Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Horizonte Médico (Lima)
versão impressa ISSN 1727-558X
Horiz. Med. vol.23 no.4 Lima out./dic. 2023 Epub 18-Dez-2023
http://dx.doi.org/10.24265/horizmed.2023.v23n4.11
Articles
Hypoxia-inducible factor in cancer
1Clínica Internacional San Borja. Lima, Peru.
2Universidad Peruana de Ciencias Aplicadas. Lima, Peru.
Oxygen and carbon dioxide are essential for breathing; variations in these gases outside of the normal range are a threat to cell survival. Hypoxia is a common condition that occurs in most malignant tumors, increases angiogenesis and defective vascularization, promotes cell proliferation and acquires an epithelial-mesenchymal transition phenotype, which causes metastasis. It also affects cancer cell metabolism and makes patients resistant to treatment by causing cell quiescence. As a result, hypoxia is a detrimental component that is linked to unfavorable outcomes in most cancer treatments. Through the activation of more than a hundred genes that control cell activity, which produce key functions for cancer development, the transcription factor known as hypoxia-inducible factor (HIF) is linked to hypoxia in cancer. This review’s main goals are to highlight the role of hypoxia in the development of cancer, identify the key molecules that interact to promote HIF expression, explain the molecular mechanisms of the pathways that lead to HIF induction, describe the cellular effects of HIF alteration, and discuss potential HIF-targeted therapies. Articles from 1990 to 2022 were reviewed in PubMed, Scopus and SciELO databases. Keywords related to cancer and HIF were searched in bibliographical references. In conclusion, HIF-1α overexpression in tumor biopsies is associated with increased patient mortality in human cancers. Potential HIF-1α-regulated target genes that may play a role in tumor progression are starting to be identified. Although hundreds of chemicals have been studied in relation to HIF in cancer, there are currently few approved HIF inhibitors available on the global market; moreover, many clinical trials, in their various stages of development, do not show encouraging results. It is likely that in the future, when there is a better understanding of the structure, molecular and biological functioning of this factor, more specific drugs for HIF inhibition will be developed.
Keywords: Hypoxia-Inducible Factor-Proline Dioxygenases; Von Hippel-Lindau Tumor Suppressor Protein; Hypoxia-Inducible Factor 1; Neoplasms
Introduction
Molecular oxygen and carbon dioxide are the main gaseous substrates in breathing organisms; variations of these gases outside the physiological range present a serious threat to cell, tissue and organism survival 1. Oxygen (O₂) is the final electron acceptor produced during food metabolism and in the electron transference process within mitochondria, which through oxidative phosphorylation generates adenosine triphosphate, the biochemical energy necessary to comply with all the cell functions 2. However, O₂ itself is transformed, in a small fraction, into free radicals able to cause biological oxidative stress and serious cell alterations. Low O₂ concentrations (hypoxia) are detrimental to the cellular environment and, thus, evolution has created physiological adaptations to withstand transient hypoxia. Nevertheless, cancer cells skillfully use these survival mechanisms to keep up with their metabolic demands associated with rapid growth and proliferation and manage to withstand harsh tumor microenvironments. The most well-studied response pathway to hypoxia involves hypoxia-inducible factor (HIF), which is a transcription factor regulated by the von Hippel-Lindau (VHL) protein and the prolyl hydroxylases enzymes 3. Zhong et al., in their publication in 1999, stated that there was an increased HIF-1α expression in diverse types of cancer and their metastases, which suggested an important role of HIF in the progression of cancer disease 4.
This review’s goals are to highlight the importance of hypoxia in the development of cancer, identify the key molecules that interact to promote transcription factor expression, explain the molecular mechanisms of the pathways that lead to HIF induction and identify the cellular effects of HIF alteration and discuss potential HIF-targeted therapies.
Search strategy
Articles from 1990 to 2022 were reviewed in PubMed, Scopus and SciELO databases. Bibliographic references with the following key words were searched: Hypoxia-Inducible Factor-Proline Dioxygenases; Von Hippel-Lindau (VHL) Syndrome Tumor Suppressor Protein; Hypoxia-Inducible Factor 1 and Neoplasms. Repetitive reviews were excluded, and original sources had priority. Articles dated before the search date range were added in the event of relevant historical research or pivotal trials with current validity.
