Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.11 n.1 Lima ene./jul. 2004
Revisión del género de arañas Caloctenus Keyserling, 1877 (Araneae, Ctenidae)
Diana Silva-Dávila*
Center for Biodiversity and Conservation, American Museum of Natural History.
Presentado: 02/07/2004
Aceptado: 20/07/2004
Abstract
Caloctenus Keyserling is examined and redefined to include small ground-dwelling spiders with an enhanced ventral spination beneath anterior tibiae/metatarsi -a combination of five-eight/six-seven paired-spines, pars cephalica well marked by a deep furrow, strongly convex pars thoracica, and body thickly coated with iridescent scales.
As relimited, Caloctenus comprises four species from northern South America; a key to identify them is provided. Males of C. aculeatus Keyserling and C. gracilitarsis Simon are described for the first time, as are two new species, C. carbonera from Venezuela, and C. oxapampa from Peru.
All other species described under this genus belong somewhere else. Caloctenus boetonensis Strand, C. celer Simon, and C. oreus Simon are transferred to Acantheis Thorell, 1891. Caloctenus fernandensis Simon is transferred to Africactenus Hyatt, 1954. Caloctenus penicilliger Simon and C. variegatus Bertkau are transferred to Enoploctenus Simon,1897. Caloctenus abyssinicus Strand is placed incertae sedis within Ctenidae.
Keywords: Taxonomy, spiders, Calocteninae, northern South America, neotropics, new species.
Resumen
Se examina y redefine el género Caloctenus Keyserling. Este grupo incluye arañas pequeñas que se caracterizan por tener el cefalotórax con las regiones cefálica y torácica bien delimitadas por surcos profundos; el cuerpo enteramente cubierto por escamas iridicentes y una combinación de 5-8/6-7 pares de espinas ventrales en las tibias/metatarsos del primer y segundo par de patas.
Caloctenus incluye cuatro especies del norte de Sudamérica, se proporciona una clave para identificarlas. Los ejemplares machos de C. aculeatus Keys. y C. gracilitarsis Simon se describen por primera vez. Se presenta dos nuevas especies: C. carbonera, de Venezuela y C. oxapampa, del Perú.
Otras especies previamente descritas bajo este género pertenecen a otros grupos. Caloctenus boetonensis Strand, C. celer Simon, y C. oreus Simon se transfieren al género Acantheis Thorell, 1891. Caloctenus fernandensis Simon se transfiere a Africactenus Hyatt, 1954. Caloctenus penicilliger Simon y C. variegatus Bertkau se transfieren a Enoploctenus Simon, 1897. Caloctenus abyssinicus Strand se considera incertae sedis en Ctenidae.
Palabras claves: Taxonomía, arañas, Calocteninae, norte de Sudamérica, neotrópico, nuevas especies.
Introduction
The family Ctenidae was created by Keyserling (1877) to include Ctenus Walckenaer, 1805 and three genera he was proposing for the first time: Oligoctenus, Acanthoctenus, and Caloctenus.
At one time (Pickard-Cambridge, 1897a-b; Simon, 1897a; Roewer, 1954; Bonnet, 1955), Caloctenus comprised as many as 16 species. Benoit (1974) justifiably removed five African species and placed them in Africactenus Hyatt, 1954. As currently listed, Caloctenus comprises eleven species (Table 1), of which six, including the type species, have been described from the Neotropics.
Since early in the history of ctenids (Keyserling, 1877; Simon, 1897a; Simon, 1909; Mello-Leitão, 1936), Caloctenus was placed in its own group and easily separated from other genera by its labium wider than long and about one third the length of the endites, a vaulted cephalothorax, seven pairs of spines beneath tibiae I-II, and anterior spinnerets not longer than the posterior ones.
Although none of these characters were unique to Caloctenus, at least the labium shape appeared to hold together a natural group; initially as the section Calocteneae (Simon, 1897a) and later recognized as the subfamily Calocteninae by various authors.
The monophyly of caloctenines was first tested (Silva Dávila, 1994) to examine the relationships among species of Caloctenus. Based on genitalic similarities, this hypothesis supported a sister-group relationship between C. aculeatus and C. gracilitarsis, and in turn, this clade sister to C. oxapampa plus C. carbonera. More recently (Silva Dávila, 2003), caloctenines were compared to a more comprehensive sample of the various ctenid groups. Both analyses (Silva Dávila, 1994, 2003) showed that caloctenines, as delimited in the literature, were polyphyletic; however, both sets of data strongly supported one distinctive clade, which was recognized as Calocteninae sensu stricto.
Caloctenines comprise five genera from South America, Sri Lanka, the Seychelles Islands, and Madagascar. Although this group is strongly supported by seven synapomorphies, all but one (long and thick anal setae) are subject to some degree of homoplasy. The phylogenetic relationships within this group are being re-examined with the description of several new species from Sri Lanka and Madagascar.
