Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.10 no.2 Lima jul./dic. 2003
ARTICULO DE REVISIÓN
Laboratorio de Micología y Biotecnología, Universidad Nacional Agraria La Molina
Aceptado: 29/12/2003
Abstract
Basic knowledge on solid state fermentation and on biofilm formation is summarized and related to cell adhesion processes. These subjects are covered from the engineering and molecular biology points of view. Contrary to the common believe, the advantages of solid state fermentation are related to the adhesion of fungi to solid particles instead of being due to the low water content. Thus, solid state fermentation and biofilm fermentation (erroneously known as adsorption immobilization) are technical variants of the same biological process, and should be referred as Surface Adhesion Fermentation.
Keywords: Biofilms, solid substrate fermentation, differential gene expression, fungi.
Resumen
Se resume el conocimiento básico sobre la fermentación en estado sólido y la formación de biopelículas y se relaciona con los procesos de adhesión celular, cubriendo puntos de vista de ingeniería y de biología molecular. Contrariamente a la creencia común, la ventaja de la fermentación en estado sólido está relacionada a la adhesión de los hongos a partículas sólidas y no al bajo contenido de agua. Por lo tanto, la fermentación en estado sólido y la fermentación en biopelículas (erradamente conocida como inmovilización por adsorción) son variantes técnicas del mismo proceso biológico y deben ser referidas como Fermentación por Adhesión a Superficies.
Palabras clave: Biopelículas, fermentación en sustrato sólido, expresión diferencial de genes, hongos.
Introduction
Many of the traditional fermented food are based on the koji process that belongs to a major technical fermentation category known as "solid substrate (state) fermentation" (SSF) differing from either submerged or immobilization fermentations in the amount of free water that they contain. For a long time it has been considered that the advantages of SSF are due to water limitation of the system so that a higher product concentration and volumetric productivity are attained. However, scale up of SSF processes is the main drawback for a more ample industrial application. On the other hand, related to fungal enzyme production, submerged fermentation (SF) is the process of choice for industrial operations due to the very well known engineering aspects such fermentation modeling, bioreactor design and process control. Also, it has been considered that there would not be biochemical differences between an enzyme produced by the same fungal strain in either SSF or SF and most of the biochemical information on some important fungal enzymes comes from submerged cultures. Enzyme market in 2005 will be between 1,700 to 2,000 million dollar and thus it represents a very important biotechnological sector in which any technological development would be rapidly adopted (Bhat, 2000; Demain, 2000).
Nowadays, biofilm processes used mainly for waste water treatment are also being considered for metabolite and enzyme production. Although fungal biofilms are less known than bacterial biofilms, they can be used for enzyme production as it has been recently showed (Villena et al., 2001). As it will be stated in the present paper, both SSF and biofilm fermentation (BF) depend on surface adhesion and a new fermentation category was recently established. Surface adhesion fermentation (SAF) was proposed by Gutierrez-Correa (2003) as this new category and it will be sustained it further in this paper.
As mentioned above, SSF is a process used for the production of fermented food, animal feed, fuel, enzymes, pharmaceuticals, which involves the growth of microorganisms (mainly fungi) on moist solid substrates in the absence of free-flowing water. SSF processes exhibit several advantages over SF, including improved product characteristics, higher product yields and productivities, easier product recovery and reduced energy requirements. Also, mixed culture SSF for enzyme production give higher yields than single culture SSF as it can be seen in figure 1 (Castillo et al., 1994; Dueñas et al., 1995; Gutierrez-Correa and Tengerdy, 1997, 1998, 1999; Gutierrez-Correa et al., 1998).
Regarded to the solid support used for SSF, two main types of processes are used. Firstly, SSF processes that use natural solid substrates like starch- or (lingo)cellulose residues or agro-industrial sources such as grains, and grain by-products, cassava, potato, rice, beans and sugar beet pulp. In theses cases substrate is used also as the source of carbon and nutrients for microbial growth (Ningam and Singh, 1996; Tengerdy and Szakacs, 2003). Secondly, SSF processes that use inert natural or artificial solid supports like sugarcane bagasse, perlite, amberlite, polyurethane foam and others. In these latter processes support is used only as an attachment structure for the microorganism. From the engineering point of view inert supports are better because they do not change their geometric and physical characteristics due to the microbial growth, allowing a better control of heat and mass transfer (Ooijkaas et al., 2000).
Enzyme production by SSF is not of wide use by manufacturers mainly due difficulties in bioreactor design, although the advantages of this form of microbial cultivation as compared to SF are well known. Growth patterns in SSF have been detailed in only few cases and these can be summarized in two phases: a) germination, germ tube elongation and mycelial branching to cover loosely most of the substrate; b) increase in mycelial density with aerial and penetrative hyphae development (Mitchell, 1992). However, as it will be described below, a previous phase namely spore adhesion to surfaces has been forgotten by most researchers. Recently, a general approach to compare productivity of fungal enzymes using SSF and SF techniques was attempted by using logistic and Luedeking-Piret equations (Viniegra-González et al., 2003). Also, many attempts to develop mathematical models for SSF have been done and current models are more rational tanking into account not only mass and heat transport processes across the substrate bed (macroscale models) but also phenomena that occur on and within individual particles (microscale models) (Mitchell et al., 2003).
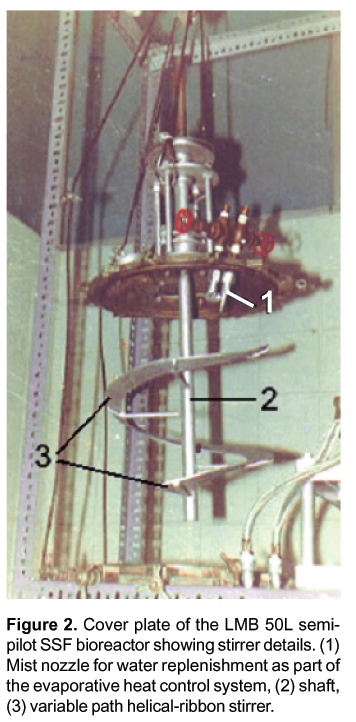
Several SSF bioreactor models have been designed following two general categories: laboratory-scale and pilot and industrial-scale (Durand, 2003; Fasidi et al., 1996). Many designs have been published that belong to the first category including static and agitated models but only few models are used in commercial production. We designed and built an agitated 50L semi-pilot computer-controlled SSF bioreactor with an evaporative heat control system and a variable path helical-ribbon stirrer Figure 2; unpublished results). In general, however, many types of SSF bioreactors can be run at the bench scale level with small quantities of substrate but their scale-up is difficult due to heat generation and heterogeneity with natural supports.
Biofilm fermentation overview
The concept of a biofilm presumes either a population or a community of microorganisms living attached to a surface. Biofilms can be developed on either biotic or abiotic surfaces from a single species or as a community derived from several species (David and O'Toole, 2000; Fenchel, 2002). This way of growth is the prevailing lifestyle of microorganisms including bacteria, yeast, filamentous fungi and even micro-animals (Armstrong et al., 2001; Gilbert and Lappin-Scott, 2000; Morris et al., 1997; Watnick and Kolter, 2000). However, there is a strong tendency in microbiology and in the scientific literature to consider within the above concept of biofilms to those developed by bacteria in complex natural communities or those developed by bacteria and yeast in natural or artificial environments of medical relevance that are responsive of persistent infections (O'Toole et al., 2000. Rickard et al., 2003; Sauer, 2003; Stickler, 1999; Wimpenny, 2000). It should be noted that adhesion and subsequent differential gene expression to generate phenotypes distinct from those of free living organisms are two unifying processes of the biofilm concept (Ghigo, 2003; O'Toole et al., 2000). Also, biofilms have been used for a long time in water treatment facilities where they were called slime, mats or sludge, but not other practical used was seen until recently. This has brought that most of the available information is on bacterial and, in recent years, on yeast biofilms.
Filamentous fungi are naturally adapted to growth on surfaces and in these conditions they show a particular physiological behavior which it is different to that in submerged culture; thus, they can be considered as biofilm forming organisms according to our former concept. Differential physiological behavior of most attached fungi corresponds principally to a higher production and secretion of enzymes and also to a morphological differentiation which is absent in submerged cultures (Akao et al., 2002; Biesebeke et al., 2002). The advantages of this form of growth have been industrially exploited by two culture systems: SSF and cell immobilization although there is a lack of knowledge on the molecular basis of growth on surfaces.