Hypoxia and cancer
Hypoxia can be defined as a state in which O₂ is not available in sufficient amounts at the tissue level to maintain adequate homeostasis. This condition is common in most malign tumors; tumor hypoxia may lead to tumor angiogenesis, which consists of tumor dysfunctional vascularization due to the need of more vascular supply because of cell proliferation and the acquisition of an epithelial-to-mesenchymal transition phenotype resulting in cell motility and metastasis. This condition alters cancer cell metabolism and may contribute to therapy resistance since it induces cell quiescence. Hypoxia stimulates a complex cell signaling network in cancer cells, including the HIF, phosphoinositide 3-kinases (PI3K), mitogen-activated protein kinases (MAPK) and nuclear factor kB (NFkB) pathways, which interact with each other and cause positive and negative feedback loops that increase or decrease hypoxic effects 5. O₂ distribution is very heterogeneous in cancer tumors, ranging from mild to severe hypoxic and even anoxic levels. Hypoxia is a negative factor that is associated with adverse results in most treatments of different cancers. The importance of hypoxia levels is well demonstrated in radiotherapy since ionizing radiation will destroy more cells in the presence of oxygen compared to hypoxic or anoxic conditions, where a steep decrease in radiosensitivity occurs 6.
Hypoxia-inducible factor (HIF)
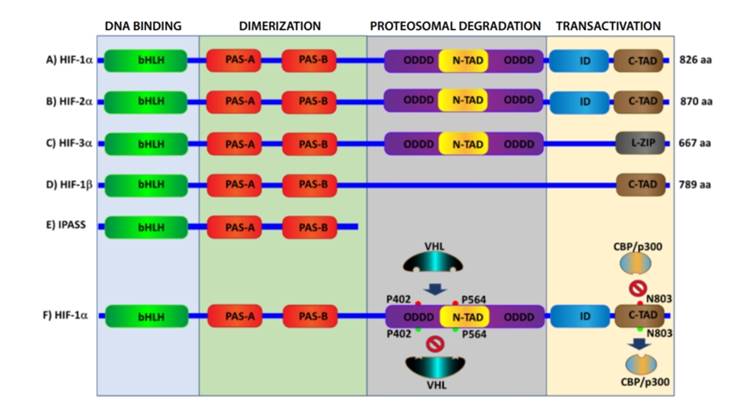
HIF is the most important transcription factor involved in the response to hypoxia through the activation of more than a hundred genes that regulate cell activity 7. Furthermore, it belongs to the superfamily of basic helixloop-helix (bHLH) proteins—with a PAS domain acting as a molecular sensor [bHLH-PAS]—which are transcriptional regulators, commonly occurring in living organisms 8. HIF is a heterodimer consisting of two subunits (Figure 1):
IF-1α subunit: it is sensitive to O₂, its expression is induced by hypoxia, has two terminal transactivation domains: NH2 (N-TAD) and COOH (C-TAD). These are responsible for HIF-1α transcriptional activity; C-TAD interacts with CBP/p300 (transcriptional coactivators associated with DNA-binding transcription factors that
modify transcription factors and chromatin by means of acetylation) to modulate HIF-1α gene transcription under hypoxic conditions; N-TAD has the oxygen-dependent degradation domain (ODDD), responsible for the stability of HIF-1α 9 (Figure 1 A, F).
HIF-2α isoform: it behaves similarly to HIF-1α; however, HIF-1α and 2α are expressed in different tissues, and it is more ubiquitous in the case of the former; HIF-1a regulates glycolytic gene expression and leads more apoptotic pathways, while HIF-2α stimulates humor growth and angiogenesis 10 (Figure 1 B).
HIF-3α isoform: it acts the opposite way to other isoforms because it generates an inhibitory PAS domain (IPAS), which prevents HIF-1α from binding DNA; therefore, it prevents hypoxia-dependent gene activation 11 (Figure 1 C, E).
HIF-1β subunit: it is also known as aryl receptor nuclear translocator (ARNT): It is constitutively expressed in cells and usually binds to the aryl hydrocarbon receptor, which facilitates its translocation to the nucleus; therefore, HIF1β is a nuclear localization factor 12 (Figure 1 D).
