Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Horizonte Médico (Lima)
versão impressa ISSN 1727-558X
Horiz. Med. vol.23 no.3 Lima jul./set. 2023 Epub 13-Set-2023
http://dx.doi.org/10.24265/horizmed.2023.v23n3.09
History
History and basic principles of transcranial magnetic stimulation
1Instituto de Neuroestimulación de Lima (Lima Neurostimulation Institute). Lima, Peru.
2Universidad de San Martín de Porres, School of Human Medicine. Lima, Peru.
Transcranial magnetic stimulation (TMS) is a noninvasive technique that uses magnetic fields to stimulate neurons in the cerebral cortex.
While electricity has previously been intended to be used in the medical field, the history of TMS dates back to the discovery of electromagnetic induction by Faraday in the 19th century. However, it was not until the 1980s when Anthony Barker developed the first TMS device at the University of Sheffield.
TMS works by means of a coil placed against the scalp, thereby producing a magnetic field. This magnetic field can pass through the skull and stimulate cortical neurons. The intensity and frequency of the magnetic field can be adjusted to target specific areas of the brain and produce excitatory and inhibitory effects.
The principles of TMS are based on the concept of neuroplasticity, which refers to the brain’s ability to change and adapt in response to new experiences and stimuli. By stimulating neurons in the brain with TMS, it is possible to cause changes in neuronal activity and connectivity, which in turn can lead to cognitive and mood changes.
Keywords: Transcranial Magnetic Stimulation; Magnetic Fields; Cortical Excitability
Introduction
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technique that uses alternating magnetic fields to produce electric pulses capable of stimulating or inhibiting cortical activity. These stimuli can be recorded by electromyography and/or electroencephalography to evidence their physiological activity 1.
Though noninvasive brain stimulation is a relatively new medical procedure, the concept of using a magnetic field or a power supply to stimulate the brain with medical purposes has a long history. The oldest ways of electrical stimulation are very different from modern noninvasive brain stimulation 2. Electrical stimulation was first mentioned in medical practice in 46 AD, when Scribonius Largus, a physician at the court of Emperor Claudius, described that the electric eel (Electrophorus electricus) (Figure 1A) was used to relieve pain caused by gout 3.
In the 17th century, Englishman William Gilbert coined the term "electricity" aimed to explain the attractive property of amber stone. In 1743, German physician Johann Gottlob Krüger told his students, "All things must have a usefulness… Since electricity must have a usefulness, and we have seen that it cannot be looked for either in theology or in jurisprudence, there is obviously nothing left but medicine." 4
At the end of the 18th century, at the University of Bologna in Italy, Luigi Galvani described that electrical stimulation caused twitching of muscles from a frog’s leg. Later, Alessandro Volta demonstrated that this effect (referred to as "galvanic") did not require direct contact with the animal specimen 5,6.
This historical review is aimed to go over the TMS biophysical principles and to mention some proven clinical applications to date and others that are still under research. For this purpose, we conducted a literature search for publications in English from the beginning of the 20th century until present.
The science of transcranial magnetic stimulation
In 1820, Danish physicist Hans Christian Oersted empirically demonstrated the relationship between electricity and magnetism 7. Later, in 1831, Michael Faraday first described the relationship between a the magnetic field and electricity. According to Faraday, an electric current may be induced in a circuit by changing the magnetic field, e.g., brief current pulses in the primary circuit or moving it relative to the secondary one. Faraday could observe that this effect was caused by the magnetic flux created by an alternating circuit and that these changes in the magnetic flux induced an electric field 8. This is called "electromotive force," which is responsible for the current flow induced by an electromagnetic field. The magnitude of this effect may be mathematically quantified and expressed by the Faraday’s law formula, which states that "the magnitude of the electromotive force in a circuit is directly proportional to the rate of transient variation of magnetic flux passing through any surface at the rim of the circuit." 9
Some years later, Nikola Tesla researched the physiological changes produced by high-frequency current flows using big coils, which produced air ionization between them and were described by the patients between the coils as a "bombardment of miniature hail stones." 10
Jacques-Arsène d'Arsonval, in 1896, was the first to develop a device similar to a modern magnetic stimulator. It consisted of a very big coil that generated 110 volts at 42 Hz and 30 amperes, which was applied to human beings in different experiments. The physiological responses and sensations reported by d'Arsonval were diverse, including vasodilation, vertigo and the presence of phosphenes. Phosphenes (perception of flashes of light without stimuli) are produced by modern TMS devices when the occipital visual cortex is stimulated. It is possible that the area had been stimulated during d'Arsonval’s experiments but, based on the technological capability of that time, it seems more likely that they were the result of direct retinal stimulation 3.
