Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Horizonte Médico (Lima)
Print version ISSN 1727-558X
Horiz. Med. vol.23 no.4 Lima Oct./Dec. 2023 Epub Dec 18, 2023
http://dx.doi.org/10.24265/horizmed.2023.v23n4.04
ORIGINAL ARTICLE
Clinical and epidemiological characteristics and impact of non-pharmacological measures on the evolution of the COVID-19 pandemic among residents of a highaltitude region of Peru
1Universidad de San Martín de Porres, School of Human Medicine, Centro de Investigación de Medicina de Altura (High Altitude Medical Research Center). Lima, Peru.
2Hospital de Lircay II-1, Ministry of Health (MINSA). Huancavelica, Peru.
Objective:
To describe the clinical and epidemiological characteristics and the impact of non-pharmacological measures (NFMs) on the evolution of the COVID-19 pandemic among residents of a high-altitude region of Peru. Materials and methods: The study was conducted in the district of Lircay (3,278 m a.s.l.), province of Angaraes, department of Huancavelica, between March 31, 2020, and January 31, 2021 (lockdown period). The Information Technology Department at Hospital Lircay MINSA II-1 provided all COVID-19 patient records used during the aforementioned period of time. The clinical and epidemiological characteristics and the impact of NFMs were evaluated by determining the transmissibility of the virus using the effective reproduction number (Rt).
Results:
A total of 1,404 COVID-19 patients from the district of Lircay, who were treated at Hospital Lircay MINSA II-1, were studied. The median age was 33 years (IQR: 24.0-45.0); 10.7 % were older adults and 70 % reported no comorbidities at diagnosis. Out of all participants, 2.16 % had a moderate or severe condition, being more frequent in males (p < 0.001). Moreover, the significantly associated comorbidities were obesity (p < 0.001) and hypertension (p = 0.004). The average case fatality rate was 0.9 %; however, in moderate-severe cases, it reached 26.7 % (p < 0.001), and the elderly population was the most affected one. The lockdown, understood as an NFM, generated an Rt of 2.98 (95 % CI: 1.54-5.14) in July, and the end of the lockdown plus the economic recovery generated an Rt of 9.94 (95 % CI: 4.19-19.45) in December. After Christmas, focused lockdown was introduced and, 14 days later, an Rt reduction of up to 0.79 (95 % CI: 0.41-1.36) was observed.
Conclusions:
In this high-altitude region, the COVID-19 overall case fatality rate was low; this could be related to the characteristics of the population (young age and low prevalence of comorbidities). Lockdown apparently proved to be an effective measure for controlling SARS-CoV-2 transmissibility.
Keywords: Mortality; COVID-19; Public Health; Altitude; Peru
Introduction
SARS-CoV-2 was discovered as the etiological agent of the Wuhan pandemic 1-3. Faced with the spread of the virus, Peru opted for mandatory social distancing (border closures, mandatory social isolation and ban on the use of transportation nationwide), but the evolution was not as expected. By the 130th day of lockdown, the number of infected persons had risen to 375,961 and the case fatality rate (CFR) had increased to 4.75% 4,5.
In this context, a heterogeneous progression of the disease was observed within the different regions of Peru. For example, by 2020, in epidemiological week 22 (EW 22), Huancavelica had 245 cases and a CFR of 0.41 % 5, while other regions reported higher rates. Although there are publications reporting a reproduction rate (R0) of 2.97 for Peru and 2.88 for Lima 6, it is not clear how the number of infected persons has evolved during the pandemic in high-altitude areas.
Initially, in the absence of effective treatments or safe and effective immunization, non-pharmacological measures (NPMs) were chosen in Peru to "mitigate" the transmission of SARS-CoV-2 7-10. In a given context, traditional public health measures such as isolation, lockdown, social distancing and reduction of economic activities are considered as NPMs. However, in Peru, both the efficacy and efficiency of NPMs were affected by the non-compliance of the population 11, the low response capacity of health centers or the early recovery of economic activities.
The present research reports the clinical and epidemiological characteristics of COVID-19 patients in an area of Huancavelica, in the Peruvian high-altitude region, and details an approximation of the impact of NPMs on the transmission of SARS-CoV-2.
Materials and methods
Study design and population
An observational, descriptive and cross-sectional study conducted in the district of Lircay, at 3,278 meters above sea level (m a.s.l.), province of Angaraes, department of Huancavelica, between March 31, 2020, and January 31, 2021. This district covers 9.3 % of the population of Huancavelica (22,991 inhabitants), according to data from the Instituto Nacional de Estadística e Informática (INEI - National Institute of Statistics and Informatics) 12.