Technology of cell immobilization was highly developed during the last two decades based on the operative advantages in the productive process instead of physiological issues. Natural adsorption on solid supports is an immobilization technique that it has been used with filamentous fungi thus neglecting its study as a way of biofilm formation. Actually, once spores are adsorbed to the support they grow attached to it thus forming a film. We prefer the term biofilm fermentation (BF) instead of cell immobilization because the microbe is an active and differential entity. BF has been applied for the production of enzymes, amino acids, organic acids, alcohol, aromas and in bioconversion processes (Anderson, 1983; Groboillot et al., 1994; Norton and Vuillemard, 1994) as well as in bioremediation an effluent biotreatment (Burton, 2001; Doggett, 2000; King and Shoda, 1999; Kasinath et al., 2003; Rodgers et al., 2003; Van Driessel and Christov, 2001).
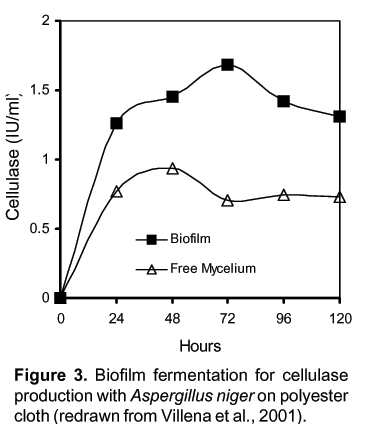
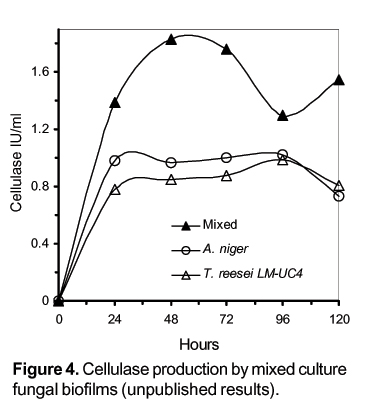
We have showed that Aspergillus and Trichoderma biofilms developed on polyester cloth produce higher cellulase titers than submerged cultures. A. niger biofilms produce 20% more cellulolytic activity than T. reesei biofilms and 140% more cellulolytic activity than submerged cultures (Villena et al., 2001) Figure 3. As in SSF, it has been possible to use mixed culture BF of Aspergillus niger and Trichoderma reesei for cellulase production Figure 4.
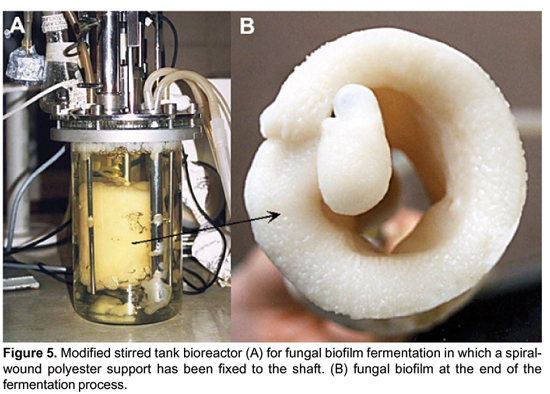
Due to the extended used of bacterial biofilms for wastewater treatment several mathematical models of growth kinetics have been developed including estimation of biokinetic parameters, mass transfer processes, flow velocity through biofilms (Benefield and Molz, 1985; Riefler et al., 1998; Stoodley et al., 1997). Also, the engineering treatment of cell immobilization including the type that actually correspond to biofilm according to our concept together with the availability of mathematical models of biofilm kinetics have paved the way for bioreactor design and process development (Vekatasubramanian et al., 1983). Fungal biofilm kinetic models need to be developed yet since they have only recently been realized as true biofilms instead of simple cell immobilization systems. Perhaps, the easy way to treat mathematically fungal biofilms is to adapt fungal pellet growth kinetics knowledge. An advantage of biofilms is that according to the type and form of the support almost all type of submerged bioreactors can be used, including the simple stirred tank, gas-lift, packed bed columns, rotating contactors and horizontal blade-stirrer bioreactor (Keshavarz et al., 1990; Pakula and Freeman, 1996). We have tested cellulase production by fungal biofilms in both a modified internal-loop air-lift reactor in which the riser tube was replaced by a spiral-wound polyester support and in a stirred-tank reactor in which a spiral-wound polyester support was attached to the shaft instead of turbines (unpublished results; Figure 5.
Cell adhesion as a biological process has been studied by cell biology particularly referred to animal cell. It has been considered it as a basic important processes for tissue and organ development that includes not only physical and chemical interactions but also vital actions such signaling and gene regulation. A number of surface protein receptor are present in animal cell including E-cadherins, integrins and others (Pece and Gutkind, 2002; Schwartz and Ginsberg, 2002). Cell adhesion is also present in plants and it may play a similar role as in animal organisms. Plant cell adhesion is desirable because of its positive effects on cell physiology and biochemistry particularly on secondary metabolite production. Hence controlled cell adhesion considered as a immobilization process (as it happened with fungi) is of interest from the process engineering point of view (Panda et al., 1989; Rao and Ravishankar, 2002). For instance, enhanced production of shikonin by Lithospermum erythrorhizon cells immobilized on polyurethane foam matrices (thus, a plant biofilm) has been attained in packed bed columns (Park et al., 1990).
Formation and maintenance of biofilms are dynamic processes that involve complex interactions of physical and biological processes. Physical properties of support like hydrophobicity, electrostatic charge and surface roughness are important at the initial adhesion step of bacteria, yeast and filamentous fungi (Bigerelle et al., 2002; Cunliffe et al., 1999; Dufrene, 2000; Webb et al., 1999). The DLVO (for Dejarguin, Landau, Verwey, and Overbeek) theory of adhesion of colloidal particles considers the van der Waals attractive interactions and the electrostatic interactions between the surfaces (Gerin et al., 1995; Rouxhet and Mozes, 1990). For the adhesion of microbial cells, the DLVO theory is usefully completed by considering the influence of cell wall macromolecules which can either bridge the cell to the surface or prevent adhesion by steric hindrance.
In bacteria cell structures like fimbriae, pili and flagella participate in the process of recognition and colonization of surfaces and extracellular polysaccharide production stabilizes adhesion (Davey and O'Toole, 2000; Ghigo, 2003; Lejeune, 2003; Purevdorj et al., 2002; Stickler, 1999; Watnick and Kolter, 2000). In yeasts, adhesion depends on the expression of surface glycoproteins, floculins or adhesins, that modify the properties of cell walls and they are differentially expressed during haploid and diploid stages, or in dimorphic yeast-like or hyphal-like stages, and also the production of extracellular polysaccharides is a quite common process (O'Toole et al., 2000; Reynolds y Fink, 2001; Sundstrom, 1999).
Adhesion in filamentous fungi is mediated principally by a class of small amphipathic proteins called hydrophobins that are produced by ascomycetes and basidiomycetes, and may also be produced by zygomycetes. Hydrophobins stabilize the adhesion of spores and mycelium to both natural and artificial hydrophobic surfaces resulting in morphogenetic signals (Mankel et al., 2002; Scholmeijer et al., 2001, 2002; Wosten y Willey, 2000). Other molecules like glycoproteins participate in adhesion as found in Colletotrichum lindemuthianum (Hughes et al., 1999). There is also evidence on the production of an extracellular matrix that enhance adhesion (Doss, 1999).
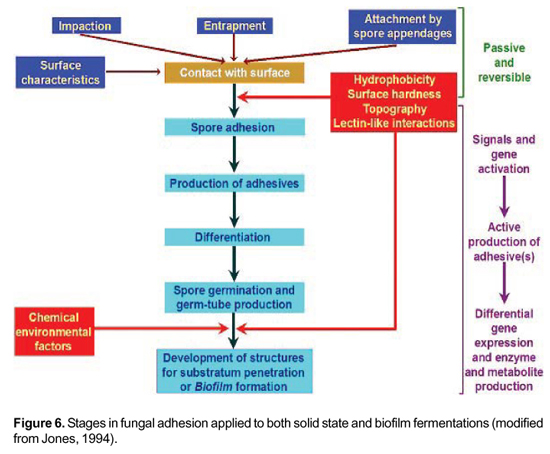
We have described the growth pattern of A. niger BF by using scanning electron microscopy. Biofilm formation can be divided into three phases: 1) adhesion, which is strongly increased by Aspergillus spore hydrophobicity; 2) initial growth and development phase from spore germination to surface colonization; 3) maturation phase in which biomass density is highly increased and an internal channel organization that assures medium flow through biofilm is clearly evident (Villena and Gutierrez-Correa, 2003). Based on early studies of Jones (1994) on fungal adhesion, we have summarized the stages of this process by including recent findings on cell signaling and differential gene expression, since the latter processes are most important in SSF and BF Figure 6.