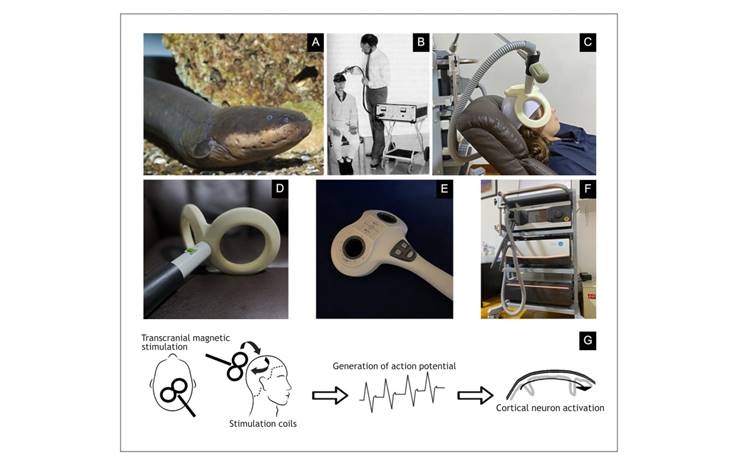
Figure 1 History and principles of TMS. (A) South American electric eel, an electric fish that can be up to 2 m long, which uses electricity in different ways: low voltages (Sach’s organ) as a sensorial organ for its environment and high voltages to detect a prey and stun it; it can also generate electricity from about 10 V at 25 Hz up to 480 V and several hundred Hz. (B) English physicist Anthony Barker with his first TMS device, with which he produced contractions of hand muscles by stimulating the motor cortex. (C) Transcranial magnetic stimulator with a double-cone coil placed on the right dorsolateral prefrontal cortex of a patient with treatment-resistant major depression, one of the indications approved by the Food and Drug Administration (FDA). (D) Neurosoft DCC-02-125 double-cone coil, 125 mm, with liquid cooling system. (E) Neurosoft FEC-03-100 figure-of-eight coil, 100 mm, with liquid cooling system. (F) Tower with Neurosoft Neuro-MSX neurostimulation biphasic device with liquid cooling system and double power supply capable of producing stimulation frequencies of up to 100 Hz and up to 2,000 Hz in theta waves. (G) TMS coil placed on the scalp covering specific cortical localizations where we want to induce a current moving from the posterior to anterior direction, perpendicular to descending pyramidal neurons and parallel to interneurons which modulate pyramidal cell activation.
Some years later, in 1902, a device designed and patented by Beer 11 in Vienna to treat depression and other neuroses was used by Thompson 12, thereby producing sight and taste sensations. Magnusson and Stevens, using two elliptic coils, produced visual flickering and luminous horizontal bars 13. The interest in this area decreased during the first half of the 20th century because of the use of other tools to study and stimulate the central nervous system as a potential treatment for psychiatric diseases.
A new attempt to directly stimulate the brain activity was carried out in the 1950s. Direct electrical stimulation through the scalp was applied in awake human subjects. However, its use was limited due to the high degree of discomfort of the procedure 14. Different types of lowintensity electrical stimulation were used during these
years, but with limited knowledge about the effects of these forms of stimulation on the brain tissue. At the beginning of the 1980s, it was demonstrated that a brief high-voltage electric pulse applied on the scalp could noninvasively stimulate the motor cortex. Nevertheless, this procedure was painful and therefore, it had little practical usefulness 15.