The population consisted of COVID-19 patients who were diagnosed with SARS-CoV-2 through a serology or antigen test at Hospital de Lircay II-1 12 of the Ministry of Health (MINSA) and registered in the Sistema Integrado para COVID-19 (SISCOVID-19 Integrated System for COVID-19) module of the district of Lircay 13. It should be noted that, in this district, there are only two health care facilities: Hospital de Lircay II-1, which covers most of the insured (88%, according to the INEI 12), and Centro de Atención Médica de Lircay (Lircay Health Care Center) of the Seguro Social de Salud (EsSalud - Social Security Health Insurance), whose number of insured population is considerably smaller. The study used a non-probabilistic and consecutive sampling, as all COVID-19 cases diagnosed in that hospital were included.
A diagnosis was considered positive if the result of the serology test detected antibodies:
Serology test: "reactive IgM," "reactive IgG" or "both reactive."
Antigen test: a result reported as "reactive" was considered positive.
Cases diagnosed by reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 were not included, because the district of Lircay did not have the necessary logistics to offer this type of test.
Variables and measurements
The evaluating medical staff collected information on the characteristics and clinical classification of the patients; the rest of the variables were included in the COVID-19 Epidemiological Record. Such information was uploaded into the SISCOVID-19 module, as provided in Ministerial Decree No. 183-2020-MINSA 14:
Mild cases: patients with general symptoms.
Moderate cases: patients with dyspnea or respiratory distress, respiratory rate (RR) ≥ 22 breaths per minute (bpm), altered level of consciousness, arterial hypotension or shock, clinical or radiological signs of pneumonia, and leukocyte count < 1,000 cells/µL.
Severe cases: patients with RR ≥ 22 bpm or partial pressure of carbon dioxide (PaCO₂) < 32 mmHg, altered level of consciousness, systolic blood pressure < 100 mmHg or mean blood pressure < 65 mmHg, clinical signs of muscle fatigue (nasal flaring, use of accessory muscles, thoracoabdominal imbalance) and serum lactate > 2 mmol/L.
Asymptomatic cases: patients with no symptoms at diagnosis.
The other clinical and epidemiological variables considered for this study were sex, age, district of origin, date of symptom onset, symptoms, risk factors (cardiovascular disease, diabetes, liver disease, obesity, tuberculosis, renal disease, liver disease, asthma, cancer), diagnostic test and outcome.
Likewise, fatality was determined with the help of the CFR and the calculation of proportions; the transmissibility of the virus was determined by calculating the effective reproduction number (Rt) in each lockdown period, thanks to the methodology described by Cori et al 15.
The data of interest for this study were collected from the SISCOVID-19 module located in the Information Technology Department at MINSA’s Hospital de Lircay II-1, for which an ad hoc card was used.
Statistical analysis
The quantitative variables were described by median and interquartile range (IQR) and the qualitative variables by absolute and relative frequencies. The CFR was estimated as the proportion of patients who died from COVID-19 during the observation period out of all those with a diagnosis of SARS-CoV-2 infection. Deaths from COVID-19 were confirmed with the Bureau of Statistics, medical history and death certificate. To compare the study variables between the asymptomatic, mild and moderate-severe cases, the statistical significance was estimated using the Kruskal-Wallis test for the quantitative and nonparametric variables, while the categorical variables were contrasted with the chi-square test. A p value less than 0.05 (p < 0.05) was considered statistically significant.
The transmissibility of the virus was evaluated throughout the study (with versus without lockdown) by estimating the Rt with the methodology described by Cori et al. 15 using a Microsoft Excel spreadsheet, published by these authors at the following link: http://tools.epidemiology. net/EpiEstim.xls.
To estimate the Rt, the research indicated a mean serial interval (SI) of 7.5 days and a standard deviation (SD) of 3.4 days, which were constant between periods and were reported in the first cases in Wuhan 10. In addition, the median Rt and its respective 95 % confidence interval were determined every 7 days. For this analysis, confirmed COVID-19 cases, who were diagnosed at Hospital de Lircay II-1 and reported the date of symptom onset, were included.
The observation period was divided into the following stages:
National lockdown: mandatory social immobilization and border closure were established throughout the territory. For this study, the starting date was March 31—when the first COVID-19 case was diagnosed in the department of Huancavelica—and the end date was June 30.