Von hippel-lindau protein (pVHL)
The von-Hippel Lindau suppressor protein (pVHL), under normoxic conditions, is the recognition component of an E3 ubiquitin ligase, which operates as a master regulator of HIF activity; the pVHL is directed to and adheres to the hydroxylated HIF-α subunit for its subsequent ubiquitination and rapid proteasomal degradation. The VHL gene, which encodes the protein under the same name, is a tumor suppressor gene which, therefore, is associated with the possibility of failure of both alleles (two strikes); it contains three exons that express messenger RNA which is capable of translating two proteins as a function of splice sites. It is not known why two VHL proteins are produced; therefore, they will be generically hereinafter referred to as pVHL. This protein is dynamically displaced between cytosol and the nucleus, depending on the transcription; however, most of the protein is in cytosol under stable conditions, with a relatively long mean life, though in some circumstances it is modified by polyubiquitination and is degraded by ubiquitin ligase WSB1. The pVHL forms a stable complex containing different proteins such as elongin B, elongin C, Cullin 2 and RBX1, which act as a ubiquitin ligase complex. Patients who inherit a defective copy of the VHL tumor suppressor gene have a higher risk of suffering from a variety of cancers including clear cell renal cell carcinoma, hemangioblastomas (tumors of the retina vessels, cerebellum and spinal cord) and paragangliomas (tumors of the sympathetic nervous system) 13.
Domains of HIF-1α (A-C) and HIF-1β (E, F) subunits. HIF-1α (F) activation and inhibition: during hypoxia, proline residues at positions 402 and 564 undergo hydroxylation by PH1-3 (red), which attracts the VHL protein that triggers ubiquitination and subsequent proteosomal destruction of HIF-1α protein; during normoxia, there is no hydroxylation (green). Therefore, there is no pVHL binding, and HIF-1α is not destroyed. Hydroxylation of asparagine residue at position 803 (red) by the enzyme FIH inhibits CBP/p300, and HIF-1α cannot reach DNA to cause gene transcription, regardless of VHL.
HIF pathway regulation
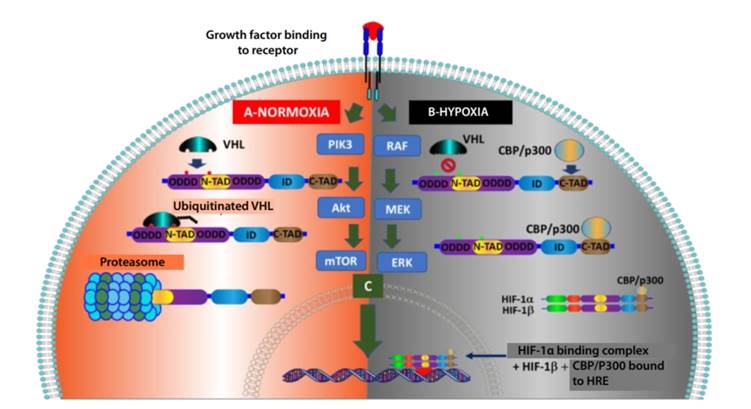
Normoxia leads to the rapid degradation of transcription factor HIF-1α (average time: 5 minutes) 14 because the VHL complex "polyubiquitinates" HIF-1α subunits and targets them for proteosomal degradation. This requires the hydroxylation of two proline residues (P402 y P564) within the ODDD of HIF-1α, which is catalyzed by PHD1-3 (Figure 1-F and Figure 2-A). Another lysine residue in the ODDD (K532) could be acetylated by the enzyme acetyl transferase ARD-1 (arrest-defective-1) and produce the same results 15. Hypoxia prevents hydroxylation of HIF-α subunits; therefore, it blocks the subsequent ubiquitination of these subunits by the VHL complex and prevents proteosomal degradation. This allows dimerization of HIF-1α and HIF-1β and, at nuclear level, binding to the hypoxia response element (HRE) of the DNA, which finally promotes the transcription of target genes by means of the promotor or enhancer of various hypoxia-inducible genes (Figure 2-B), including erythropoietin (EPO), vascular endothelial growth factor (VEGF), glucose transporters (GLUT) and glycolytic enzymes, genes involved in iron metabolism and survival of cancer stem cells 16.