Modern magnetic stimulation
In 1985, Anthony Barker (Sheffield, England) (Figure 1B) developed a device that could generate a sufficiently powerful magnetic field to stimulate the cortical activity in the human brain through the scalp. This was the beginning of modern application of TMS 16. This stimulator drew the attention of neurophysiologists and neurologists since the device allowed studying nerve conduction from the pyramidal neurons in the cortex to the periphery. A very important breakthrough in TMS was the development of stimulators capable of emitting repetitive pulses at frequencies of 1 Hz or greater. Subsequent studies soon demonstrated that repetitive stimulation with such pulses could potentially modulate the excitability of the cortex, either increasing or reducing the cortical activity. As expected, this drew more interest because of the potential therapeutic use of these devices.
The first therapeutic application of these stimulators was for the modulation of mood in patients with depressive disorders. Studies among healthy subjects demonstrated that mood might be positively changed; also, preliminary clinical studies among patients with depression showed encouraging results in the mid-1990s 17. Since then, randomized clinical trials have researched the use of TMS in a variety of neuropsychiatric disorders, thereby underpinning the science used to develop research protocols.
Basic principles
TMS is based on Michael Faraday’s law of magnetic induction, which demonstrates that current can be induced in another circuit when it is brought near a circuit in which a time-varying current is flowing. This change in the initial electric current produces a magnetic field that induces a secondary current in the adjacent conducting material 18.
An electric charge of very high intensity (several thousand amperes) is stored in capacitors in the TMS device and discharged rapidly so that current flows through the stimulator coil and is switched on at high speed (100 microseconds). This process of charge and discharge of capacitors is controlled by a thyristor-type semiconductor that can manage high amounts of voltage as well as current. This varying electric field produces a significant magnetic field (of approximately 2 tesla) capable of inducing an electric field in the superficial layers of the cerebral cortex (Figure 1C). If this electric field is of sufficient strength, it can cause depolarization of pyramidal neurons; therefore, it directly or indirectly activates dendrites and interneurons 9.
This process occurs efficiently because there is no effective resistance to the passage of the magnetic field from the scalp to skull. Thus, TMS works as a way of inducing electric current in the brain without requiring direct stimulation to the scalp. However, the distance between the stimulation coil and brain tissue involves a rapid decrease in the strength of the magnetic field based on the distance 19.
The stimulator circuitry determines the type of pulse emitted by the TMS device. The biphasic type is sinusoidal, of shorter duration and can produce TMS at high frequencies for therapeutic use. The monophasic type has a rapid rise followed by a slow decay back to the baseline and has been typically used in research. Monophasic and biphasic pulses may have different effects on neuronal activity and cannot be used indiscriminately and interchangeably 20.
There is a variety of coils used for TMS (Figure 1D). Initially, circular coils were used, and the windings of the coils were concentrated in a band on the outside of a circle, usually between 7 and 10 cm in diameter. This type of coil had reduced stimulation focus as the peak stimulation area was under the ring. One of the most frequently used types of coils—both in research and as therapeutic tools—is the "figure-of-eight" coil" 21. It consists of two circular coils that are arranged in parallel (Figure 1E). This produces a better stimulation focus, where the central point of stimulation is located under the joint of both circular coils. Figure-of-eight coils produce an area of cortical activation of approximately 2-3 cm2 and a depth of about 2 cm. These coils are held over the cortex at 45 degrees from the midline and perpendicular to the central sulcus. This induces a current in the posterior to the anterior direction that is perpendicular to the descending pyramidal neurons and parallel to interneurons that regulate pyramidal cell activation 22. Figure-of-eight coils may be made with an air core (space between the windings of the coil) or with an iron core, and they have the advantage of requiring less power to produce more potent magnetic fields and generating less heat. Heat generation may be managed with cooling systems using air or liquid to dissipate the heat generated inside the coils (Figure 1F).
In addition, there are novel coils where figure-of-eight coils may be angled (double-cone coils) in order to stimulate deeper brain areas 23. As example we can mention H-coils, consisting of multiple coil windings to generate stimulation areas at a depth of up to 6 cm below the cerebral cortex 24. Manufacturers are still developing devices for different coils; hence, new options are very likely to be available in the next few years.