Both hypotheses (Silva Dávila, 1994, 2003) show a sister group relationship between Caloctenus and Gephyroctenus Mello-Leitão, 1936. The latter is a more speciose taxon restricted to the Amazonian lowland forests; a taxonomical revision of Gephyroctenus is also in progress (Brescovit, pers. comm.).
Interestingly, a new genus from Madagascar appears to be the sister group to the South American caloctenines. Luckly, exhaustive field work in recent years is providing a more complete set of data to re-examine the phylogenetic relationships among caloctenines and help to clarify their distribution patterns; which are so far, characterized by narrow endemisms at generic and species level.
Material and methods
Specimens were examined following standard procedures for spiders. All measurements are in millimeters. Species descriptions are based upon a single individual, locality noted in parentheses. Spines on legs III-IV appear to be highly variable, so only spination patterns for pedipalps and legs I-II are here described. When right male pedipalps are indicated, the images have been rotated as to appear left.
Tracheae were examined in one adult female of Caloctenus gracilitarsis. The dorsal cuticle of the abdomen was partly removed and the abdomen transferred to saturated KOH, boiled for 5-10 minutes to digest the soft tissue, rinsed in distilled water, stained in ethanol-chlorazol black solution, and finally transferred to 75 percent ethanol for examination with a stereomicroscope (Griswold, 1991).
A compound microscope with drawing tube/photographic camera was used to examine and draw further details of the male and female genitalia. Digital images were made with a Leica stereoscope adapted to a digital camera and put together with automontage software by Syncroscopy Ltd.
Abbreviations
AC,aciniform gland spigots; AER, anterior ocular row at their greatest width; AME, anterior median eyes; ALE, anterior lateral eyes; ALS, anterior lateral spinnerets; AN, annuli of subtegulum; BH, basal hematodocha; C, conductor; CD, copulatory duct; CO, copulatory opening; CY, cylindrical gland spigots; CyL, cymbial lobe; Eb, embolic base; Ep, ventral process of embolus base; FD, fertilization duct; LL, lateral lobes of epigynum; MA, tegular median apophysis; MAP, major ampullate gland spigots; mAP, minor ampullate gland spigots; mp, epigynal mating plug; MS, median sector of epigynum; N, nubbin; OQA, ocular quadrangle, width of anterior median eye row; OQL, ocular quadrangle length at their greatest distance from AME to PME in frontal view; OQP, ocular quadrangle, width of posterior median eye row; P, petiole; PI, piriform gland spigots; PME, posterior median eye; PLE, posterior lateral eye; PER, posterior ocular row at their greatest width; PLS, posterior lateral spinnerets; PMS, posterior median spinnerets; RTA, retrolateral tibial apophysis; S, spermatheca; ST, subtegulum; T, tegulum; Ta, tartipore; TP, tegular process; TS, tracheal spiracle; VL, tibial ventral lobe.
Museum Collections
AMNH - American Museum of Natural History, New York (N. Platnick); BMNH - Natural History Museum, London (J. Beccaloni, P. Hillyard); CAS - California Academy of Sciences, San Francisco (C. Griswold); MCZ - Museum of Comparative Zoology, Harvard University, Cambridge (G. Giribet, H. W. Levi, L. Liebensperger); MNHN - Museum National d'Histoire Naturelle, Paris (C. Rollard, J. Hertault); MUSM - Museo de Historia Natural, Lima (G. Lamas, D. Silva); MZS - Museo Zoologico de «La Specola», Florence (L. Bartolozzi); SM - Senckenberg Museum, Frankfurt am Main (M. Grasshoff); UCV Universidad Central de Venezuela (courtesy of A. Brescovit).
Taxonomy
Caloctenus Keyserling
Caloctenus Keyserling 1877: 682, 696 (type species, by original designation, Caloctenus aculeatus Keys., 1877:697, holotype female in BMNH, examined).- Roewer, 1954: 667.- Bonnet, 1955: 936.- Platnick, 2004.
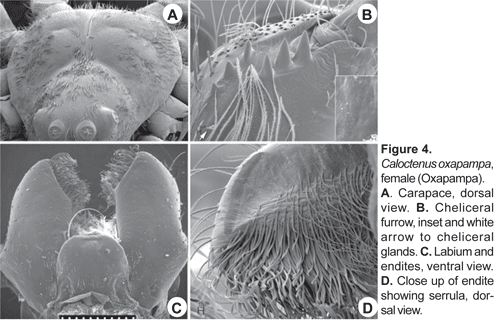
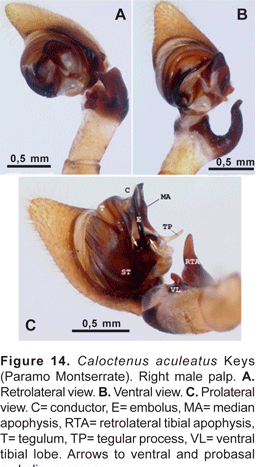
Diagnosis.- Males and females differ from other ctenids in the ventral spination on tibiae/metatarsi I-II, five to eight /six to seven pairs of long spines; broad carapace with deep transversal median and longitudinal lateral furrows (figs. 1A-C; 4A); dark-colored body coated with iridescent scales (figs. 2A-B, 3B-C, 4A); male palpal tibiae strongly sclerotized at apex and often with large ventral lobe (figs. 14 b-C, 16A, 19B-C, 22B-C), median apophysis with an apical beak (figs. 14A, 17B, 20B, 22D); and epigynal folds fused to various degrees (figs. 15A, 18A, 21A, 24A).