The ample analytical study of bacterial biofilms has demonstrated that adhesion launches the expression of a set of genes that ends with the typical biofilm phenotype, particularly with an enhanced resistance to antimicrobial agents (Davey and O'Toole, 2000; Goldberg, 2002; Stickler, 1999; Watnick and Kolter, 2000). Differential expression implies the activation or repression of genes that comprise between 1% to 38% of the bacterial genome. In gram-negative bacteria like E. coli, differential expression comprises 230 genes while in Pseudomonas 1% of its genome is differentially expressed when growing as biofilm. In gram-positive bacteria like Bacillus it has been found that 519 genes are expressed during biofilm formation (Ghigo, 2003; Oosthuizen et al., 2002; Sauer, 2003; Schoolnik and Yildiz, 2000).
In yeast like Candida genes of the ALS (agglutinin-like) family that code for proteins related to adhesion to surfaces are differentially expressed. Likewise, it is possible that biofilm resistance to antimicrobial agents may be due to a higher expression of drug resistance gene families (Chandra et al., 2001). Moreover, Candida albicans biofilms are structured communities composed of a mixture of yeast cells and hyphal elements, suggesting a pivotal role for the dimorphic switch in the development of biofilms and the Efg1 regulator protein being responsible for the filamentous growth (Ramage et al., 2002). In Saccharomyces cerevisiae FLO11 gene has been identified as responsible for cell adhesion and the formation of aggregates and biofilm (Reynolds and Fink, 2001).
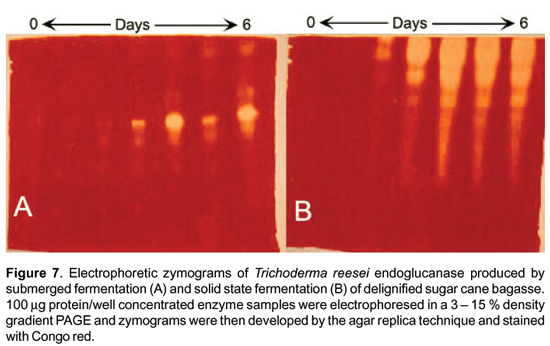
As mentioned above, biofilms of filamentous fungi have not been realized until recently thus biochemical and molecular information is lacking. However, as SSF processes have been traditionally related to water limitation and not to adhesion, it is considered that there are not biochemical differences between enzymes produced by SSF and those produced by SF; thus, work in this subject is scarce. Molecular comparison of cellulases produced by Trichoderma reesei in SF and SSF showed that those produced in SSF were strongly different from those produced in SF Figure 7; unpublished work), the latter being of low molecular weight.
Due to the lack of knowledge on the molecular biology of fungal biofilms we can only speculate that as in SSF a similar differential gene expression will be found in biofilm fermentation. Techniques of functional genomics will help in testing this hypothesis and if so biofilm fermentation would be more suited for the scale up to industrial operations than SSF since many of the engineering concepts of SF can be easily adapted to this type of fermentation. We should now consider a new major technical fermentation category, named "surface adhesion fermentation" (SSF and biofilm fermentation).
G.K.Villena is supported by doctoral scholarships from INCAGRO and CONCYTEC.
Akao, T.; K. Gomi, K. Goto, N. Okazaki and O. Akita. 2002. Subtractive cloning of cDNA from Aspergillus oryzae differentially regulated between solid-state culture and liquid (submerged) culture. Curr. Genet. 41:275-281. [ Links ]
Anderson, J. G. 1983. Immobilized cell and film reactor systems for filamentous fungi. In "The Filamentous Fungi" (J.E. Smith, D.R. Berry, B. Kristiansen, eds.), Vol. 4, Chapter 6, E. Arnold, London. [ Links ]
Armstrong, E.; L. Yan, K.G. Boy, P.C. Wright and J.G. Burgess. 2001. The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461:37-40. [ Links ]
Asther, M.; M. Haon, S. Roussos, E. Record, M. Delattre, L. Lesage-Meessen, M. Labat and M. Asther. 2002. Feruloyl esterase from Aspergillus niger: a comparison of the production in solid state and submerged fermentation. Process Biochem. 38:685-691. [ Links ]
Benefield, L. and F. Molz. 1985. Mathematical simulation of a biofilm process. Biotechnol. Bioeng. 27:921-931. [ Links ]
Bhat, M. K. 2000. Cellulases and related enzymes in Biotechnology. Biotechnol. Adv. 18:355-383. [ Links ]
Biesebeke, R.; G. Ruijter, Y. S. P. Rahardjo, M. J. Hoogschagen, M. Heerikhuisen, A. Levin, K. G. A. van Driel, M.A.L. Schutyser, J. Dijksterhuis, Y. Zhu, F. J. Weber, W. M. de Vos, K. A. M. J. J. van den Hondel, A. Rinzema and P. Punt. 2002. Aspergillus oryzae in solid-state and submerged fermentations: progress report on a multidisciplinary project. FEMS Yeast Res. 2:245-248. [ Links ]
Bigerelle, M.; K. Anselme, E. Dufresne, P. Hardouin and A. Lost. 2002. An unscaled parameter to measure the order of surfaces: a new surface elaboration to increase cells adhesion. Biomolec. Engineering 19: 79-83. [ Links ]
Busrton, S. G. 2001. Development of bioreactors for application of biocatalysts in biotransformations and bioremediation. Pure App. Chem. IUPAC 73:77-83. [ Links ]
Castillo, M. R.; M. Gutierrez-Correa, J. C. Linden and R. P. Tengerdy. 1994. Mixed culture solid substrate fermentation for cellulolytic enzyme production. Biotechnol. Let. 16:967-972. [ Links ]
Chandra, J.; D. M. Jun, P. K. Mukherjee, L. L. Hoyer, T. McCormick and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture and drug resistance. J. Bacteriol. 183:5385-5394. [ Links ]
Cunliffe, D.; C. A. Smart, C. Alexander and E. N. Vulfson. 1999. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 65:4995-5002. [ Links ]
Davey, M. E. and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Molec. Biol. Rev. 64:847-867. [ Links ]
Demain, A. L. 2000. Microbial Biotechnology. Trends Biotechnol. 18:26-31. [ Links ]
Doelle, H. W.; D. A. Mitchell and C. E. Rolz (eds.). 1992. Solid Substrate Cultivation. Elsevier Science Pub., New York. [ Links ]
Dogget, M. S. 2000. Characterization of fungal biofilms within a municipal water distribution system. Appl. Environ. Microbiol. 66:1249-1251. [ Links ]
Doss R. P. 1999. Composition and enzymatic activity of the extracellular matrix secreted by germlings of Botrytis cinerea. Appl. Environ. Microbiol. 65:404-408. [ Links ]
Dueñas, R.; R. P. Tengerdy and M. Gutierrez-Correa. 1995. Cellulase production by mixed fungal solid substrate fermentation of sugar cane bagasse. World J. Microbiol. Biotechnol. 11:333-337. [ Links ]
Dufrene, Y. F. 2000. Direct characterization of the physicochemical properties of fungal spores using functionalized AFM probes. Biophysical J. 78:3286-3291. [ Links ]
Durand, A. 2003. Bioreactor designs for solid state fermentation. Biochem. Eng. J. 13:113-125. [ Links ]
Fasidi, I. O.; O. S. Isikhuemhen and F. Zadrazil. 1996. Bioreactors for solid state fermentation of lignocellulosics. J. Scientific Ind. Res. 55: 450-456. [ Links ]
Fenchel, T. 2002. Microbial behavior in a heterogeneous world. Science 296:1068-1071. [ Links ]
Gerin, P.; M.N. Bellon-Fontaine, M. Asther and P.G. Rouxhet. 1995. Immobilization of fungal spores by adhesion. Bioetchnol. Bioeng. 47:677-687. [ Links ]
Ghigo, J. M. 2003. Are There Biofilm-Specific Physiological Pathways Beyond A Reasonable Doubts?. Research in Microbiology. 154:1-8. [ Links ]
Gilbert, P. and H. Lappin-Scott. 2000. Biofilms: united they stand, divided they fall. Microbiol. Today 27:136-137. [ Links ]
Goldberg, J. 2002. Biofilms and antibiotic resistance: a genetic linkage. Trends Microbiol. 10:264. [ Links ]
Groboillot, A.; D. Boadi, D. Poncelet and R. Neufeld. 1994. Immobilization of cells for application in the food industry. Crit. Rev. Biotechnol. 14:75-102. [ Links ]
Gutierrez-Correa, M. 2003. Opinion on surface adhesion fermentation. Agri-Food Res. News 1:11-12. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1999. Cellulolytic enzyme production by fungal mixed culture solid substrate fermentation. Agro-food-Industry Hi-Tech 10:6-8. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1998. Xylanase production of fungal mixed culture solid substrate fermentation on sugar cane bagasse. Biotechnol. Let. 20:45-47. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1997. Production of cellulase on sugar cane bagasse by fungal mixed culture solid substrate fermentation. Biotechnol. Let. 19:665-667. [ Links ]
Gutierrez-Correa, M.; L. Portal, P. Moreno and R. P. Tengerdy. 1998. Mixed culture solid substrate fermentation of Trichoderma reesei with Aspergillus niger on sugar cane bagasse. Bioresource Technol. 68:173-178. [ Links ]
Hata, Y.; Ishida, H., Ichikawa, E., Kawaro, A., Suginami, K., and Imayasu, S. 1998. Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 207:127-134. [ Links ]