Another important mechanism for the oxygen-dependent downregulation of the HIF-1α pathway, which does not involve the p-VHL, is the control of HIF-1α transactivation. Therefore, under normoxic conditions, hydroxylation of an oxygen-dependent asparagine residue (at N803) by HIF-1α by factor-inhibiting HIF-1 (FIH-1), also known as asparaginyl hydroxylase, blocks the interaction between both domains: C-TAD of HIF-1α and coactivators CBP/p300. Therefore, it prevents the subsequent HIF-1α-mediated gene transcription 17 (Figure 1-F).
It has been demonstrated that some proteins that participate in different cell signaling pathways—such as mTOR 18, PI3K 19—some growth factors that activate the RAS protein of the MAPK pathway 20, platelet-derived growth factor (PDGF) 21, insulin 22 and insulin-like growth factor (IGF) 23 may increase HIF-1α translation (Figure 2-C).
During normoxia, pVHL is attracted by HIF-1α and starts the ubiquitination process, which is followed by degradation. Consequently, it does not allow the binding complex to reach the nucleus and cause gene transduction. B) During hypoxia, VHL does not bind to HIF-1α; therefore, it does not degrade and does not bind to HIF-1β and CBP/P300 to reach the nucleus to activate the HRE and start gene transcription. C) Activations caused by important signaling pathways such as PIK3 and MAPK in malign cells may result in direct gene transduction regardless of O₂ conditions.
Consequences of HIF-1α activation in cancer cells
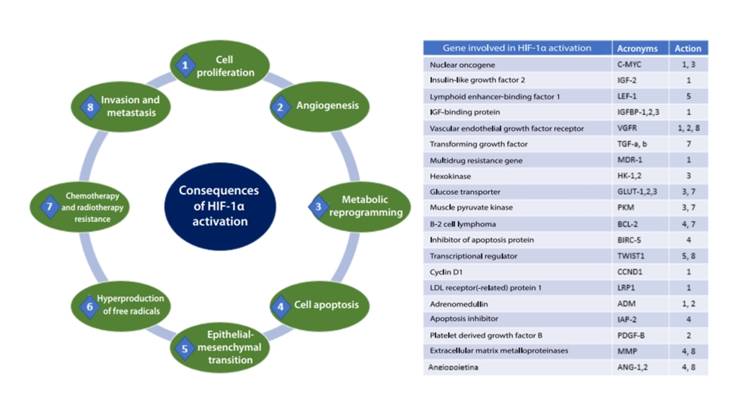
Approximately 60 % of solid tumors have < 1 % of O2, which results in intratumor hypoxia. Hence, HIF-1α is activated, thereby leading to trigger some essential functions to develop cancer such as cell proliferation and apoptosis, angiogenesis, cellular metabolic reprogramming, invasion and metastasis, which allow increased tumor survival. (Figure 3)
Tumor proliferation and apoptosis
For most cell types, hypoxia induces decreased cell proliferation by hypoxic stress. However, some populations maintain cell proliferation in the face of hypoxia, particularly in cancer. Therefore, they serve a physiological function as in the maintenance of stem cell population that reside in a hypoxic niche 24. HIF-regulated genes in various tumors include VEGF, EPO, IGF-2 and transforming growth factor α (TGFα); these genes influence tumor progression 25.
There is evidence of the controversial role of HIF-1 to prevent cell death by stimulating cell proliferation or even apoptosis induction in normal cells. Oxygen concentration and ATP level determine whether the cell will undergo apoptosis or not; also, hypoxia can inhibit the mitochondrial electron transport chain, cause activation of Bax or Bak, which leads to cytochrome C release and caspase-9 activation to apoptosis 26. Hypoxia is a common phenomenon in solid tumors; however, cells adapt to this environmental stress, so after repeated periods of hypoxia, selection of resistance to hypoxia-induced apoptosis occurs. These resistant tumors may probably be more aggressive and even more resistant to treatment. HIF-1 may induce antiapoptotic proteins such as IAP-2, whereas Bax proapoptotic protein can be downregulated. During hypoxia, an intricate balance exists between factors that induce or counteract apoptosis, or even stimulate proliferation. Understanding these regulation mechanisms may result in more specific treatments for solid tumors 27.