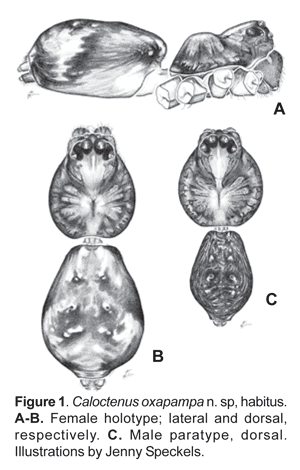
Description.- Small to medium-sized spiders, total length 3,10-7,17. Sexual dimorphism slight (figs. 1B-C). Live specimens show shades of green and brown (figs. 13A-B), in preserved specimens the carapace is brown, with a pale orange median band and dark brown radial streaks (figs. 1B-C, 2A-B); black pigment around eyes except ALE's; chelicerae light orange-brown, anterior surface with dark longitudinal reticulations, boss pale orange; labium light orange-brown at base, pale amber at tip; sternum pale yellow; legs and pedipalps pale orange with irregular dark brown reticulations (figs. 2A-B).
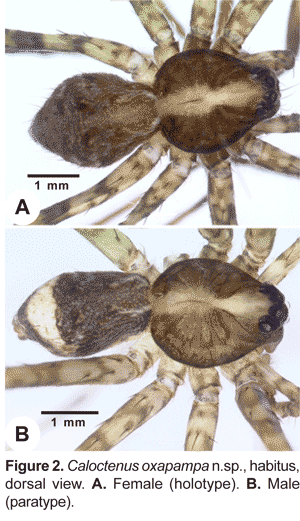
Carapace (figs. 1A-C, 2A-B, 3A) with pars cephalica well marked by deep furrows, pars thoracica strongly convex, thoracic fovea longitudinal and deep. Carapace length 0,81 to 1,32 times width. Ocular area dark and broad, eight eyes in three rows, 2-4-2 (figs. 1A-C; 3A), second row recurved; ALE oval, lenses greatly reduced (fig. 1A; 3A); OQP/OQA 1,28-1,70. Clypeus low, height 0,60 to 1,44 times AME diameter. Chelicerae stout, with conspicuous boss (fig. 1A); chilum absent; fang furrow with three promarginal and three to four retromarginal teeth (fig.4B). Labium 0,67-0,91 times wider than long (fig. 4C); endites converging slightly anteriorly (fig. 4C), length 1,60 to 2,73 times width; serrula subapical (fig. 4D). Sternum heart-shaped, length 1,15-2,51, width 1,18-2,69.
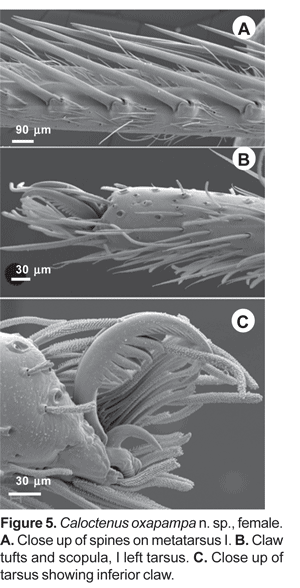
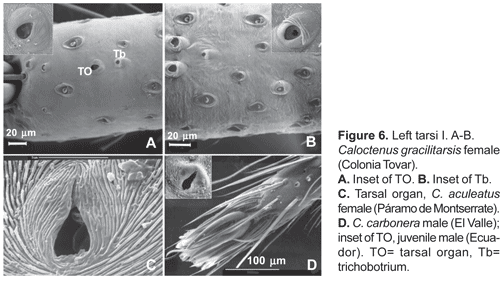
Legs slender, male femur I length/carapace width 1,02-1,78; leg formula 4123, occasionally 1423; trochanters deeply notched. Anterior femora with a row of prolateral spines on greatly enlarged sockets (fig. 3D); tibiae and metatarsi I-II have long, overlapping spines on enlarged sockets (fig. 5A). Femora of legs and pedipalps with dorsal and lateral spines, ventral spinules may be present; palpal patella with dorsal and lateral spines, patellae I-IV frequently with one dorsal spine; tibiae I-II with 5-8 ventral-paired spines, rarely with one to two pairs of lateral spines; metatarsi I-II with 6-7 paired-ventral spines, rarely with one to two pairs of lateral spines; tibiae III-IV with variable number of dorsal, lateral, and ventral spines. Tarsal scopula sparse (fig. 5B). Two or three tarsal claws (fig. 5C), with ridged surface; superior tarsal claws pectinate with 6 to 9 teeth, inferior tarsal claw smooth (fig. 5C). Claw tuft hairs sparse (figs. 5B-C). Tarsal organ capsulate and seemingly sexually dimorphic (fig. 6A-D). Tarsi with two to three irregular rows of trichobothria; trichobothrial hood with two to four transverse ridges (fig.6B).