Repetitive transcranial magnetic stimulation
TMS devices were initially developed to evoke transient cortical activity through the emission of a single pulse. Nevertheless, technological development allowed generating repetitive pulses, usually between 1 and 20 Hz. Repetitive TMS (rTMS) has enabled noninvasive stimulation aimed to modulate the brain activity. Most of the devices could achieve higher frequencies of stimulation by using bipolar stimulus pulses which, in contrast to unipolar stimulating devices used at present, were shorter in duration and required less energy to produce neuronal excitability. Thus, capacitors can charge and discharge rapidly, which allows higher stimulation rates 25 (Figure 1G).
Repetitive stimulation may either activate or inhibit cortical activity, depending on the stimulation frequency. Lowfrequency (~1 Hz) stimulation for 15 minutes may induce a transient inhibition or reduce cortical activity 26. The mechanisms of such inhibition are still unclear, although they are similar to long-term depression (LTD), in which lowfrequency stimulation reduces synaptic activity in cellular experiments. In contrast, stimulation at frequencies over 1 Hz increases cortical activation, analogous to the process of long-term potentiation (LTP) 27. This may be due to a transient increase in the efficacy of excitatory synapses. The improvement of neuronal plasticity may also be a mechanism by which rTMS changes the brain activity. Cortical plasticity involves adaptative neo-synapse as a response to environmental inputs. Synaptic plasticity has been conceptualized as the cellular mechanism of learning and memory. According to Hebb, this plasticity is represented by changes in the synaptic strength in response to the coordinated activation of cells, which manifest as LTD and LTP 28. LTP depends, in part, upon the activation of double-gated N-methyl-D-aspartic acid (NMDA) receptor that operates as a molecular "coincidence" detector. These calcium-permeable glutamate receptors are able to contribute to the long-term augmentation of post-synaptic signal once activated by an input sufficient to depolarize the membrane and relieve magnesium (Mg2+)-dependent inhibition. rTMS may cause cortical neurons to generate a constant and repeated activation of coactive cells, thereby producing plasticity in the cortex 28,29.
Currently, TMS is approved by the FDA for the treatment of refractory depression and obsessive-compulsive disorder (30). Different stimulation paradigms are studied with encouraging results for the management of pain, tinnitus, movement disorders, post-stroke rehabilitation, schizophrenia, addictions, autism spectrum disorder, epilepsy, multiple sclerosis, Parkinson’s disease, Alzheimer’s disease and others 31,32.
Conclusions
We still lack knowledge about how these concepts underpin the effects of TMS when applied to cortical circuits, especially when used to treat pathologies such as depression. Its therapeutic effects may be related to local changes in cortical excitability resulting from changes in the capacity of the stimulated brain region (e.g., the dorsolateral prefrontal cortex) to regulate other disease-related brain regions, or changes in the strength of connections between important brain regions driven by repeated stimulation, or changes in the activity of distal brain regions altered through transsynaptic activation. Furthermore, as these mechanisms play a role, they may be specific for each disease or type of stimulation. Therefore, the rTMS mechanism may be different in diverse pathologies.
Despite all these still unanswered questions, rTMS has become a valuable tool to research the role of cortical areas in the brain function as well as a safe and effective therapeutic tool. Its use will continue to expand with the progress of clinical applications of rTMS and the development of new experimental methods allowing to combine it with others that assess the brain activity, such as functional magnetic imaging or electroencephalography in real time.