Economic recovery: period in which the Peruvian Government ended the national lockdown and the country’s main economic activities resumed (July 1 to 31).
Focused lockdown: period in which mandatory social immobilization was established only in some regions of the country due to the increase in COVID-19 cases (August 1 to September 17).
Lifting of the focused lockdown: from this period to the end of the study, Lircay did not return to lockdown (September 18 to November 31).
Start of year-end holidays shopping (December 1 to 23).
Year-end holidays (December 24 to 31).
Post "2020 year-end" holidays period (January 1 to 31).
Because the initial reports had indicated a mean SI of 7.5 days 10, it was assumed that a significant effect on the Rt curve would occur from day 14 (two weeks) after each stage was established. Thus, a drop or increase in the Rt, two weeks after the beginning of these study stages, would indicate a positive or negative influence of the evolution of the pandemic in Lircay.
Ethical considerations
The research was evaluated and approved by Hospital de Lircay II-1’s Management, through MEMORANDUM No. 00423A-2020/GOB.REG.HVCA/RSA-HL/D, on November 30, 2020, and was registered in the online platform of Proyectos de Investigación en Salud (PRISA - Health Research Projects) of the Instituto Nacional de Salud del Perú (INS - National Health Institute of Peru), with code EI00001712. Even though the study did not require the approval of an ethics committee, since only statistical and epidemiological data and not medical histories were analyzed, all the pertinent confidentiality measures were adopted. Likewise, the preparation of the report of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Results
Out of 1,454 records of COVID-19 patients diagnosed at Hospital de Lircay II-1, 50 were removed. Out of these, 48 were deleted because they had two positive tests on different dates and two were eliminated because they died at home with a diagnosis of COVID-19 post-mortem and without previous clinical care; therefore, 1,404 records were left (n = 1404). On the other hand, only 129 records included symptoms and date of symptom onset.
Table 1 presents the epidemiological and clinical characteristics of the study subjects, whose profile consisted of a median age of 33.0 (IQR: 24.0-45.0); age range of 30 to 59 years (49 %, n = 688); female predominance (59 %, n = 828); no comorbidity (73.2 %, n = 1,028); reactive IgM and IgG diagnostic tests (53.3 %, n = 749); headache, general malaise and cough as main symptoms; clinical classification with predominance of asymptomatic patients (n = 1,091), followed by mild (n = 276) and moderate-severe (n = 37) cases.
Table 1 Clinical and epidemiological characteristics of patients diagnosed with COVID-19 at Hospital de Lircay II-1, Angaraes, Huancavelica, Peru (March 31, 2020-January 31, 2021)
| Clinical classification | |||||
|---|---|---|---|---|---|
| All patients N = 1,404 | Asymptomatic N = 1,091 | Mild N = 276 | Moderate-severe N = 37 | p value | |
| Age | 33.0 (24.0-45.0) | 32.0 (24.0-43.0) | 35.0 (24.0-50.0) | 60.0 (44.0-71.00) | < 0.001* |
| 0-11 years | 58 (4.1) | 43 (3.9) | 13 (4.7) | 2 (5.4) | < 0.001 |
| 12-17 years | 39 (2.8) | 27 (2.5) | 12 (4.3) | 0 (0.0) | |
| 18-29 years | 469 (33.4) | 386 (35.4) | 81 (29.3) | 2 (5.4) | |
| 30-59 years | 688 (49.0) | 549 (50.3) | 125 (45.3) | 14 (37.8) | |
| ≥ 60 years | 150 (10.7) | 86 (7.9) | 45 (16.3) | 19 (51.4) | |
| Sex | |||||
| Male | 576 (41.0) | 418 (38.3) | 133 (48.2) | 25 (67.6) | < 0.001 |
| Female | 828 (59.0) | 673 (61.7) | 143 (51.8) | 12 (32.4) | |
| District of origin | |||||
| Lircay | 1,364 (97.2) | 1,058 (97.0) | 272 (98.6) | 34 (91.9) | 0.056 |