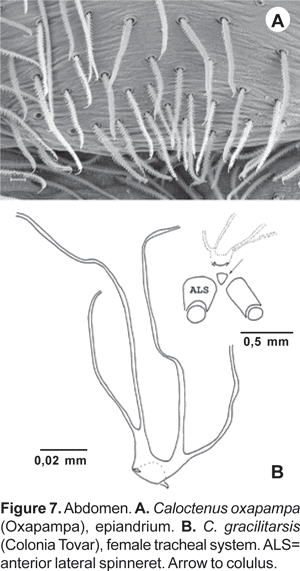
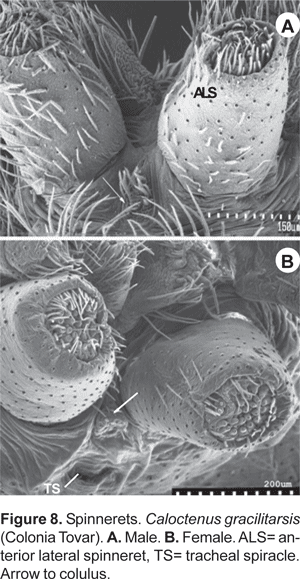
Abdomen widest posteriorly (figs. 1B-C), coated with iridescent scales, sparse white plumose hairs, club-shaped red hairs, and macrosetae (figs. 2A-B, 3B-C). Epiandrium lacking spigots (fig. 7A) Tracheal system (fig. 7B); consisting of a pair of slender tubes nearly as long as abdomen, and a lateral shorter branch arising from each tube, tracheal spiracle closer to colulus (fig. 7B, 8B); colulus a short, hairy lobe (figs. 7B, 8A-B); anal tubercle with long, thick ventral setae (fig. 9A).
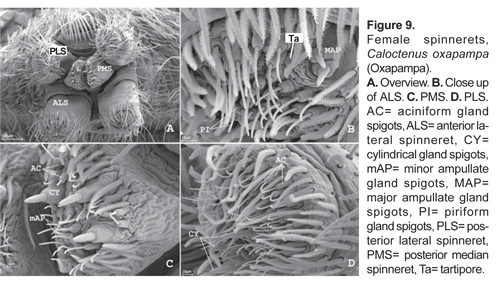
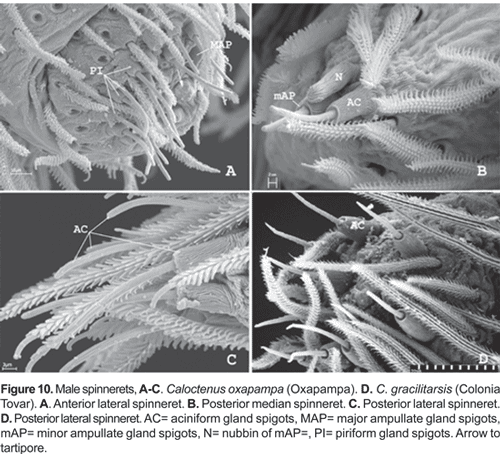
Six spinnerets (fig. 9A). Female ALS (fig. 9B) with 2 major ampullate gland spigots, a tartipore, and more than 20 piriform gland spigots; PMS (fig. 9C) with two minor ampullate gland spigots, three-four cylindrical gland spigots, and more than 20 aciniform gland spigots; PLS (fig. 9D) with three-four cylindrical gland spigots and more than 20 aciniform gland spigots. Male ALS (fig. 10A) with single major ampullate gland spigot, nubbin, tartipore, and more than 20 piriform gland spigots; PMS (fig. 10B) with single minor ampullate gland spigot, nubbin, and reduced field of aciniform gland spigots; PLS (figs. 10C-D) with reduced field of aciniform gland spigots.