Hughes, H. B., R. Carzaniga, S. L. Rawlings, J. R. Green and R. O'Connell. 1999. Spore surface glycoproteins of Colletotrichum lindemuthianum are recognized by a monoclonal antibody which inhibits adhesion to polystyrene. Microbiology 145:1927-1936. [ Links ]
Jones, E. B. G. 1994. Fungal adhesion. Mycol. Res. 98:961-981. [ Links ]
Kasinath, A.; C. Novothy, K. Suobododud, K.C. Patel and V. Sasek. 2003. Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed bed reactor. Enzyme Microb. Technol. 32:167-173. [ Links ]
Keshavarz, T.; R. Eglin, E. Walker, C. Bucke, G. Holt, A. T. Bull and M. D. Lilly. 1990. The large-scale immobilization of Penicillium chrysogenum: Batch and continuous operation in an air-lift reactor. Biotechnol. Bioeng. 36:763-770. [ Links ]
King, S. J. and M. Shoda. 1999. Batch decolorization of molasses by suspended and immobilized fungus Geotricum candidum Dec 1. J. Bioscience Bioeng. 88:586-589. [ Links ]
Lejeune, P. 2003. Contamination of abiotic surfaces: what a colonizing bacterium sees and how to blur it. Trends Microbiol. 11:179-184. [ Links ]
Mankel, A.; K. Krausse and E. Kothe. 2002. Identification of a hydrophobin gene that is developmentally regulated in the ectomycorrhizal fungus Tricholoma terreum. Appl. Environ. Microbiol. 68:1408-1413. [ Links ]
Mitchell, D. A.; O. F. von Meien and N. Krieger. 2003. Recent developments in modeling of solid-state fermentation: heat and mass transfer in bioreactors. Biochem. Eng. J. 13:137-147. [ Links ]
Mitchell, D. A. 1992. Growth patterns, growth kinetics and the modelling of growth in solid-state cultivation. In "Solid Substrate Cultivation" (Doelle, H.W., D.A. Mitchell and C.E. Rolz, eds.), p.87-114. Elsevier Science Pub., New York. [ Links ]
Morris, C. E.; J.M. Monier and M. A. Jacques. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576. [ Links ]
Ningam, P. and D. Singh. 1996. Processing of agricultural wastes in solid state fermentation for cellulolytic enzymes production. J. Scientific Ind. Res. 55:457-463. [ Links ]
Norton, S. and J. Vuillemard. 1994. Food bioconversions and metabolic production using immobilized cell technology. Crit. Rev. Biotechnol. 14:193-224. [ Links ]
Ooijkaas, L. P., F. J. Weber, R. M. Buitelaar, J. Tramper and A. Rinzema. 2000. Defined media and inert supports: their potential as solid-state fermentation production systems. Trends Biotechnol. 18:356-360. [ Links ]
Oosthuizen, M. C., B. Steyn, J. Theron, P. Cosette, D. Lindsay, A. von Holy and V.S. Brözel. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770-2780. [ Links ]
O'Toole, G., H. B. Kaplay and R. Kolter. 2000. Biofilm formation as microbial development. Ann. Rev. Microbiol. 54:49-79. [ Links ]
Pakula, R. and A. Freeman. 1996. A new continuous biofilm bioreactor for immobilized oil-degrading filamentous fungi. Biotechnol. Bioeng. 49:20-25. [ Links ]
Panda, A. K., S. Mishra, V. S. Bisaria and S. S. Bhojwani. 1989. Plant cell reactors - a perspective. Enzyme Microb. Technol. 11:386-397. [ Links ]
Park, Y. H., W. T. Seo and J. R. Liu. 1990. Enhanced production of shikonin by Lithospermum erythrorhizon cells immobilized in polyurethane foam matrices. J. Ferment. Bioeng. 70:317-321. [ Links ]
Pece, S. and J. S. Gutkind. 2002. E-cadherin and hakai: signalling, remodeling or destruction. Nature Cell Biol. 4: E72-E74. [ Links ]
Purevdorj, B.; J. W. Costerton and P. Stoodley. 2002. Influence of hydrodynamics and cell signalingon the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68:4457-4464. [ Links ]
Ramage, G.; K. VandeWalle, J. L. López-Ribot and B. L. Wickes. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Let. 214:95-100. [ Links ]
Rao, S. R. and G. A. Ravishankar, 2002. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol. Adv. 20:101-153. [ Links ]
Reynolds, T. B. and G. R. Fink. 2001. Bakers' yeast, a model for fungal biofilm formation. Science 291:878-881. [ Links ]
Rickard, A. H.; P. Gilbert, N. J. High, P. E. Kolenbrander and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11:94-100. [ Links ]
Riefler, R. G., D. P. Ahlfeld and B. F. Smets. 1998. Respirometric assay for biofilm kinetics estimation: parameter identifiability and retrievability. Biotechnol. Bioeng. 57:35-45. [ Links ]
Rodgers, M.; X-M. Zhan and B. Gallagher. 2003. A pilot plant study using vertical moving biofilms process to treat municipal wastewater. Bioresource Technol. 89:139-143. [ Links ]
Rouxhet, P. G. and N. Mozes. 1990. Physical chemistry of the interface between attached micro-organisms and their support. Wat. Sci. Tech. 22:1-16. [ Links ]
Sauer, K. 2003. The genomics and proteomics of biofilm formation. Genome Biol, 4:219. [ Links ]
Scholtmeijer, K., J. G. H. Wessels and H. A. B. Wösten. 2001. Fungal hydrophobins in medical and technical applications. Appl. Microbiol. Biotechnol. 56:1-8. [ Links ]
Scholtmeijer, K., M. I. Janssen, B. Gerssen, M. L. de Vocht, B. M. van Leeuwen, T. G. van Kooten, H. A. B. Wösten and J. H. Wessels. 2002. Surface modifications created by using engineered hydrophobins. Appl. Environ. Microbiol. 68:1367-1373. [ Links ]
Schoolnik, G. K. and F. H. Yildiz. 2000. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and two lifestyles. Genome Biology I(3):reviews 1016.1-1016.3. [ Links ]
Schwartz, M. A. and M. H. Ginsberg. 2002. Networks and crosstalk: integrin signalling spreads. Nature Cell Biol. 4:E65-E68. [ Links ]
Soccol, C. R. and L. P. S. Vandenberghe. 2003. Overview of applied solid-state fermentation in Brazil. Biochem. Eng. J. 13:205-218. [ Links ]
Stickler, D. 1999. Biofilms. Curr. Op. Microbiol. 2: 270-275. [ Links ]
Stoodley; P., S. Yang, H. Lappin-Scott and Z. Lewandowski. 1997. Relationship between mass transfer coefficient and liquid flow velocity in heterogenous biofilms using microelectrodes and confocal microscopy. Biotechnol. Bioeng. 56:681-688. [ Links ]
Sundstrom, P. 1999. Adhesins in Candida albicans. Curr. Opinion Microbiol. 2:353-357. [ Links ]
Suryanarayan, S. 2003. Current industrial practice in solid state fermentations for secondary metabolite production: the Biocon India experience. Biochem. Eng. J. 13:189-195. [ Links ]
Tengerdy, R. P. and G. Szakacs. 2003. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 13:169-179. [ Links ]
Tengerdy, R. P. 1996. Cellulase production by solid substrate fermentation. J. Scientific Ind. Res. 55:313-316. [ Links ]
Vallim, M. A., Janse, B. J. H., Gaskell, J., Pizzirani-Kleiner, A.A., and Cullen, D. 1998. Phanerochaete chrysosporium cellobiohydrolase and cellobiose dehydrogenase transcripts in wood. Appl. Environ. Microbiol. 64:1924-1928. [ Links ]
Van Driessel, B. and L. Christov. 2001. Decolorization of bleach plant effluent by Mucoralean and white-rot fungi in a rotating biological contactor reactor. J. Bioscience Bioeng. 92:271-276. [ Links ]
Venkatasubramanian, K.; S. B. Karkare and W.R. Vieth. 1983. Chemical engineering analysis of immobilized-cell systems. Appl. Biochem. Bioeng. 4:311-349. [ Links ]
Viniegra-González, G., E. Favela-Torres, C. N. Aguilar, S. J. Romero-Gómez, G. Díaz-Godínez and C. Augur. 2003. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 13:157-167. [ Links ]
Villena, G. K., Moreno, P. and Gutierrez-Correa, M. 2001. Cellulase production by fungal biofilms on polyester cloth. Agro-food-Industry Hi-Tech Jan./Feb., pp. 32-35. [ Links ]
Villena, G. K. and M. Gutierrez-Correa. 2003. Biopelículas de Aspergillus niger para la producción de celulasas: algunos aspectos estructurales y fisiológicos. Rev. Peru. Biol. 10:78-87. [ Links ]
Watnick, P. and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [ Links ]
Webb, J.S., H.C. Van der Mei, M. Nixon, I.M. Eastwood, M. Greenhalgh, S.J. Read, G.D. Robson and P.S. Handley. 1999. Plasticizers increase adhesion of the deteriogenic fungus Aureobasidium pullulans to polyvinyl chloride. Appl. Environ. Microbiol. 65: 3575-3585. [ Links ]
Winpenny, J. 2000. An overview of biofilms as functional communities. In "Community Structure and Cooperation in Biofilms" (D.G. Allison, P. Gilbert, H. Lappin-Scott and M. Wilson, eds.), Cambridge Univ. Press, U.K. [ Links ]
Wosten, H. and J. Willey. 2000. Surface active proteins enable microbial aerial hyphae to grow in the air. Microbiology 146:767-773. [ Links ]
*Laboratorio de Micología y Biotecnología, Universidad Nacional Agraria La Molina, Apartado 456 Lima 1, Perú.