| Others | 40 (2.8) | 33 (3.0) | 4 (1.4) | 3 (8.1) | |
| Comorbidities | |||||
| No comorbidity | 1,028 (73.2) | 818 (75.0) | 196 (71.0) | 14 (37.8) | < 0.001 |
| COPD | 3 (0.2) | 2 (0.2) | 1 (0.4) | 0 (0.0) | 0.814 |
| HBP | 11 (0.8) | 4 (0.4) | 6 (2.2) | 1 (2.7) | 0.004 |
| Obesity | 11 (0.8) | 4 (0.4) | 4 (1.4) | 3 (8.1) | < 0.001 |
| T2DM | 6 (0.4) | 4 (0.4) | 2 (0.7) | 0 (0.0) | 0.661 |
| Asthma | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0.866 |
| Diagnostic test a | |||||
| Reactive IgM | 185 (13.2) | 141 (12.9) | 35 (12.7) | 9 (24.3) | < 0.001 |
| Reactive IgM and IgG | 749 (53.3) | 561 (51.4) | 168 (60.9) | 20 (54.1) | |
| Reactive IgG | 423 (30.1) | 383 (35.1) | 35 (12.7) | 5 (13.5) | |
| Reactive antigen test | 47 (3.3) | 6 (0.5) | 38 (13.8) | 3 (8.1) | |
| Symptoms b | |||||
| General malaise | 69 (53.5) | --- | 50 (72.5) | 19 (27.5) | N/A |
| Headache | 66 (51.2) | --- | 56 (84.9) | 10 (15.1) | N/A |
| Cough | 65 (50.4) | --- | 50 (76.9) | 15 (23.1) | N/A |
| Fever or chills | 59 (45.7) | --- | 42 (71.2) | 17 (28.8) | N/A |
| Odynophagia | 57 (44.2) | --- | 46 (80.7) | 11 (19.3) | N/A |
| Dyspnea | 39 (30.2) | --- | 15 (38.5) | 24 (61.5) | N/A |
| Chest pain | 26 (20.2) | --- | 18 (69.2) | 8 (30.7) | N/A |
| Diarrhea | 19 (14.7) | --- | 14 (73.7) | 5 (26.3) | N/A |
| Rhinorrhea | 14 (10.9) | --- | 10 (71.4) | 4 (28.6) | N/A |
| Nausea or vomiting | 10 (7.8) | --- | 8 (80.0) | 2 (20.0) | N/A |
| Muscle pain | 9 (7.0) | --- | 9 (100.0) | 0 (0.0) | N/A |
| Arthralgias | 8 (6.2) | --- | 8 (100.0) | 0 (0.0) | N/A |
| Abdominal pain | 5 (3.9) | --- | 5 (100.0) | 0 (0.0) | N/A |
| CFR (%) | 13 (0.9) | 1 (0.1) | 3 (1.1) | 9 (24.3) | < 0.001* |
a Clinical classification according to Ministerial Decree No. 183-2020-MINSA (14); laboratory and radiological data were not available at diagnosis.
b Only 129 records that included symptoms and date of symptom onset were considered. The percentage for the "all patients" column considered N = 129, and the percentage for the "mild" and "moderate-severe" clinical classification columns considered the total reported in the "all patients" column.
The continuous quantitative data are shown as median (IQR) and the qualitative variables as numbers (percentages). To compare the variables between the asymptomatic, mild and moderate-severe patients, chi-square tests were used for the qualitative variables.
*The Kruskal-Wallis test was used to determine the statistical significance since it did not present a Gaussian distribution.
N/A: not applicable.
Table 1 and Figure 1 show an average CFR of 0.9 % (n = 13), a higher percentage of moderate-severe clinical cases (24.3 %, n = 9) and a highly affected elderly population.
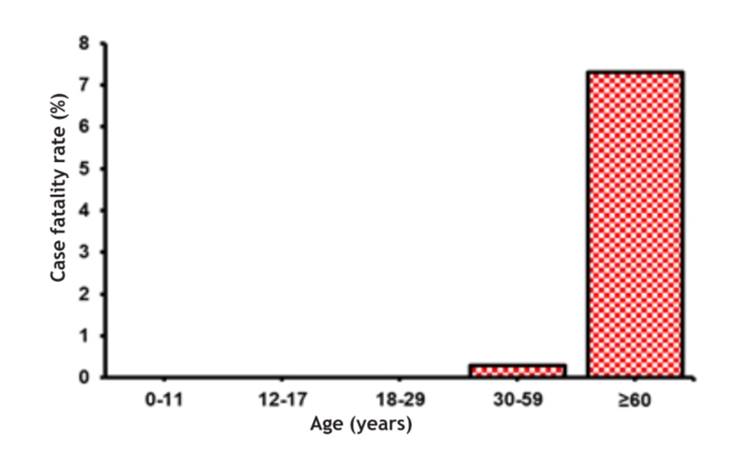
Figure 1 COVID-19 CFR according to the age of diagnosed cases, Hospital de Lircay II-1, Angaraes, Huancavelica, Peru (March 31, 2020- January 31, 2021)
The transmissibility of the virus throughout the study was determined by the Rt (Figure 2). It revealed an estimated Rt of 0.88 as of July 12 (95 % CI: 0.26-2.10). In contrast, the beginning of the progressive economic recovery, around July 1, caused an increase in the transmissibility of the virus, with the Rt increasing up to 2.98 (95 % CI: 1.54-5.14) on July 17 and up to 4.72 (95 % CI: 2.93-7.12) on August 1. On the contrary, focused lockdown resulted in a decrease in the Rt to 0.79 (95 % CI: 0.41-1.36), two weeks after the implementation of said measure. Likewise, during this period, the transmissibility rate remained below 1. However, once again, the lifting of the lockdown and some restrictions caused an increase in the fluctuating Rt.