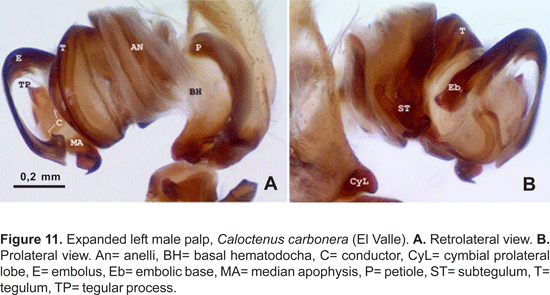
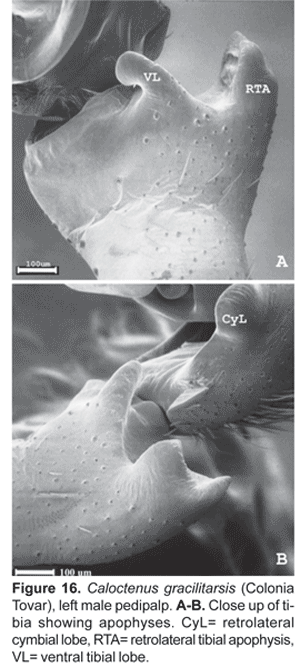
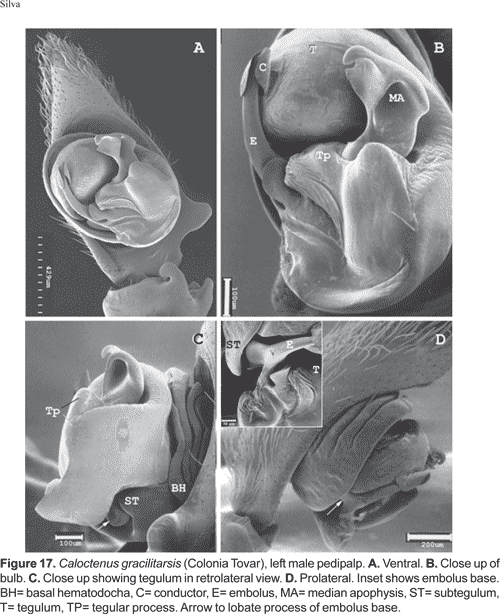
Male palpal tibia with large retroapical apophysis and ventral lobe (figs. 14 B-C, 16A, 19B-C, 22B-C). Cymbium projecting prolaterally into a small basal lobe (fig. 11B), variously produced retrolaterally (figs. 14A-B, 17A, 19B, 20A, 22A), petiole nearly as large as alveolus (fig. 11A); subtegulum cup-shaped, with 4-5 annuli (figs. 11A-B), prolaterally with a basal process (fig. 11B); tegulum often variously produced at embolus and median apophysis base (figs. 14B, 17A-B, 19B); ST-T locking mechanism conspicuous when partly or totally expanded (figs. 11B, 22D); embolus arising on prolateral side of tegulum, coniform (fig. 17A) or spiral-like (fig. 20A), embolic base projecting prolaterally into a basal process (figs. 11B, 22D); median apophysis with an apical beak, often thin and translucent (figs. 17B, 22D); small, hyaline conductor, arising from mesoapex of tegulum (fig. 17A, 20B, 23A); sperm duct simple, encircles tegulum (figs. 11A, 14B, 19A, 22A-B).
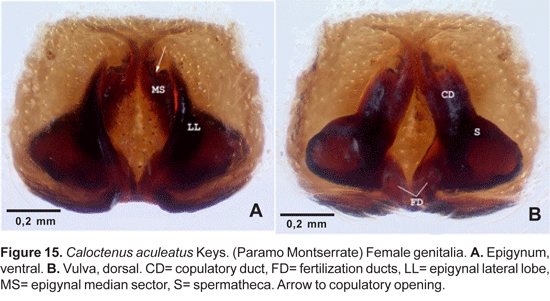
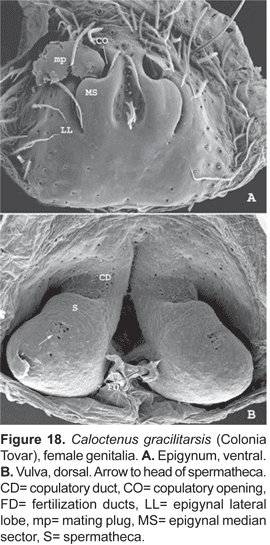
Epigynum either divided into median sector and lateral lobes (fig. 15A) or median sector partly (fig. 18A) or entirely fused to lateral lobes (figs. 21A, 24A); lateral lobes lacking teeth; vulva with copulatory ducts somewhat elongated (figs. 15B, 18B), slightly convoluted (fig. 21B), or very short (figs. 24B, 25B). Copulatory duct openings either antero-mesal (figs. 15A, 18A), antero-lateral (fig. 21A), or posterior (fig. 24A, 25A); spermathecal head defined by few large pores (figs. 18B, 25B); fertilization ducts posterior (figs. 15B, 18B, 25B), or antero-mesal (fig. 21B).