E-mail Marcel Gutiérrez-Correa: mgclmb@lamolina.edu.pe
Surface adhesion fermentation: a new fermentation category
Fermentación por adhesión a superficies: una nueva categoría fermentativa
Marcel Gutiérrez-Correa* y Gretty K. Villena
Laboratorio de Micología y Biotecnología, Universidad Nacional Agraria La Molina
Presentado: 22/12/2003
Aceptado: 29/12/2003
Abstract
Basic knowledge on solid state fermentation and on biofilm formation is summarized and related to cell adhesion processes. These subjects are covered from the engineering and molecular biology points of view. Contrary to the common believe, the advantages of solid state fermentation are related to the adhesion of fungi to solid particles instead of being due to the low water content. Thus, solid state fermentation and biofilm fermentation (erroneously known as adsorption immobilization) are technical variants of the same biological process, and should be referred as Surface Adhesion Fermentation.
Keywords: Biofilms, solid substrate fermentation, differential gene expression, fungi.
Resumen
Se resume el conocimiento básico sobre la fermentación en estado sólido y la formación de biopelículas y se relaciona con los procesos de adhesión celular, cubriendo puntos de vista de ingeniería y de biología molecular. Contrariamente a la creencia común, la ventaja de la fermentación en estado sólido está relacionada a la adhesión de los hongos a partículas sólidas y no al bajo contenido de agua. Por lo tanto, la fermentación en estado sólido y la fermentación en biopelículas (erradamente conocida como inmovilización por adsorción) son variantes técnicas del mismo proceso biológico y deben ser referidas como Fermentación por Adhesión a Superficies.
Palabras clave: Biopelículas, fermentación en sustrato sólido, expresión diferencial de genes, hongos.
Introduction
Many of the traditional fermented food are based on the koji process that belongs to a major technical fermentation category known as "solid substrate (state) fermentation" (SSF) differing from either submerged or immobilization fermentations in the amount of free water that they contain. For a long time it has been considered that the advantages of SSF are due to water limitation of the system so that a higher product concentration and volumetric productivity are attained. However, scale up of SSF processes is the main drawback for a more ample industrial application. On the other hand, related to fungal enzyme production, submerged fermentation (SF) is the process of choice for industrial operations due to the very well known engineering aspects such fermentation modeling, bioreactor design and process control. Also, it has been considered that there would not be biochemical differences between an enzyme produced by the same fungal strain in either SSF or SF and most of the biochemical information on some important fungal enzymes comes from submerged cultures. Enzyme market in 2005 will be between 1,700 to 2,000 million dollar and thus it represents a very important biotechnological sector in which any technological development would be rapidly adopted (Bhat, 2000; Demain, 2000).
Nowadays, biofilm processes used mainly for waste water treatment are also being considered for metabolite and enzyme production. Although fungal biofilms are less known than bacterial biofilms, they can be used for enzyme production as it has been recently showed (Villena et al., 2001). As it will be stated in the present paper, both SSF and biofilm fermentation (BF) depend on surface adhesion and a new fermentation category was recently established. Surface adhesion fermentation (SAF) was proposed by Gutierrez-Correa (2003) as this new category and it will be sustained it further in this paper.
Solid State Fermentation Overview
As mentioned above, SSF is a process used for the production of fermented food, animal feed, fuel, enzymes, pharmaceuticals, which involves the growth of microorganisms (mainly fungi) on moist solid substrates in the absence of free-flowing water. SSF processes exhibit several advantages over SF, including improved product characteristics, higher product yields and productivities, easier product recovery and reduced energy requirements. Also, mixed culture SSF for enzyme production give higher yields than single culture SSF as it can be seen in figure 1 (Castillo et al., 1994; Dueñas et al., 1995; Gutierrez-Correa and Tengerdy, 1997, 1998, 1999; Gutierrez-Correa et al., 1998).
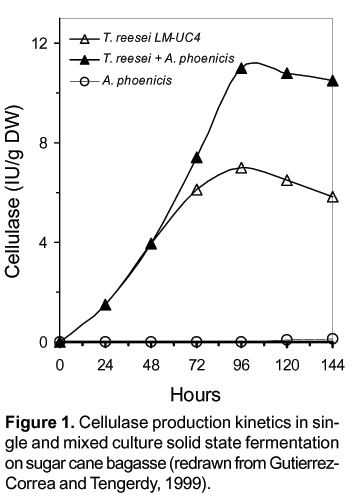
Since SSF processes have been used for centuries there is a great number of references and many excellent reviews have also been published (Doelle et al., 1992; Soccol et al., 2003; Suryanarayan, 2003; Tengerdy, 1996).
Regarded to the solid support used for SSF, two main types of processes are used. Firstly, SSF processes that use natural solid substrates like starch- or (lingo)cellulose residues or agro-industrial sources such as grains, and grain by-products, cassava, potato, rice, beans and sugar beet pulp. In theses cases substrate is used also as the source of carbon and nutrients for microbial growth (Ningam and Singh, 1996; Tengerdy and Szakacs, 2003). Secondly, SSF processes that use inert natural or artificial solid supports like sugarcane bagasse, perlite, amberlite, polyurethane foam and others. In these latter processes support is used only as an attachment structure for the microorganism. From the engineering point of view inert supports are better because they do not change their geometric and physical characteristics due to the microbial growth, allowing a better control of heat and mass transfer (Ooijkaas et al., 2000).
Enzyme production by SSF is not of wide use by manufacturers mainly due difficulties in bioreactor design, although the advantages of this form of microbial cultivation as compared to SF are well known. Growth patterns in SSF have been detailed in only few cases and these can be summarized in two phases: a) germination, germ tube elongation and mycelial branching to cover loosely most of the substrate; b) increase in mycelial density with aerial and penetrative hyphae development (Mitchell, 1992). However, as it will be described below, a previous phase namely spore adhesion to surfaces has been forgotten by most researchers. Recently, a general approach to compare productivity of fungal enzymes using SSF and SF techniques was attempted by using logistic and Luedeking-Piret equations (Viniegra-González et al., 2003). Also, many attempts to develop mathematical models for SSF have been done and current models are more rational tanking into account not only mass and heat transport processes across the substrate bed (macroscale models) but also phenomena that occur on and within individual particles (microscale models) (Mitchell et al., 2003).
Several SSF bioreactor models have been designed following two general categories: laboratory-scale and pilot and industrial-scale (Durand, 2003; Fasidi et al., 1996). Many designs have been published that belong to the first category including static and agitated models but only few models are used in commercial production. We designed and built an agitated 50L semi-pilot computer-controlled SSF bioreactor with an evaporative heat control system and a variable path helical-ribbon stirrer Figure 2; unpublished results). In general, however, many types of SSF bioreactors can be run at the bench scale level with small quantities of substrate but their scale-up is difficult due to heat generation and heterogeneity with natural supports.