Angiogenesis
A tumor diameter can grow few millimeters by using vessels from adjacent cells. Nevertheless, increased cell proliferation and/or decreased apoptosis in solid tumors causes severe hypoxia, regions with low pH and lower nutrient flow. Therefore, the process of new vessel formation from those existing (angiogenesis) is essential for solid tumor progression 28. HIF may reduce the expression of a high number of proangiogenic factors, including VEGF with the corresponding receptors—platelet-derived growth factor B (PDGF-B), plasminogen activator inhibitor 1 (PAI-1), TIE-2 receptor, matrix metalloproteinases (MMP-2 and MMP-9) and angiopoietins (ANG-1 and ANG-2). VEGF-A is a potent endothelial mitogen, the most potent in the context of cancer since it is highly expressed in many human tumors 29,30.
Cellular metabolic reprogramming
Metabolic changes in cancer cells, particularly prevalence of anaerobic glycolytic metabolism over oxidative phosphorylation (OXPHOS) in the mitochondria in the aerobic process in various types of cancer (even with plenty of oxygen), proposed by Otto Warburg over a century ago 31, was an important benchmark in distinguishing between differentiated and cancer cells. In this process, known as "metabolic reprogramming," the tumor microenvironment plays an essential role through oncogene activation, epithelial-mesenchymal transition and drug resistance, all of which are hallmarks of aggressive cancer behavior 32.
HIF-1 stimulates the expression and activation of glycolytic enzyme isoforms, thereby supporting the Warburg effect by potentiating macromolecular biosynthesis and energy-producing pathways in human cancers. Such enzymes are pyruvate kinase M1 and M2, hexokinase 1 and 2 (HK1-2), glucose transporters 1 and 3 (GLUT 1-3), lactate dehydrogenase-A (LDH-A), aldolase A and C and phosphofructokinase, among others 33. HIF-1a induces glycolysis and actively restricts mitochondrial function and the use of oxygen since it promotes the activity of pyruvate dehydrogenase kinase 1 (PDK-1) 34. The critical consequence of this glycolytic shift is acidosis of the tumor microenvironment, which provides a higher number of metabolic intermediaries that stimulate tumor progression and aggressiveness 35.
The MYC oncogene encodes a transcription factor (c-Myc), which associates altered cell metabolism with tumorigenesis; the c-Myc protein regulates various genes related to metabolic reprogramming, such as GLUT1, LDH-A and HK2. HIF-1α inhibits the c-Myc activity under physiological conditions, but in the event of cancer c-Myc operates with HIF-1 to induce PDK-1 and HK2 expression, thereby leading to angiogenesis and aerobic glycolysis [70] 36.
Invasion and metastasis
Members of the HIF family play important roles in all the key stages of metastatic dissemination, including local migration within the tumor and invasion of the surrounding stromal tissue through the induction of an epithelialmesenchymal transition (EMT)-like process, remodeling of extracellular matrix, intravasation and extravasation, survival and dissemination through circulation, generation of premetastatic niches, etc., to promote tumor growth and colonization of distant organs and maintain tumor cell dormancy 37. Many reports have revealed that HIF-1α expression in cancer increases invasion and induces loss of E-cadherin 38.
HIF-1 directs the expression of many EMT regulators, and is one of the factors that contribute to tumor metastasis. The degree of tumor invasiveness and metastasis depends on HIF-1-regulated transcriptional activation of some metalloproteinases and lysyl oxidase, which degrade and remodel the extracellular matrix. Moreover, HIF-1 target genes include permeability factors—such as VEGF—which promote intravasation of cancer into vessels 39.
Cancer stem cells (CSCs) are a population of cells with similar characteristics to those of stem cells, and are associated with the occurrence, recurrence, metastasis and chemoradiation resistance of cancer. HIF-1 seems to play an important, or even, fundamental role in the generation and maintenance of CSCs, but the specific mechanism remains unclear 40.
HIF as a therapeutic target for cancer
HIF-1α overexpression in tumor biopsies is associated with higher mortality among human patients with bladder, brain, breast, cervix, colon, endometrium, lung, oropharynx, pancreas, skin and stomach cancers. Studies have started to identify possible HIF-1α-regulated target genes that may play a role in tumor progression. Nevertheless, a specific subset of HIF-1α genes differs according to the type of cancer 41. It has been demonstrated that many drugs currently in use—such as amphotericin B, metformin and some non-steroidal anti-inflammatory drugs—and even natural substances such as turmeric 42 influence the activity of this important pathway for the development of cancer; however, this article includes only a quick review of drugs that are currently in a relevant clinical research stage or that have been approved by the most important regulatory authorities worldwide.