Composition.- Four species, two of them are new.
Misplaced species.- An examination of the type specimens of Caloctenus celer Simon and Caloctenus penicilliger Simon shows they belong somewhere else. Caloctenus penicilliger belongs in Enoploctenus Simon, while C. celer is a member of Acantheis Thorell. The type specimens of Caloctenus distinctus Caporiacco and C. luteovittatus Simon, are both immatures of Enoploctenus. The type specimen of Caloctenus variegatus Bertkau is lost and, according to Brescovit (in litt.), is a penultimate stage of a female Enoploctenus cyclothorax Bertkau. I have not been able to locate the type specimen of Caloctenus fernandensis Simon, an adult female; however, Simon's remarks (1910: 362) indicate that this species belongs in Africactenus Hyatt; no other African ctenid genus has an epigynum projecting anteriorly in two lobes and six pairs of ventral spines beneath tibiae I-II.
The type specimen of Caloctenus abyssinicus Strand is lost. The original description (Strand 1917: 41) is based on an immature female and does not allow a positive identification. Also, a cheliceral furrow with a combination of two prolateral and four retromarginal teeth is unusual for the family. Caloctenus abyssinicus is placed incertae sedis, within Ctenidae.
Natural history. Based on collection records of C. gracilitarsis and C. aculeatus, Simon (1897a:120) remarks that Caloctenus inhabited the highest mountains and cloud forests of South America. Labels of recent collections indicate these spiders are found at elevations over 1800 m, running about on the ground surface of cloud forests or adjacent habitats (roadsides).
Peck and Peck (collection labels), by using flight interception traps along altitudinal transects in Venezuela, found males of C. carbonera in rain forests at 1200 m as well as in cloud forests from 2250 to 2400 m. Brignoli (1972:81) records one female of C. gracilitarsis from a cave in Miranda, near Caracas (Venezuela); this record suggests a preference for dimly lit and humid environments.
Peruvian specimens were first collected at Oxapampa on the ground of a steep slope with secondary vegetation at about 1900 m; at present, the type locality has been replaced by eucalyptus trees and a water reservoir (fig. 12A). The type specimens were running at daytime in a lycosid-like fashion. Most recently, after a more exhaustive search only a few were found in the litter of a steep slope (fig. 12B-D).
They were found together with other ctenids (Ctenus s.l., Enoploctenus sp.) and various other spiders.
These cryptic spiders (fig. 13) are very fast runners. Also,it was observed that once annoyed, one female raised its first pair of legs from time to time.
Distribution. Known from Colombia, Ecuador, Peru, and Venezuela (Map 1). The record from Ecuador (Tungurahua: Rio Pastaza Canon [ca. 01° 15' S, 78° 30' W], 1700 m, 4.xi.1987. W. Clarke-Macintyre, AMNH), corresponds to a juvenile male.
Key to the species of Caloctenus
1a. Males.................................................................2
1b. Females...............................................................5
2a. Cymbium with large retrolateral projection (fig. 19B); palpal tibia shorter than cymbium (fig. 19A-C); retromargin of cheliceral furrow with 3 stout teeth
.......................................................................... carbonera
2b. Cymbium otherwise; palpal tibia as long or longer (figs. 14A, 22A) than cymbium (fig. 14A); retromargin of cheliceral furrow with 4 teeth (fig. 3B)
..........................................................................3
3a. Embolus base projecting into a large ventral process (figs. 22B-D, 23A-D), palpal tibia with retrobasal thorn (fig. 22A-B); all tarsi with 3 claws
..........................................................................oxapampa
3b. Embolus base otherwise; palpal tibia lacking modified spines, all tarsi with 2 claws
..........................................................................4
4a. RTA large, strongly curved, apex pointed outwards (fig. 14B); tegulum with narrow digitiform projection at median apophysis base (fig. 14B-C)
..........................................................................aculeatus
4b. RTA short, apex truncated, bifid (fig. 16A-B); tegulum not so projected (fig. 17A-B)
..........................................................................gracilitarsis
5a. Median sector and lateral lobes fused medially (fig.18A), tibia I with 5 pairs of ventral spines
..........................................................................gracilitarsis
5b. Median sector and lateral lobes otherwise, tibia I with more than 5 pairs of ventral spines
..........................................................................6
6a. Epigynal median sector arrowhead-shaped, inner margin of lateral lobes heavily sclerotized (fig. 15A)
..........................................................................aculeatus
6b. Epigynal median sector and lateral lobes otherwise; epigynum lightly sclerotized.