Biofilm fermentation overview
The concept of a biofilm presumes either a population or a community of microorganisms living attached to a surface. Biofilms can be developed on either biotic or abiotic surfaces from a single species or as a community derived from several species (David and O'Toole, 2000; Fenchel, 2002). This way of growth is the prevailing lifestyle of microorganisms including bacteria, yeast, filamentous fungi and even micro-animals (Armstrong et al., 2001; Gilbert and Lappin-Scott, 2000; Morris et al., 1997; Watnick and Kolter, 2000). However, there is a strong tendency in microbiology and in the scientific literature to consider within the above concept of biofilms to those developed by bacteria in complex natural communities or those developed by bacteria and yeast in natural or artificial environments of medical relevance that are responsive of persistent infections (O'Toole et al., 2000. Rickard et al., 2003; Sauer, 2003; Stickler, 1999; Wimpenny, 2000). It should be noted that adhesion and subsequent differential gene expression to generate phenotypes distinct from those of free living organisms are two unifying processes of the biofilm concept (Ghigo, 2003; O'Toole et al., 2000). Also, biofilms have been used for a long time in water treatment facilities where they were called slime, mats or sludge, but not other practical used was seen until recently. This has brought that most of the available information is on bacterial and, in recent years, on yeast biofilms.
Filamentous fungi are naturally adapted to growth on surfaces and in these conditions they show a particular physiological behavior which it is different to that in submerged culture; thus, they can be considered as biofilm forming organisms according to our former concept. Differential physiological behavior of most attached fungi corresponds principally to a higher production and secretion of enzymes and also to a morphological differentiation which is absent in submerged cultures (Akao et al., 2002; Biesebeke et al., 2002). The advantages of this form of growth have been industrially exploited by two culture systems: SSF and cell immobilization although there is a lack of knowledge on the molecular basis of growth on surfaces.
Technology of cell immobilization was highly developed during the last two decades based on the operative advantages in the productive process instead of physiological issues. Natural adsorption on solid supports is an immobilization technique that it has been used with filamentous fungi thus neglecting its study as a way of biofilm formation. Actually, once spores are adsorbed to the support they grow attached to it thus forming a film. We prefer the term biofilm fermentation (BF) instead of cell immobilization because the microbe is an active and differential entity. BF has been applied for the production of enzymes, amino acids, organic acids, alcohol, aromas and in bioconversion processes (Anderson, 1983; Groboillot et al., 1994; Norton and Vuillemard, 1994) as well as in bioremediation an effluent biotreatment (Burton, 2001; Doggett, 2000; King and Shoda, 1999; Kasinath et al., 2003; Rodgers et al., 2003; Van Driessel and Christov, 2001).
We have showed that Aspergillus and Trichoderma biofilms developed on polyester cloth produce higher cellulase titers than submerged cultures. A. niger biofilms produce 20% more cellulolytic activity than T. reesei biofilms and 140% more cellulolytic activity than submerged cultures (Villena et al., 2001) Figure 3. As in SSF, it has been possible to use mixed culture BF of Aspergillus niger and Trichoderma reesei for cellulase production Figure 4.
Due to the extended used of bacterial biofilms for wastewater treatment several mathematical models of growth kinetics have been developed including estimation of biokinetic parameters, mass transfer processes, flow velocity through biofilms (Benefield and Molz, 1985; Riefler et al., 1998; Stoodley et al., 1997). Also, the engineering treatment of cell immobilization including the type that actually correspond to biofilm according to our concept together with the availability of mathematical models of biofilm kinetics have paved the way for bioreactor design and process development (Vekatasubramanian et al., 1983). Fungal biofilm kinetic models need to be developed yet since they have only recently been realized as true biofilms instead of simple cell immobilization systems. Perhaps, the easy way to treat mathematically fungal biofilms is to adapt fungal pellet growth kinetics knowledge. An advantage of biofilms is that according to the type and form of the support almost all type of submerged bioreactors can be used, including the simple stirred tank, gas-lift, packed bed columns, rotating contactors and horizontal blade-stirrer bioreactor (Keshavarz et al., 1990; Pakula and Freeman, 1996). We have tested cellulase production by fungal biofilms in both a modified internal-loop air-lift reactor in which the riser tube was replaced by a spiral-wound polyester support and in a stirred-tank reactor in which a spiral-wound polyester support was attached to the shaft instead of turbines (unpublished results; Figure 5.
Cell adhesion as a biological process has been studied by cell biology particularly referred to animal cell. It has been considered it as a basic important processes for tissue and organ development that includes not only physical and chemical interactions but also vital actions such signaling and gene regulation. A number of surface protein receptor are present in animal cell including E-cadherins, integrins and others (Pece and Gutkind, 2002; Schwartz and Ginsberg, 2002). Cell adhesion is also present in plants and it may play a similar role as in animal organisms. Plant cell adhesion is desirable because of its positive effects on cell physiology and biochemistry particularly on secondary metabolite production. Hence controlled cell adhesion considered as a immobilization process (as it happened with fungi) is of interest from the process engineering point of view (Panda et al., 1989; Rao and Ravishankar, 2002). For instance, enhanced production of shikonin by Lithospermum erythrorhizon cells immobilized on polyurethane foam matrices (thus, a plant biofilm) has been attained in packed bed columns (Park et al., 1990).
Formation and maintenance of biofilms are dynamic processes that involve complex interactions of physical and biological processes. Physical properties of support like hydrophobicity, electrostatic charge and surface roughness are important at the initial adhesion step of bacteria, yeast and filamentous fungi (Bigerelle et al., 2002; Cunliffe et al., 1999; Dufrene, 2000; Webb et al., 1999). The DLVO (for Dejarguin, Landau, Verwey, and Overbeek) theory of adhesion of colloidal particles considers the van der Waals attractive interactions and the electrostatic interactions between the surfaces (Gerin et al., 1995; Rouxhet and Mozes, 1990). For the adhesion of microbial cells, the DLVO theory is usefully completed by considering the influence of cell wall macromolecules which can either bridge the cell to the surface or prevent adhesion by steric hindrance.
In bacteria cell structures like fimbriae, pili and flagella participate in the process of recognition and colonization of surfaces and extracellular polysaccharide production stabilizes adhesion (Davey and O'Toole, 2000; Ghigo, 2003; Lejeune, 2003; Purevdorj et al., 2002; Stickler, 1999; Watnick and Kolter, 2000). In yeasts, adhesion depends on the expression of surface glycoproteins, floculins or adhesins, that modify the properties of cell walls and they are differentially expressed during haploid and diploid stages, or in dimorphic yeast-like or hyphal-like stages, and also the production of extracellular polysaccharides is a quite common process (O'Toole et al., 2000; Reynolds y Fink, 2001; Sundstrom, 1999).
Adhesion in filamentous fungi is mediated principally by a class of small amphipathic proteins called hydrophobins that are produced by ascomycetes and basidiomycetes, and may also be produced by zygomycetes. Hydrophobins stabilize the adhesion of spores and mycelium to both natural and artificial hydrophobic surfaces resulting in morphogenetic signals (Mankel et al., 2002; Scholmeijer et al., 2001, 2002; Wosten y Willey, 2000). Other molecules like glycoproteins participate in adhesion as found in Colletotrichum lindemuthianum (Hughes et al., 1999). There is also evidence on the production of an extracellular matrix that enhance adhesion (Doss, 1999).
We have described the growth pattern of A. niger BF by using scanning electron microscopy. Biofilm formation can be divided into three phases: 1) adhesion, which is strongly increased by Aspergillus spore hydrophobicity; 2) initial growth and development phase from spore germination to surface colonization; 3) maturation phase in which biomass density is highly increased and an internal channel organization that assures medium flow through biofilm is clearly evident (Villena and Gutierrez-Correa, 2003). Based on early studies of Jones (1994) on fungal adhesion, we have summarized the stages of this process by including recent findings on cell signaling and differential gene expression, since the latter processes are most important in SSF and BF Figure 6.
The ample analytical study of bacterial biofilms has demonstrated that adhesion launches the expression of a set of genes that ends with the typical biofilm phenotype, particularly with an enhanced resistance to antimicrobial agents (Davey and O'Toole, 2000; Goldberg, 2002; Stickler, 1999; Watnick and Kolter, 2000). Differential expression implies the activation or repression of genes that comprise between 1% to 38% of the bacterial genome. In gram-negative bacteria like E. coli, differential expression comprises 230 genes while in Pseudomonas 1% of its genome is differentially expressed when growing as biofilm. In gram-positive bacteria like Bacillus it has been found that 519 genes are expressed during biofilm formation (Ghigo, 2003; Oosthuizen et al., 2002; Sauer, 2003; Schoolnik and Yildiz, 2000).
In yeast like Candida genes of the ALS (agglutinin-like) family that code for proteins related to adhesion to surfaces are differentially expressed. Likewise, it is possible that biofilm resistance to antimicrobial agents may be due to a higher expression of drug resistance gene families (Chandra et al., 2001). Moreover, Candida albicans biofilms are structured communities composed of a mixture of yeast cells and hyphal elements, suggesting a pivotal role for the dimorphic switch in the development of biofilms and the Efg1 regulator protein being responsible for the filamentous growth (Ramage et al., 2002). In Saccharomyces cerevisiae FLO11 gene has been identified as responsible for cell adhesion and the formation of aggregates and biofilm (Reynolds and Fink, 2001).