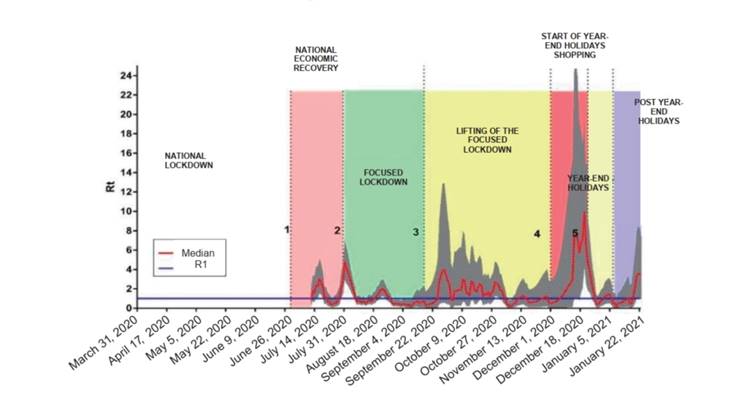
Figure 2 Rt for the COVID-19 pandemic according to diagnosed cases, Hospital de Lircay II-1, Angaraes, Huancavelica, Peru (March 31, 2020-January 22, 2021). The shaded area represents the 95 % confidence interval for the median. The numbers on the dotted lines represent the following: 1: June 30, 2020; 2: July 31, 2020; 3: September 18, 2020; 4: December 1, 2020; and 5: December 23, 2020.
Discussion
The COVID-19 population of Lircay, at 3,278 m a.s.l., had epidemiological characteristics similar to those reported in other studies; however, they also showed important clinical differences. Early publications from China indicated that the infection was common in adult and elderly patients, with ages ranging from 30 to 79 1 years and median ages of 49 16 and 47 17 years. In the present study, 82.4 % of the COVID-19 patients aged between 18 and 59 years with a median age of 33 years. A study 18 conducted in Huaraz, Peru, at 3,052 m a.s.l. found that COVID-19 patients had a median age of 41 years, while in another report 19 from Huancayo, Peru, at 3,250 m a.s.l., the mean age was 49 years. The difference between these findings was probably due to the demographic distribution of the population in the high Andean region.
In the present study, COVID-19 in symptomatic patients was characterized by general malaise, headache and cough. Early reports in China indicated that cough was a frequent symptom in patients with SARS-CoV-2 infection 16,17; however, its frequency was slightly exceeded by general malaise (53.5 %) and headache (51.2 %) 16,17. Other publications indicate that, in high Andean areas of the country, 49.5 % 18 to 70.26 % 19 of the symptomatic patients had cough. The discrepancy with other national publications could be due to the fact that, in this study, 77 % of the cases were classified as asymptomatic, which could have caused a bias by missing data in MINSA’s statistical computer system.
The average CFR of this study was 0.9 %, which is lower than that reported for the first COVID-19 cases in China. Thus, since the outbreak in Wuham, CFRs were low and in the range of 1.4 % 17 to 2.3 % 16. In Peru, Díaz-Lazo et al. 19 reported a rate of 14.21 in the high altitude region of Huancayo, Peru; meanwhile, Flores et al. 20 found a CFR of 32 per 100,000 inhabitants in the department of Huancavelica between March and September 2020.
On the other hand, Ortiz-Prado et al. 21 claimed that, in Ecuador, in regions below 2,500 m a.s.l., mortality rates were 24 % higher than at higher altitudes. In contrast, the CFR in hospitalized patients was 18.27 % in Santiago, Chile 22; 12.7 % in Fortaleza, Brazil 23; and only 5 % in Buenos Aires, Argentina 24.