..........................................................................7
7a. Median sector and lateral lobes fused anteriorly (fig. 21A); fertilization ducts anterior (fig. 21B); all tarsi with two claws
..........................................................................carbonera
7b. Median sector and lateral lobes fused posteriorly (fig. 24A); fertilization ducts posterior (figs. 24B, 25B); all tarsi with three claws
..........................................................................oxapampa
Caloctenus aculeatus Keyserling
Caloctenus aculeatus Keys., 1877: 697 (female holotype from Bogota, Colombia, in BMNH, no. 2921, examined).- Roewer, 1954: 667.- Bonnet, 1955: 936.- Platnick, 2004.
Diagnosis. Males differ from other species in the narrow, digitiform tegular process at base of median apophysis (fig. 14A-C). Females can be recognized by the arrowhead-shaped epigynal median sector (MS) and lateral lobes (LL) with heavily sclerotized inner margins (fig. 14A).
Male (Paramo Montserrate). Total length 5,47. Carapace dark brown, markings typical. Abdomen dorsum and sides light gray-brown, 2 posterodorsal chevrons, venter yellow-gray. Carapace 2,59 long, 2,15 wide; ocular area 0,57 long, 0,99 wide; diameter of eyes AM:AL:PM:PL, 0,16:0,07:0,26:0,24; AME-AME 0,4 times AME diameter; PME separation 0,44 times PME diameter; clypeal height 0,20. Sternum 2,48 long, 2,52 wide; labium 0,33 long, 0,42 wide; endites 0,82 long, 0,40 wide. Femur I length 1,39 times carapace width. Spination: palpus -femur d0-1-1, p0-0-1, r0-0-1, patella d0-0-1, p1-0-0, tibia p1-1-0, r0-1-0; leg I -femur d0-1-1-0, p0-2-1-1, r0-1-0-1, v0-0-1-0, patella d0-0-1, tibia v2-2-2-2-2-2-1, metatarsus v2-2-2-2-2-1; leg II -femur d0-1-1-1, p1-1-1-0, r1-1-1-0, patella d0-0-1, tibia v2-2-2-2-2-2-2, metatarsus v2-2-2-2-2; leg III -femur d0-1-1-1, p1-1-1-0,r1-1-1-0, patella d0-0-1, tibia d0-0-1-0, p0-1-1-0, r0-1-1-0, v2-2-0-2, metatarsus d0-0-1-0, p1-1-0-1, r1-1-0-1, v0-1-1-1; leg IV -femur d1-1-0-1, p1-1-0-1, r1-0-1-1, patella d0-0-1, tibia d0-0-1-0, p0-1-1-0, r0-1-1-0, v2-2-0-0, metatarsus d0-1-0-0, p1-1-0-1, r1-1-0-1, v1-1-1-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 2,99 | 2,81 | 2,59 | 3,32 | 1,20 |
| Patella | 1,06 | 1,02 | 0,91 | 0,95 | 0,62 |
| Tibia | 3,25 | 2,73 | 2,30 | 3,07 | 0,51 |
| Metatarsus | 3,23 | 2,74 | 2,63 | 3,87 | — |
| Tarsus | 1,06 | 0,91 | 0,91 | 1,17 | 1,28 |
| Total | 11,59 | 10,21 | 9,34 | 12,38 | 3,61 |
Palpal tibia (figs. 14A-C) stout, with large ventral lobe, length 0,17 times femur I; RTA (figs. 14A-C) large, broad, and curved, directed outwards. Cymbium retrolaterally projecting into a small subbasal lobe (figs. 14A-B); tegulum with a slender hyaline process at median apophysis base (figs. 14B-C); MA origin retro-mesal (fig. 14B); embolus coniform, arising from a large sclerotized process on prolateral side of tegulum (fig. 14C); conductor (figs. 14A-C) hyaline, origin mesal.
Female (holotype).- Total length 4,71. Markings as in male except paler. Carapace 2,15 long, 1,79 wide, 0,68 high; ocular area 0,55 long, 0,91 wide, diameter of eyes AM:AL:PM:PL, 0,11:0,06:0,22:0,22; AME-AME nearly AME diameter; PME separation 0,68 times PME diameter; clypeal height 0,13; sternum 1,02 long, 1,02 wide; endites 0,68 long, 0,34 wide. Femur I 1,08 times carapace width. Leg III missing. Spination: palpus -femur d0-0 -2-1, p0-0-2-0, r0-0-1-0, patella d1-0-0-1, p1-0-0, tibia d1-0-0-1, p2-0-0-0, tarsus p2-1-0-0, r1-1-0-0; leg I -femur d0-1-1-1, p0-2-1-1, r1-1-1-1, patella d-0-0-1, tibia p0-0-1-1-0, r0-0-1-1-0, v2-2-2-2-2-2, metatarsus p0-0-1-1-0, r0-0-1-1-0, v2-2-2-2-2-2; leg II -femur d0-1-0-1-0-1, p2-1-1-1-1-1, r1-0-0-0-1-0, tibia v2-2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 1,93 | 1,86 | — | 2,23 | 0,77 |
| Patella | 0,73 | 0,77 | — | 0,66 | 0,40 |
| Tibia | 1,86 | 1,61 | — | 1,86 | 0,49 |
| Metatarsus | 1,72 | 1,57 | — | 2,48 | — |
| Tarsus | 0,53 | 0,51 | — | 0,75 | 1,31 |
| Total | 6,77 | 6,32 | — | 7,98 | 2,97 |
Epigynum (fig. 15A) with median sector shaped like an arrowhead; inner margins of lateral lobes heavily sclerotized, lateral lobes convex, slightly protruding anteriorly. Vulva (fig. 15B) with elongate copulatory ducts; spermathecae nearly rounded, head not defined; fertilization ducts posterior, directed upwards.