As mentioned above, biofilms of filamentous fungi have not been realized until recently thus biochemical and molecular information is lacking. However, as SSF processes have been traditionally related to water limitation and not to adhesion, it is considered that there are not biochemical differences between enzymes produced by SSF and those produced by SF; thus, work in this subject is scarce. Molecular comparison of cellulases produced by Trichoderma reesei in SF and SSF showed that those produced in SSF were strongly different from those produced in SF Figure 7; unpublished work), the latter being of low molecular weight.
Concluding remarks
Due to the lack of knowledge on the molecular biology of fungal biofilms we can only speculate that as in SSF a similar differential gene expression will be found in biofilm fermentation. Techniques of functional genomics will help in testing this hypothesis and if so biofilm fermentation would be more suited for the scale up to industrial operations than SSF since many of the engineering concepts of SF can be easily adapted to this type of fermentation. We should now consider a new major technical fermentation category, named "surface adhesion fermentation" (SSF and biofilm fermentation).
Acknowledgement
G.K.Villena is supported by doctoral scholarships from INCAGRO and CONCYTEC.
Literature cited
Akao, T.; K. Gomi, K. Goto, N. Okazaki and O. Akita. 2002. Subtractive cloning of cDNA from Aspergillus oryzae differentially regulated between solid-state culture and liquid (submerged) culture. Curr. Genet. 41:275-281. [ Links ]
Anderson, J. G. 1983. Immobilized cell and film reactor systems for filamentous fungi. In "The Filamentous Fungi" (J.E. Smith, D.R. Berry, B. Kristiansen, eds.), Vol. 4, Chapter 6, E. Arnold, London. [ Links ]
Armstrong, E.; L. Yan, K.G. Boy, P.C. Wright and J.G. Burgess. 2001. The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461:37-40. [ Links ]
Asther, M.; M. Haon, S. Roussos, E. Record, M. Delattre, L. Lesage-Meessen, M. Labat and M. Asther. 2002. Feruloyl esterase from Aspergillus niger: a comparison of the production in solid state and submerged fermentation. Process Biochem. 38:685-691. [ Links ]
Benefield, L. and F. Molz. 1985. Mathematical simulation of a biofilm process. Biotechnol. Bioeng. 27:921-931. [ Links ]
Bhat, M. K. 2000. Cellulases and related enzymes in Biotechnology. Biotechnol. Adv. 18:355-383. [ Links ]
Biesebeke, R.; G. Ruijter, Y. S. P. Rahardjo, M. J. Hoogschagen, M. Heerikhuisen, A. Levin, K. G. A. van Driel, M.A.L. Schutyser, J. Dijksterhuis, Y. Zhu, F. J. Weber, W. M. de Vos, K. A. M. J. J. van den Hondel, A. Rinzema and P. Punt. 2002. Aspergillus oryzae in solid-state and submerged fermentations: progress report on a multidisciplinary project. FEMS Yeast Res. 2:245-248. [ Links ]
Bigerelle, M.; K. Anselme, E. Dufresne, P. Hardouin and A. Lost. 2002. An unscaled parameter to measure the order of surfaces: a new surface elaboration to increase cells adhesion. Biomolec. Engineering 19: 79-83. [ Links ]
Busrton, S. G. 2001. Development of bioreactors for application of biocatalysts in biotransformations and bioremediation. Pure App. Chem. IUPAC 73:77-83. [ Links ]
Castillo, M. R.; M. Gutierrez-Correa, J. C. Linden and R. P. Tengerdy. 1994. Mixed culture solid substrate fermentation for cellulolytic enzyme production. Biotechnol. Let. 16:967-972. [ Links ]
Chandra, J.; D. M. Jun, P. K. Mukherjee, L. L. Hoyer, T. McCormick and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture and drug resistance. J. Bacteriol. 183:5385-5394. [ Links ]
Cunliffe, D.; C. A. Smart, C. Alexander and E. N. Vulfson. 1999. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 65:4995-5002. [ Links ]
Davey, M. E. and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Molec. Biol. Rev. 64:847-867. [ Links ]
Demain, A. L. 2000. Microbial Biotechnology. Trends Biotechnol. 18:26-31. [ Links ]
Doelle, H. W.; D. A. Mitchell and C. E. Rolz (eds.). 1992. Solid Substrate Cultivation. Elsevier Science Pub., New York. [ Links ]
Dogget, M. S. 2000. Characterization of fungal biofilms within a municipal water distribution system. Appl. Environ. Microbiol. 66:1249-1251. [ Links ]
Doss R. P. 1999. Composition and enzymatic activity of the extracellular matrix secreted by germlings of Botrytis cinerea. Appl. Environ. Microbiol. 65:404-408. [ Links ]
Dueñas, R.; R. P. Tengerdy and M. Gutierrez-Correa. 1995. Cellulase production by mixed fungal solid substrate fermentation of sugar cane bagasse. World J. Microbiol. Biotechnol. 11:333-337. [ Links ]
Dufrene, Y. F. 2000. Direct characterization of the physicochemical properties of fungal spores using functionalized AFM probes. Biophysical J. 78:3286-3291. [ Links ]
Durand, A. 2003. Bioreactor designs for solid state fermentation. Biochem. Eng. J. 13:113-125. [ Links ]
Fasidi, I. O.; O. S. Isikhuemhen and F. Zadrazil. 1996. Bioreactors for solid state fermentation of lignocellulosics. J. Scientific Ind. Res. 55: 450-456. [ Links ]
Fenchel, T. 2002. Microbial behavior in a heterogeneous world. Science 296:1068-1071. [ Links ]
Gerin, P.; M.N. Bellon-Fontaine, M. Asther and P.G. Rouxhet. 1995. Immobilization of fungal spores by adhesion. Bioetchnol. Bioeng. 47:677-687. [ Links ]
Ghigo, J. M. 2003. Are There Biofilm-Specific Physiological Pathways Beyond A Reasonable Doubts?. Research in Microbiology. 154:1-8. [ Links ]
Gilbert, P. and H. Lappin-Scott. 2000. Biofilms: united they stand, divided they fall. Microbiol. Today 27:136-137. [ Links ]
Goldberg, J. 2002. Biofilms and antibiotic resistance: a genetic linkage. Trends Microbiol. 10:264. [ Links ]
Groboillot, A.; D. Boadi, D. Poncelet and R. Neufeld. 1994. Immobilization of cells for application in the food industry. Crit. Rev. Biotechnol. 14:75-102. [ Links ]
Gutierrez-Correa, M. 2003. Opinion on surface adhesion fermentation. Agri-Food Res. News 1:11-12. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1999. Cellulolytic enzyme production by fungal mixed culture solid substrate fermentation. Agro-food-Industry Hi-Tech 10:6-8. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1998. Xylanase production of fungal mixed culture solid substrate fermentation on sugar cane bagasse. Biotechnol. Let. 20:45-47. [ Links ]
Gutierrez-Correa, M. and R. P. Tengerdy. 1997. Production of cellulase on sugar cane bagasse by fungal mixed culture solid substrate fermentation. Biotechnol. Let. 19:665-667. [ Links ]
Gutierrez-Correa, M.; L. Portal, P. Moreno and R. P. Tengerdy. 1998. Mixed culture solid substrate fermentation of Trichoderma reesei with Aspergillus niger on sugar cane bagasse. Bioresource Technol. 68:173-178. [ Links ]
Hata, Y.; Ishida, H., Ichikawa, E., Kawaro, A., Suginami, K., and Imayasu, S. 1998. Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 207:127-134. [ Links ]
Hughes, H. B., R. Carzaniga, S. L. Rawlings, J. R. Green and R. O'Connell. 1999. Spore surface glycoproteins of Colletotrichum lindemuthianum are recognized by a monoclonal antibody which inhibits adhesion to polystyrene. Microbiology 145:1927-1936. [ Links ]
Jones, E. B. G. 1994. Fungal adhesion. Mycol. Res. 98:961-981. [ Links ]
Kasinath, A.; C. Novothy, K. Suobododud, K.C. Patel and V. Sasek. 2003. Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed bed reactor. Enzyme Microb. Technol. 32:167-173. [ Links ]
Keshavarz, T.; R. Eglin, E. Walker, C. Bucke, G. Holt, A. T. Bull and M. D. Lilly. 1990. The large-scale immobilization of Penicillium chrysogenum: Batch and continuous operation in an air-lift reactor. Biotechnol. Bioeng. 36:763-770. [ Links ]