The CFR of the present study was considerably increased in the most severe cases (CFR of 24.3 % for moderate- severe cases). Previous reports in hospitalized Peruvian patients from sea level regions show slightly higher CFRs, from 29.4 % 25 to 32.8 % 26. Apparently, the lethality of the virus is lower in this high Andean region, but it should be considered that not all the inhabitants were treated at Hospital de Lircay II-1.
Although a lower COVID-19 CFR at a higher altitude could be plausible, such results—and especially those of this study—could be conditioned by factors such as the characteristics of the population (lower age and low prevalence of comorbidities), demographic factors (low population density) and lesser or greater social mobility or other measures (greater or lesser use of masks, use of non-conventional medicines). In addition, part of the study population was treated in other conventional health systems (EsSalud and private health centers) and possibly in non-conventional systems.
An asymptomatic patient was one whose diagnosis was confirmed by a COVID-19 test, being RT-PCR the most commonly employed 27-29. A report of absence of symptoms during a follow-up period (5 days before to 14 days after testing) was prepared 27, which was enough to differentiate asymptomatic from presymptomatic cases. In this research, it was found that 77.7 % of COVID-19 cases in Lircay reported no symptoms at diagnosis. According to some studies, the percentage of asymptomatic infections accounted for 17 % 27 to 32% 30; however, research conducted in Cuba reported up to 80 % 31 while in Peruvian sea-level regions 25.7 % 32. The high percentage of asymptomatic patients reported in the present study is probably due to the fact that, at the time of the research, an "asymptomatic case" was confirmed, in some cases, by a molecular test and, in others, by a positive COVID-19 antigen test or IgM or IgM/IgG reactive serology test 33,34.
The present research has used the Rt estimation as well as previous research carried out in China 15,10 to evaluate the evolution of the pandemic in Lircay. It was found that the early national economic recovery and the lifting of the national lockdown, around the beginning of July 2020, and the start of the year-end holidays shopping (December 2020) increased SARS-CoV-2 transmissibility. The increased number of cases after the economic recovery, promoted by the Peruvian Government, did not consider the heterogeneous spread of the virus. By the beginning of July 2020, at the national level, the "wave" of infections seemed to be decreasing, which would have motivated the lifting of the most severe restrictions.
The results of this study show that, during the pre-economic recovery phase, diagnosed cases were low and did not reach a peak in diagnosis rate. Thus, a low number of infections would lead to a high number of susceptible people who, in addition to the lifting of the national lockdown, would have led to an increase in the transmissibility of the virus.
Focused lockdown was established as a pandemic control measure, which was evidenced by a decreased Rt. The progressive lifting of mandatory social isolation, associated with social distancing measures and reduced capacity of public places, kept the Rt below 1 during November.
Paradoxically, after the increase in transmissibility at the beginning of December, the Rt decreased at the beginning of the holidays around December 24.
A similar phenomenon was observed in the study by Pan et al. 10: during the first period of the outbreak in Wuhan (January 1 to 10, 2020), due to the Chinese Lunar New Year mass travel, the Rt dropped even though there was no restriction of any kind. In addition, the total number of cases reported in each province in China was associated with the total number of travelers leaving Wuhan 35-37. Thus, the decreased transmissibility in Lircay at the end of December could possibly have been caused by the migration of people from the district to other regions to celebrate the year-end holidays, with the resulting theoretical export of COVID-19 cases to the destination sites.
One of the main limitations of the study was the use of the SISCOVID-19 database as a source of information. Although it is a great online resource that helped to integrate data at the national level, the epidemiological records were incomplete and most of the information was obtained from such database. Likewise, those files only included the results of serology or antigen tests, since the results of molecular tests (RTPCR) were recorded on another online platform and the number of such tests was very small.
Another limitation was the lack of certainty about the characteristics of other non-pharmacological interventions, such as the use of barriers like masks and face masks, or non-conventional interventions specific to the regions in the Peruvian inland. Extrapolation to the total population of Lircay is impossible due to the certainty that the supply of care was also covered by EsSalud and, probably, by private health centers.
Finally, although the number of comorbidities in the study was low, there is no certainty about the presence or absence of communicable and non-communicable conditions specific to the region, or pathologies related to high altitude, such as chronic mountain sickness, or other cardiovascular conditions that are more frequent at higher altitude levels.