REFERENCES
1. Cambiaghi M, Sconocchia S. Scribonius Largus (probably before 1CEafter 48CE). J Neurol. 2018;265(10):2466-68. [ Links ]
2. Cao KX, Ma ML, Wang CZ, Iqbal J, Si JJ, Xue YX, et al. TMS-EEG: An emerging tool to study the neurophysiologic biomarkers of psychiatric disorders. Neuropharmacology. 2021;197:108574. [ Links ]
3. Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation treatment for depressive disorders. En: Repetitive transcranial magnetic stimulation treatment for depressive disorders. Springer; 2013. p. 1-123. [ Links ]
4. Janik EL, Jensen MB. Every man his own electric physician: T. Gale and the history of do-it-yourself neurology. J Neurol Res Ther. 2016;1(2):17-22. [ Links ]
5. Raghavan M, Fee D, Barkhaus PE. Generation and propagation of the action potential. Handb Clin Neurol. 2019;160:3-22. [ Links ]
6. Becker RO, Marino AA. The origins of electrobiology. En: Electromagnetism and life. United States of America: State University of New York Press; 1982. p. 3-22. [ Links ]
7. Caneva KL. Ampere, the etherians, and the oersted connexion. Br J Hist Sci. 1980;13(2):121-38. [ Links ]
8. Baigrie BS. Electricity and magnetism: a historical perspective. Greenwood Publishing Group; 2007. 194 p. [ Links ]
9. Lefaucheur JP. Transcranial magnetic stimulation. En: Levin KH, Chauvel P, editores. Handbook of Clinical Neurology. Elsevier; 2019. p. 559-80. (Clinical Neurophysiology: Basis and Technical Aspects; vol. 160). [ Links ]
10. Cheney M. Tesla, man out of time. Prentice Hall; 1981. 358 p. [ Links ]
11. Beer B. Uber das auftretten einer objectiven lichtempfindung in magnetischen felde. Wien klin Wochenschr. 1902;15:108-9. [ Links ]
12. Thompson SP. A physiological effect of an alternating magnetic field. Proc R Soc Lond B Biol Sci. 1997;82(557):396-8. [ Links ]
13. Magnusson CE, Stevens HC. Visual sensations caused by changes in the strength of a magnetic field. Am J Physiol. 1911;29(2):124-36. [ Links ]
14. Gualtierotti T, Paterson AS. Electrical stimulation of the unexposed cerebral cortex. J Physiol. 1954;125(2):278-91. [ Links ]
15. Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285(5762):227. [ Links ]
16. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-7. [ Links ]
17. George MS, Wassermann EM, Post RM. Transcranial magnetic stimulation: a neuropsychiatric tool for the 21st century. J Neuropsychiatry Clin Neurosci. 1996;8(4):373-82. [ Links ]
18. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008-39. [ Links ]
19. Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071-107. [ Links ]
20. Goetz SM, Luber B, Lisanby SH, Murphy DL, Kozyrkov IC, Grill WM, Peterchev AV. Enhancement of neuromodulation with novel pulse shapes generated by controllable pulse parameter transcranial magnetic stimulation. Brain Stimul. 2016;9(1):39-47. [ Links ]
21. Schecklmann M, Schmauï¿1/2er M, Klinger F, Kreuzer PM, Krenkel L, Langguth B. Resting motor threshold and magnetic field output of the figure-of-8 and the double-cone coil. Sci Rep. 2020;10(1):1644. [ Links ]
22. Amassian VE, Deletis V. Relationships between animal and human corticospinal responses. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:79-92. [ Links ]
23. Monteiro DC, Cantilino A. Use of a double-cone coil in transcranial magnetic stimulation for depression treatment. Neuromodulation. 2019;22(8):867-70. [ Links ]
24. Bersani FS, Minichino A, Enticott PG, Mazzarini L, Khan N, Antonacci G, et al. Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: A comprehensive review. Eur Psychiatry. 2013;28(1):30-9. [ Links ]
25. Chen AR, Fitzgerald PB, Blumberger and DM. TMS neurophysiology. En: A practical guide to transcranial magnetic stimulation neurophysiology and treatment studies. Oxford, New York: Oxford University Press; 2022. p. 8-10. [ Links ]
26. Valero-Cabre A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381-404. [ Links ]
27. Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin Neurophysiol. 2017;128(11):2318-29. [ Links ]
28. Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58(4):208-13. [ Links ]
29. Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. 2023;48(1):191-208. [ Links ]
30. Cohen SL, Bikson M, Badran BW, George MS. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimula. 2022;15(1):73-5. [ Links ]
31. Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131(2):474-528. [ Links ]
32. Burke MJ, Fried PJ, Pascual-Leone A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. Handb Clin Neurol. 2019;163:73-92. [ Links ]
Received: March 14, 2023; Revised: April 07, 2023; Accepted: May 04, 2023











 texto em
texto em 