King, S. J. and M. Shoda. 1999. Batch decolorization of molasses by suspended and immobilized fungus Geotricum candidum Dec 1. J. Bioscience Bioeng. 88:586-589. [ Links ]
Lejeune, P. 2003. Contamination of abiotic surfaces: what a colonizing bacterium sees and how to blur it. Trends Microbiol. 11:179-184. [ Links ]
Mankel, A.; K. Krausse and E. Kothe. 2002. Identification of a hydrophobin gene that is developmentally regulated in the ectomycorrhizal fungus Tricholoma terreum. Appl. Environ. Microbiol. 68:1408-1413. [ Links ]
Mitchell, D. A.; O. F. von Meien and N. Krieger. 2003. Recent developments in modeling of solid-state fermentation: heat and mass transfer in bioreactors. Biochem. Eng. J. 13:137-147. [ Links ]
Mitchell, D. A. 1992. Growth patterns, growth kinetics and the modelling of growth in solid-state cultivation. In "Solid Substrate Cultivation" (Doelle, H.W., D.A. Mitchell and C.E. Rolz, eds.), p.87-114. Elsevier Science Pub., New York. [ Links ]
Morris, C. E.; J.M. Monier and M. A. Jacques. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576. [ Links ]
Ningam, P. and D. Singh. 1996. Processing of agricultural wastes in solid state fermentation for cellulolytic enzymes production. J. Scientific Ind. Res. 55:457-463. [ Links ]
Norton, S. and J. Vuillemard. 1994. Food bioconversions and metabolic production using immobilized cell technology. Crit. Rev. Biotechnol. 14:193-224. [ Links ]
Ooijkaas, L. P., F. J. Weber, R. M. Buitelaar, J. Tramper and A. Rinzema. 2000. Defined media and inert supports: their potential as solid-state fermentation production systems. Trends Biotechnol. 18:356-360. [ Links ]
Oosthuizen, M. C., B. Steyn, J. Theron, P. Cosette, D. Lindsay, A. von Holy and V.S. Brözel. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770-2780. [ Links ]
O'Toole, G., H. B. Kaplay and R. Kolter. 2000. Biofilm formation as microbial development. Ann. Rev. Microbiol. 54:49-79. [ Links ]
Pakula, R. and A. Freeman. 1996. A new continuous biofilm bioreactor for immobilized oil-degrading filamentous fungi. Biotechnol. Bioeng. 49:20-25. [ Links ]
Panda, A. K., S. Mishra, V. S. Bisaria and S. S. Bhojwani. 1989. Plant cell reactors - a perspective. Enzyme Microb. Technol. 11:386-397. [ Links ]
Park, Y. H., W. T. Seo and J. R. Liu. 1990. Enhanced production of shikonin by Lithospermum erythrorhizon cells immobilized in polyurethane foam matrices. J. Ferment. Bioeng. 70:317-321. [ Links ]
Pece, S. and J. S. Gutkind. 2002. E-cadherin and hakai: signalling, remodeling or destruction. Nature Cell Biol. 4: E72-E74. [ Links ]
Purevdorj, B.; J. W. Costerton and P. Stoodley. 2002. Influence of hydrodynamics and cell signalingon the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68:4457-4464. [ Links ]
Ramage, G.; K. VandeWalle, J. L. López-Ribot and B. L. Wickes. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Let. 214:95-100. [ Links ]
Rao, S. R. and G. A. Ravishankar, 2002. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol. Adv. 20:101-153. [ Links ]
Reynolds, T. B. and G. R. Fink. 2001. Bakers' yeast, a model for fungal biofilm formation. Science 291:878-881. [ Links ]
Rickard, A. H.; P. Gilbert, N. J. High, P. E. Kolenbrander and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11:94-100. [ Links ]
Riefler, R. G., D. P. Ahlfeld and B. F. Smets. 1998. Respirometric assay for biofilm kinetics estimation: parameter identifiability and retrievability. Biotechnol. Bioeng. 57:35-45. [ Links ]
Rodgers, M.; X-M. Zhan and B. Gallagher. 2003. A pilot plant study using vertical moving biofilms process to treat municipal wastewater. Bioresource Technol. 89:139-143. [ Links ]
Rouxhet, P. G. and N. Mozes. 1990. Physical chemistry of the interface between attached micro-organisms and their support. Wat. Sci. Tech. 22:1-16. [ Links ]
Sauer, K. 2003. The genomics and proteomics of biofilm formation. Genome Biol, 4:219. [ Links ]
Scholtmeijer, K., J. G. H. Wessels and H. A. B. Wösten. 2001. Fungal hydrophobins in medical and technical applications. Appl. Microbiol. Biotechnol. 56:1-8. [ Links ]
Scholtmeijer, K., M. I. Janssen, B. Gerssen, M. L. de Vocht, B. M. van Leeuwen, T. G. van Kooten, H. A. B. Wösten and J. H. Wessels. 2002. Surface modifications created by using engineered hydrophobins. Appl. Environ. Microbiol. 68:1367-1373. [ Links ]
Schoolnik, G. K. and F. H. Yildiz. 2000. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and two lifestyles. Genome Biology I(3):reviews 1016.1-1016.3. [ Links ]
Schwartz, M. A. and M. H. Ginsberg. 2002. Networks and crosstalk: integrin signalling spreads. Nature Cell Biol. 4:E65-E68. [ Links ]
Soccol, C. R. and L. P. S. Vandenberghe. 2003. Overview of applied solid-state fermentation in Brazil. Biochem. Eng. J. 13:205-218. [ Links ]
Stickler, D. 1999. Biofilms. Curr. Op. Microbiol. 2: 270-275. [ Links ]
Stoodley; P., S. Yang, H. Lappin-Scott and Z. Lewandowski. 1997. Relationship between mass transfer coefficient and liquid flow velocity in heterogenous biofilms using microelectrodes and confocal microscopy. Biotechnol. Bioeng. 56:681-688. [ Links ]
Sundstrom, P. 1999. Adhesins in Candida albicans. Curr. Opinion Microbiol. 2:353-357. [ Links ]
Suryanarayan, S. 2003. Current industrial practice in solid state fermentations for secondary metabolite production: the Biocon India experience. Biochem. Eng. J. 13:189-195. [ Links ]
Tengerdy, R. P. and G. Szakacs. 2003. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 13:169-179. [ Links ]
Tengerdy, R. P. 1996. Cellulase production by solid substrate fermentation. J. Scientific Ind. Res. 55:313-316. [ Links ]
Vallim, M. A., Janse, B. J. H., Gaskell, J., Pizzirani-Kleiner, A.A., and Cullen, D. 1998. Phanerochaete chrysosporium cellobiohydrolase and cellobiose dehydrogenase transcripts in wood. Appl. Environ. Microbiol. 64:1924-1928. [ Links ]
Van Driessel, B. and L. Christov. 2001. Decolorization of bleach plant effluent by Mucoralean and white-rot fungi in a rotating biological contactor reactor. J. Bioscience Bioeng. 92:271-276. [ Links ]
Venkatasubramanian, K.; S. B. Karkare and W.R. Vieth. 1983. Chemical engineering analysis of immobilized-cell systems. Appl. Biochem. Bioeng. 4:311-349. [ Links ]
Viniegra-González, G., E. Favela-Torres, C. N. Aguilar, S. J. Romero-Gómez, G. Díaz-Godínez and C. Augur. 2003. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 13:157-167. [ Links ]
Villena, G. K., Moreno, P. and Gutierrez-Correa, M. 2001. Cellulase production by fungal biofilms on polyester cloth. Agro-food-Industry Hi-Tech Jan./Feb., pp. 32-35. [ Links ]
Villena, G. K. and M. Gutierrez-Correa. 2003. Biopelículas de Aspergillus niger para la producción de celulasas: algunos aspectos estructurales y fisiológicos. Rev. Peru. Biol. 10:78-87. [ Links ]
Watnick, P. and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [ Links ]
Webb, J.S., H.C. Van der Mei, M. Nixon, I.M. Eastwood, M. Greenhalgh, S.J. Read, G.D. Robson and P.S. Handley. 1999. Plasticizers increase adhesion of the deteriogenic fungus Aureobasidium pullulans to polyvinyl chloride. Appl. Environ. Microbiol. 65: 3575-3585. [ Links ]
Winpenny, J. 2000. An overview of biofilms as functional communities. In "Community Structure and Cooperation in Biofilms" (D.G. Allison, P. Gilbert, H. Lappin-Scott and M. Wilson, eds.), Cambridge Univ. Press, U.K. [ Links ]
Wosten, H. and J. Willey. 2000. Surface active proteins enable microbial aerial hyphae to grow in the air. Microbiology 146:767-773. [ Links ]
Correspondencia
*Laboratorio de Micología y Biotecnología, Universidad Nacional Agraria La Molina, Apartado 456 Lima 1, Perú.
E-mail Marcel Gutiérrez-Correa: mgclmb@lamolina.edu.pe