Digoxin. This well-known cardiac glycoside has an anticarcinogenic effect in vitro and in vivo in various solid tumors since it inhibits HIF-1α production 43; and is in Phase 2 clinical trial for breast cancer (https://clinicaltrials.gov/ ct2/show/NCT01763931).
Ganetespib. Formerly known as STA-9090, it is a Hsp90 inhibitor, a molecular and ubiquitous chaperone that plays an essential role in various cellular processes, including cell cycle control, cell survival, hormones and other signaling pathways, which is very important in cellular stress response and a key player in cellular homeostasis maintenance. This drug increases proteasome-mediated Hsp90 degradation and prevents the activation of multiple oncogenic proteins, including HIF-1α 44. It is currently studied in a Phase 3 clinical trial in patients with advanced non-small cell lung cancer together with docetaxel (https://www.clinicaltrials.gov/ct2/show/NCT01798485).
SLC-0111 and DTP348. They are carbonic anhydrase IX (CAIX) inhibitors. CAIX is a hypoxia-inducible enzyme that promotes survival, proliferation and invasion of cancer cells through HIF activation. In addition, CAIX regulates cellular pH and expresses exclusively on the cell surface of tumor cells and is one of the key factors that influence the survival and metastasis of cancer cell 45. SLC-0111 is in a Phase I clinical trial (https://clinicaltrials.gov/ ct2/show/NCT02215850); DTP348 is a CAIX inhibitor and radiosensitizer that is studied in cancer in a Phase I clinical trial (https://clinicaltrials.gov/ct2/show/NCT02216669).
PX-478. It is an experimental HIF-1α inhibitor that has shown antitumor activity against several aggressive human tumor xenografts 46. It is in a Phase I trial as an oral agent (https://clinicaltrials.gov/ct2/show/NCT00522652); but its exact mechanism to improve radiosensitivity as a clinical radiation enhancer remains to be identified 47.
Tanespimycin (17AAG). It is a benzoquinone antineoplastic antibiotic derived from the antineoplastic antibiotic geldanamycin. This molecule binds to and inhibits the functions of heat shock protein 90 (HSP90), which promotes proteasomal degradation of oncogenic signaling proteins that may be overexpressed by tumor cells 48. At present, it is studied in diverse cancers in different phases. (https://clinicaltrials.gov/ct2/results?cond=&term=17AAG&cntry=&state=&city=&dist=).
Bortezomib. It is a proteasome inhibitor that plays a vital role in cellular protein turnover, which is essential for the homeostasis of cells. It reversibly binds to the chymotrypsin-like subunit of the 26S proteasome and prevents the degradation of various proapoptotic transcription factors in neoplastic cells. Its accumulation will activate the programmed cell death via caspasemediated pathways. It also promotes the hydroxylation of proline residues in positions 402 and 564 by PHD, which leads to the repression of HIF-1 transcriptional activity and the inhibition of erythropoietin, vascular endothelial growth factor and carbonic anhydrase IX genes 49. It was approved by the Food and Drug Administration (FDA) for the treatment of multiple myeloma and mantle cell lymphoma 50.
Belzutifan (MK-6482). It is the second generation of a small molecule that inhibits the HIF-2α complex. Thus, it slows or stops the growth of VHL-related tumors. Belzutifan was approved by the FDA for the treatment of certain von Hippel-Lindau (VHL) disease-associated cancers in August 2021 51. The Phase 2 MK-6482-004 trial, conducted with 61 patients with VHL disease-associated renal cell cancer (RCC) (some of which had central nervous system (CNS) hemangioblastomas (CNSHs) and/or pancreatic neuroendocrine tumors (pNETs) was a pivot trial for such approval. RCC had a global response rate of 49 % (CI 95 %: 36-62), 56 % of responders had a response duration ≥ 12 months. The 24 patients with CNSHs and the 12 patients with measurable pNETs had a global response rate of 63 % (CI 95 %; 41-81) and 83 % (CI 95%, 52-98), respectively. The most frequent adverse reactions, including reported laboratories anomalies in ≥ 20 %, were anemia, fatigue, increased creatinine, headache, dizziness, increased glucose and nausea 52.