REFERENCES
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [ Links ]
2. Tan W, Zhao X, Ma X, Wang W, Niu P, Xu W, et al. A Novel Coronavirus Genome Identified in a Cluster of Pneumonia Cases Wuhan, China 2019-2020. CCDCW. 2020;2(4):61-2. [ Links ]
3. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it [Internet]. Geneva: WHO. [citado el 21 de mayo de 2023]. Disponible en: https://www.who.int/es/emergencies/diseases/novel-coronavirus-2019/technicalguidance/naming-the-coronavirus-disease-(covid-2019)-and-thevirus-that-causes-it [ Links ]
4. Johns Hopkins University & Medicine. COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center. 2021 [citado el 21 de mayo de 2023]. Disponible en: https://coronavirus.jhu.edu/map.html [ Links ]
5. Instituto Nacional de Salud y Centro Nacional de Epidemiologia, Prevencion y Control de Enfermedades del Ministerio de Salud. COVID-19 en el Peru [Internet]. Lima: MINSA. 2021 [citado el 21 de mayo de 2023]. Disponible en: https://covid19.minsa.gob.pe/sala_situacional.asp [ Links ]
6. Torres-Roman JS, Kobiak IC, Valcarcel B, Diaz-Velez C, La Vecchia C. The reproductive number R0 of COVID-19 in Peru: An opportunity for effective changes. Travel Med Infect Dis. 2020;37:101689. [ Links ]
7. Hartley DM, Perencevich EN. Public Health Interventions for COVID-19: Emerging Evidence and Implications for an Evolving Public Health Crisis. JAMA. 2020;323(19):1908-9. [ Links ]
8. Yang R, Gui X, Xiong Y. Comparison of Clinical Characteristics of Patients with Asymptomatic vs Symptomatic Coronavirus Disease 2019 in Wuhan, China. JAMA Netw Open. 2020;3(5):e2010182. [ Links ]
9. Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH, et al. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern Med. 2020;180(9):1156-63. [ Links ]
10. Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of Public Health Interventions with the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA. 2020;323(19):1915-23. [ Links ]
11. Zapata R, Santos G, Estrada M, Tovar A, Atamain Y, Chacon K, et al. La dura travesia de los mas pobres: pandemia y desempleo expulsan a miles de migrantes [Internet]. Ojo Publico. 2020 [citado el 21 de mayo de 2023]. Disponible en: https://ojo-publico.com/1786/desplazados-por-la-pandemia-la-travesia-los-mas-pobres [ Links ]
12. Instituto Nacional de Estadistica e Informatica. Resultados Definitivos de los Censos Nacionales 2017 [Internet]. Lima: INEI. 2017 [citado el 21 de mayo de 2023]. Disponible en: https://censo2017.inei.gob.pe/resultados-definitivos-de-los-censos-nacionales-2017/ [ Links ]
13. Ministerio de Salud. Resolucion Ministerial N.ï¿1/2 546-2011-MINSA [Internet]. Lima: MINSA; 2011 [citado el 21 de mayo de 2023] p. 148. Disponible en: https://www.gob.pe/institucion/minsa/normaslegales/243402-546-2011-minsa [ Links ]
14. Ministerio de Salud. Resolucion Ministerial N.ï¿1/2 183-2020-MINSA [Internet]. Lima: MINSA; 2020 [citado el 21 de mayo de 2023] p. 24. Disponible en: https://www.gob.pe/institucion/minsa/normaslegales/473230-183-2020-minsa [ Links ]
15. Cori A, Ferguson NM, Fraser C, Cauchemez S. A New Framework and Software to Estimate Time-Varying Reproduction Numbers During Epidemics. Am J Epidemiol. 2013;178(9):1505-12. [ Links ]
16. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [ Links ]
17. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):170820. [ Links ]
18. Vera-Ponce VJ, Mendez-Aguilar P, Ichiro-Peralta C, Failoc-Rojas VE, Valladares-Garrido MJ. Factores asociados a seropositividad para SARS-CoV-2 en pacientes atendidos en un hospital de zona altoandina peruana. Rev Cuerpo Med HNAAA. 2021;14:8-12. [ Links ]
19. Diaz-Lazo A, Montalvo R, Lazarte E, Aquino E. Caracterizacion clinica y epidemiologica de los pacientes con COVID-19 en un hospital situado en la altura. Horiz Med. 2021;21(2). [ Links ]
