation of neovascularization by S-glutathionylation via the Wnt5a/sFlt-1 pathway. Biochem Soc Trans. 2014;42:1665-70. [ Links ]
37. Ren X, Zou L, Lu J, Holmgren A. Selenocystein in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Rad Biol Med. 2018;127:238-47. [ Links ]
38. Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:736837. [ Links ]
39. Flogel U, Fago A, Rassaf T. Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides. J Exp Biol. 2010;213(Pt16):2726-33. [ Links ]
40. Theil EC. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg Chem. 2013;52(21):12223-33. [ Links ]
41. Heger Z, Rodrigo MA, Krizkova S, Ruttkay-Nedecky B, Zalewska M, Del Pozo EM, et al. Metallothionein as a scavenger of free radicals new cardioprotective therapeutic agent or initiator of tumor chemoresistance? Curr Drug Targets. 2016;17(12):1438-51. [ Links ]
42. Genova ML, Lenaz G. New developments on the functions of coenzyme Q in mitochondria. Biofactors. 2011;37:330-54. [ Links ]
43. Fujisawa S, Kadoma Y. Kinetic evaluation of polyamines as radical scavengers. Anticancer Res. 2005;25(2A):965-9. [ Links ]
44. Miller E, Mrowicka M, Malinowska K, Mrowicki J, Saluk-Juszczak J, Kedziora J. Effects of whole-body cryotherapy on oxidative stress in multiple sclerosis patients. J Therm Biol. 2010;35(8):406-10. [ Links ]
45. Eid C, Hemadi M, Ha-Duong NT, El Hage J. Iron uptake and transfer from ceruloplasmin to transferrin. Biochim Biophys Acta. 2014;1840(6):1771-81. [ Links ]
46. Hineno A, Kaneko K, Yoshida K, Ikeda S. Ceruloplasmin protects against rotenone-induced oxidative stress and neurotoxicity. Neurochem Res. 2011;36(11):2127-35. [ Links ]
47. Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. 2020;11:582680. [ Links ]
48. Taverna M, Marie A, Mira J, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3(1):4. [ Links ]
49. Ziberna L, Martelanc M, Franko M, Passamonti S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci Rep. 2016;6:29240. [ Links ]
50. Burrow H, Kanwar RK, Mahidhara G, Kanwar JR. Effect of seleniumsaturated bovine lactoferrin (Se-bLF) on antioxidant enzyme activities in human gut epithelial cells under oxidative stress. Anticancer Agents Med Chem. 2011;11(8):762-71. [ Links ]
51. Langhans W. Food components in health promotion and disease prevention. J Agric Food Chem. 2018;66(10):2287-94. [ Links ]
52. Rasoulia H, Farzaeib MH, Khodarahmia R. Polyphenols and their benefits: A review. Int J Food Prop. 2017;20(Sup 2):S1700-41. [ Links ]
53. Juurlink BHJ, Azouz HJ, Aldalati AMZ, AlTinawi BMH, Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr J. 2014;13:63. [ Links ]
54. Alam MA. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front Nutr. 2019;6:121. [ Links ]
55. Khawand TE, Courtois A, Valls J, Richard T, Krisa S. A review of dietary stilbenes: sources and bioavailability. Phytochem Rev. 2018;17:100729. [ Links ]
56. Rodriguez-Garcia C, Sanchez-Quezada C, Toledo E, DelgadoRodriguez M, Gaforio JJ. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules. 2019;24(5):917. [ Links ]
57. Wu C, Xu H, Heritier J, Andlrauer W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012;132:144-9. [ Links ]
58. Khan MK, Zill-E-Huma, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compost Anal. 2014;33(1):85-104. [ Links ]
59. Ali F, Rahul, Naz F, Jyoti S, Siddique YH. Health functionality of apigenin: A review. Int J Food Prop. 2017;20(6):1197-238. [ Links ]
60. Maatta-Riihinen KR, Kahkonen MP, Torronen AR, Heinonen IM. Catechins and procyanidins in berries of Vaccinium species and their antioxidant activity. J Agric Food Chem. 2005;53:8485-91. [ Links ]
61. Speer H, Dï¿1/2Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health-A focus on oxidative stress, inflammation and disease. Antioxidants (Basel). 2020;9(5):366. [ Links ]
62. Salinas CM, Lopez-Sobaler AM. Beneficios de la soja en la salud femenina. Nutr Hosp. 2017;34(Sup 4):36-40. [ Links ]
63. Khalid M, Saeed-ur-Rahman, Bilal M, Iqbal HMN, Huang D. Biosynthesis and biomedical perspectives of carotenoids with special reference to human health-related applications. Biocatal Agric Biotechnol. 2019;17:399-407. [ Links ]
64. Njus D, Kelley PM, Tu Y, Schlegelb BH. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic Biol Med. 2020;159:37-43. [ Links ]
65. Dosedel M, Jirkovsky E, Macakova K, Kujovska L, Javorska L, Pourova J et al. Vitamin C-Sources, physiological role, kinetics, deficiency, use, toxicity and determination. Nutrients. 2021;13(2):615. [ Links ]
66. Traber MG, Atkinson J. Vitamin E, Antioxidant and Nothing More. Free Radic Biol Med. 2007;43(1):4-15. [ Links ]
67. Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278(5706):737-8. [ Links ]
68. Chun J, Lee J, Ye L, Exler J, Eitenmiller RR. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J Food Compost Anal. 2006;19(2-3):196-204. [ Links ]
Received: November 24, 2022; Revised: February 08, 2023; Accepted: February 24, 2023











 texto en
texto en