Conclusions
Since HIF1α overexpression in cancer was found, much research has been conducted on the importance of this response pathway to hypoxia in human biology; even the 2019 Nobel Prize in Physiology or Medicine was awarded to Kaelin, Ratcliffe and Semenza for their pioneer work on oxygen detection and adaptation to hypoxia 52. Hypoxia is an essential characteristic of tumor microenvironment in many types of cancer, and it is able to generate important capacities in cells that allow them to growth in hostile environments and develop both radiotherapy and chemotherapy resistance. Tumor cells respond to change in oxygen through HIF transcriptional factor to coordinate a large number of attacks and essential cell functions.
However, although hundreds of compounds have been studied, there are currently few HIF inhibitors in clinical trials in different phases or approved trials in the world market. It was found that various HIF-1 inhibitors improve the therapeutic efficacy of anticancer drugs. Therefore, anticancer drug resistance has been overcome with advantages and limitations. Nevertheless, the identification of hypoxic markers and genomic analyses are urgently required to allow HIF-1 adapt to specific types of cancer and individual patients.
Future studies on HIF-1α will be essential to improve our understanding about different phenomena and develop new technologies, treatments and interventions. Without continuous research and investigation, there is the risk of becoming stagnant and missing out on potential breakthroughs that may involve significant implications for society. Future studies on HIF could provide significant contributions. First, they would help to develop new treatments for diseases that involve HIF deregulation. Second, they could explain the mechanisms by which HIF regulates various physiological processes. This could have important implications to understand how the body responds to hypoxia (low oxygen levels) and how such responses could improve in certain settings. Finally, research on HIF may have more implications concerning the understanding of cell signaling and overall gene expression, and the study could be a contribution to better understand these networks and develop new approaches to manage them.
REFERENCIAS BIBLIOGRÁFICAS
1. Cummins EP, Strowitzki MJ, Taylor CT. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol Rev. 2020;100(1):463-88. [ Links ]
2. Cogliati S, Cabrera-Alarcon JL, Enriquez JA. Regulation and functional role of the electron transport chain supercomplexes. Biochem Soc Trans. 2021;49(6):2655-68. [ Links ]
3. Kim LC, Simon MC. Hypoxia-inducible factors in cancer. Cancer Res. 2022;82(2):195-6. [ Links ]
4. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830-5. [ Links ]
5. Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [ Links ]
6. Hompland T, Fjeldbo CS, Lyng H. Tumor hypoxia as a barrier in cancer therapy: Why levels matter. Cancers (Basel). 2021;13(3):499. [ Links ]
7. Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268-83. [ Links ]
8. Kolonko M, Greb-Markiewicz B. bHLH-PAS Proteins: Their structure and intrinsic disorder. Int J Mol Sci. 2019;20(15):3653. [ Links ]
9. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510-4. [ Links ]
10. Keith B, Johnson RS, Simon MC. HIF1a and HIF2a: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12(1):9-22. [ Links ]
11. Torii S, Sakaki K, Otomo M, Saka K, Yasumoto K, Sogawa K. Nucleocytoplasmic shuttling of IPAS by its unique nuclear import and export signals unshared with other HIF-3a splice variants. J Biochem. 2013;154(6):561-7. [ Links ]
12. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/ CBP. J Biol Chem. 2001;276(16):12645-53. [ Links ]
13. Kaelin WGJ. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865-73. [ Links ]
14. Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxiainducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271-5. [ Links ]
15. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469-80. [ Links ]
16. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxiainducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551-78. [ Links ]
17. Dann CE3, Bruick RK, Deisenhofer J. Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway. Proc Natl Acad Sci U S A. 2002;99(24):15351-6. [ Links ]
18. Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108(10):4129-34. [ Links ]
19. Agani F, Jiang B. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13(3):245-51. [ Links ]
20. Carrera S, Senra J, Acosta MI, Althubiti M, Hammond EM, de Verdier PJ, et al. The role of the HIF-1a transcription factor in increased cell division at physiological oxygen tensions. PLoS One. 2014;9(5):e97938. [ Links ]
21. Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GC, Poon RT, et al. An Akt/ hypoxia-inducible factor-1alpha/platelet-derived growth factor-BB autocrine loop mediates hypoxia-induced chemoresistance in liver cancer cells and tumorigenic hepatic progenitor cells. Clin Cancer Res. 2009;15(10):3462-71. [ Links ]
22. Biswas S, Mukherjee R, Tapryal N, Singh AK, Mukhopadhyay CK. Insulin regulates hypoxia-inducible factor-1a transcription by reactive oxygen species sensitive activation of Sp1 in 3T3-L1 preadipocyte. PLoS One. 2013;8(4):e62128. [ Links ]
23. Sinha S, Koul N, Dixit D, Sharma V, Sen E. IGF-1 induced HIF-1a-TLR9 cross talk regulates inflammatory responses in glioma. Cell Signal. 2011;23(11):1869-75. [ Links ]
24. Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxiainducible factors. Am J Physiol Cell Physiol. 2015;309(12):C775-82. [ Links ]
25. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207-14. [ Links ]
26. Kumar H, Choi D. Hypoxia inducible factor pathway and physiological adaptation: A cell survival pathway? Mediators Inflamm. 2015;2015:584758. [ Links ]
27. Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-in hypoxia induced apoptosis. J Clin Pathol. 2004;57(10):1009-14. [ Links ]
28. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182-6. [ Links ]
29. Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217-57. [ Links ]
30. Prager GW, Poettler M, Unseld M, Zielinski CC. Angiogenesis in cancer: Anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1(1):14-25. [ Links ]
31. Otto AM. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016;4:5. [ Links ]
32. Sharma A, Sinha S, Shrivastava N. Therapeutic targeting hypoxiainducible factor (HIF-1) in cancer: Cutting gordian knot of cancer cell metabolism. Front Genet. 2022;13:849040. [ Links ]
33. Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347-53. [ Links ]
34. He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1a on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23(1):174-80. [ Links ]
35. Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13(9):611-23. [ Links ]
36. Dang CV, Kim J, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51-6. [ Links ]
37. Araos J, Sleeman JP, Garvalov BK. The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti-tumour strategies. Clin Exp Metastasis. 2018;35(7):563-99. [ Links ]
38. Zhang S, Zhou X, Wang B, Zhang K, Liu S, Yue K, et al. Loss of VHL expression contributes to epithelial-mesenchymal transition in oral squamous cell carcinoma. Oral Oncol. 2014;50(9):809-17. [ Links ]
39. Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108(39):16369-74. [ Links ]
40. Zhang Q, Han Z, Zhu Y, Chen J, Li W. Role of hypoxia inducible factor-1 in cancer stem cells (Review). Mol Med Rep. 2021;23(1):17. [ Links ]
41. Ghosh R, Samanta P, Sarkar R, Biswas S, Saha P, Hajra S, et al. Targeting HIF-1a by natural and synthetic compounds: A promising approach for anti-cancer therapeutics development. Molecules. 2022;27(16):5192. [ Links ]
42. Semenza GL. Pharmacologic targeting of hypoxia-inducible factors. Annu Rev Pharmacol Toxicol. 2019;59:379-403. [ Links ]
43. Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8(1):36-49. [ Links ]
44. White PT, Subramanian C, Zhu Q, Zhang H, Zhao H, Gallagher R, et al. Novel HSP90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery. 2016;159(1):142-51. [ Links ]
45. Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210-9. [ Links ]
46. Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3(3):233-44. [ Links ]
47. Palayoor ST, Mitchell JB, Cerna D, Degraff W, John-Aryankalayil M, Coleman CN. PX-478, an inhibitor of hypoxia-inducible factor1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123(10):2430-7. [ Links ]
48. National Center for Biotechnology Information. PubChem Compound Summary for CID 6505803, Tanespimycin [Internet]. National Library of Medicine. 2023 [citado el 21 de mayo de 2023]. Disponible en: https://pubchem.ncbi.nlm.nih.gov/compound/6505803 [ Links ]
49. Huang Z, Wu Y, Zhou X, Xu J, Zhu W, Shu Y, et al. Efficacy of therapy with bortezomib in solid tumors: a review based on 32 clinical trials. Future Oncol. 2014;10(10):1795-807. [ Links ]
50. Sharma A, Preuss CV. Bortezomib. En: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [ Links ]
51. Fallah J, Brave MH, Weinstock C, Mehta GU, Bradford D, Gittleman H, et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clin Cancer Res. 2022;28(22):4843-48. [ Links ]
52. Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von HippelLindau disease. N Engl J Med. 2021;385(22):2036-46. [ Links ]
Received: January 21, 2023; Revised: April 26, 2023; Accepted: May 06, 2023










 texto em
texto em