20. Flores MG, Soto A, De la cruz JA. Distribucion regional de mortalidad por COVID-19 en Peru. Rev Fac Med Hum. 2021;21(2):326-34. [ Links ]
21. Ortiz-Prado E, Fernandez RP, Vasconez E, Simbana-Rivera K, CorreaSancho T, Lister A, et al. Analysis of Excess Mortality Data at Different Altitudes During the COVID-19 Outbreak in Ecuador. High Alt Med Biol. 2021;22(4):406-16. [ Links ]
22. Araujo M, Ossandon P, Abarca AM, Menjiba AM, Munoz AM. Pronostico de pacientes hospitalizados por COVID-19 en un centro terciario en Chile: estudio de cohorte. Medwave. 2020;20(10): e8066. [ Links ]
23. Sanhueza-Sanzana C, Oliveira IW, Freitas RL, Kendall C, Mendes A, Sansigolo LRF. Social inequalities associated with COVID-19 case fatality rate in Fortaleza, Ceara state, Brazil, 2020. Epidemiol Serv Saude (Online). 2021;30(3):e2020743. [ Links ]
24. Castro HM, Canale HL, Ferreyro BL, Prieto MA, Massimino BE, Funtowicz G, et al. Caracteristicas clinicas de la enfermedad por Coronavirus 2019 en un centro de Argentina. Cohorte retrospective. Medicina (Buenos Aires). 2020;80(Supl. VI):35-43. [ Links ]
25. Acosta G, Escobar G, Bernaola G, Alfaro J, Taype W, Marcos C, et al. Caracterizacion de pacientes con COVID-19 grave atendidos en un hospital de referencia nacional del Peru. Rev Peru Med Exp Salud Publica. 2020;37(2):253-8. [ Links ]
26. Yupari-Azabache I, Bardales-Aguirre L, Rodriguez-Azabache J, Barros-Sevillano JS, Rodriguez-Diaz A. Factores de riesgo de mortalidad por COVID-19 en pacientes hospitalizados: Un modelo de regresion logistica. Rev Fac Med Hum. 2021;21(1):19-27. [ Links ]
27. Byambasuren O, Cardona M, Bell K, Clark J, McLaws ML, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Can. 2020;5(4):223-34. [ Links ]
28. White EM, Santostefano CM, Feifer RA, Kosar CM, Blackman C, Gravenstein S, et al. Asymptomatic and Presymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection Rates in a Multistate Sample of Skilled Nursing Facilities. JAMA Intern Med. 2020;180(12):1709-11. [ Links ]
29. Lee S, Kim T, Lee E, Lee C, Kim H, Rhee H, et al. Clinical Course and Molecular Viral Shedding Among Asymptomatic and Symptomatic Patients With SARS-CoV-2 Infection in a Community Treatment Center in the Republic of Korea. JAMA Intern Med. 2020;180(11):1447-52. [ Links ]
30. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782-93. [ Links ]
31. Noriega V, Pria MC, Corral A, Alvarez M, Bonet M. La infeccion asintomatica por el SARS-CoV-2: evidencias para un estudio poblacional en Cuba. Rev Cub Salud Publica. 2021;46(Supl Especial):1-16. [ Links ]
32. Diaz-Velez C, Failoc-Rojas VE, Valladares-Garrido MJ, Colchado J, Carrera-Acosta L, Becerra M, et al. SARS-CoV-2 seroprevalence study in Lambayeque, Peru. June-July 2020. PeerJ. 2021;9:e11210. [ Links ]
33. Ministerio de Salud. Resolucion Ministerial N.ï¿1/2 905-2020-MINSA[Internet]. Lima: MINSA; 2020 [citado el 21 de mayo de 2023] p. 29. Disponible en: https://www.gob.pe/institucion/minsa/normas-legales/1322786905-2020-minsa [ Links ]
34. Ministerio de Salud. Resolucion Ministerial N.ï¿1/2 972-2020-MINSA [Internet]. Lima: MINSA; 2020 [citado el 21 de mayo de 2023] p. 31. Disponible en: https://www.gob.pe/institucion/minsa/normaslegales/1366422-972-2020-minsa [ Links ]
35. Kraemer MUG, Yang CH, Gutierrez B, Wu CH, Klein B, Pigott DM, et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493-7. [ Links ]
36. Tian H, Liu Y, Li Y, Wu CH, Chen B, Kraemer MUG, et al. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638-42. [ Links ]
37. Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395-400. [ Links ]
Received: January 06, 2023; Revised: March 16, 2023; Accepted: May 15, 2023











 text in
text in 