Material examined. Colombia: Santa Fe de Bogota [ca. 04° 35' N, 74° 04' W; 2619 m], 7.i.1890 (BMNH, no. 2921), female holotype; Paramo Montserrate (Weinmannia forest) [ca. 04° 37' N, 74° 02' W], xii.1978 (A. Bernal, MCZ 33292), 1 female; Paramo Montserrate, iv.1979 (A. Bernal, MCZ 30753), 1 male.
Distribution.- Known from Bogota, Colombia (Map 1).
Caloctenus gracilitarsis Simon
Caloctenus gracilitarsis Simon, 1897a: 496 (2 syntype females from Colonia Tovar (MNHN 11025) plus 2 syntype females and a juvenile male (11118) from Caracas, Venezuela.- Roewer, 1954: 667.- Bonnet, 1955: 936.- Caporiacco, 1955: 397 (male palp and epigynum illustrated, fig. 55).- Brignoli, 1972: 381 (female genitalia illustrated, figs. 39-40).- Platnick, 2004.
Diagnosis. Males are distinguished by the short, slightly bifid RTA (fig. 16A-B); tegulum projecting upwards at median apophysis base (fig. 17A-C). Females have a median sector with shallow hairy fossa and epigynal lobes fused at half epigynum length (fig. 18A).
Male (Colonia Tovar). Total length 5,79. Carapace light brown, markings typical. Abdomen pale brown, posteriorly with a yellowish transversal band. Carapace 2,80 long, 2,26 wide; ocular area 0,62 long, 0,97 wide; diameter of eyes AM:AL:PM:PL, 0,17:0,14:0,22:0,27; AME-AME 0,4 times AME diameter; PME separation 0,4 times PME diameter; clypeal height 0,24. Sternum 1,28 long, 1,32 wide; labium 0,34 long, 0,42 wide; endites 0,86 long, 0,39 wide. Femur I length 1,7 times carapace width. Spination: palpus -femur d0-1-1, p0-0-1, r0-0-1, patella p0-0-1, tibia p1-1-0, r0-1-0; leg I -femur d1-1-1-0, p1-0-2-0, r1-1-1-1, tibia d0-1-0-0-0, p0-0-1-1-0, r0-0-0-1-0, v0-2-2-2-2-2, metatarsus p0-0-0-0-1-1, r0-0-0-0-1-1, v2-2-2-2-2-2; leg II -femur d1-1-1-0, p1-1-0-1, r1-1-1-1, tibia d1-1-0-0-0, p0-0-1-1-0, r0-0-0-0-1, v0-2-2-2-2-2, metatarsus p0-0-0-0-1-1, r0-0-0-0-1-1, v2-2-2-2-2-2; leg III -femur d1-1-1-0, p1-1-1-0,r1-1-1-0, patella p0-1, tibia d1-1-0-0, p0-1-0-1, r0-1-0-1, v2-0-2-2, metatarsus d2-0-0-0, p0-0-1-1, r0-0-1-1, v1-2-2-2; leg IV -femur d1-1-0-1, p1-1-1-0, r1-1-1-1, patella d0-1, tibia d0-0-1-1, p0-1-0-0, r0-1-0-1, v2-0-2-2, metatarsus d2-0-0-0, p1-1-0-1, r1-0-1-1, v2-2-0-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 3,92 | 3,69 | 3,37 | 4,26 | 1,45 |
| Patella | 1,23 | 1,22 | 1,01 | 1,03 | 0,58 |
| Tibia | 4,17 | 3,66 | 3,33 | 4,02 | 0,71 |
| Metatarsus | 4,55 | 4,23 | 3,91 | 5,28 | — |
| Tarsus | 1,49 | 1,35 | 1,30 | 1,69 | 1,26 |
| Total | 15,36 | 14,15 | 12,92 | 16,28 | 4,00 |
Palpal tibia (fig. 16A-B) stout with apex heavily sclerotized and large ventral lobe, length 0,11 times femur I; RTA short, slightly bifid and convex (fig. 16A-B).
Cymbium projecting retrolaterally into short, subbasal lobe (fig. 17A); tegulum (fig. 17A-B) with small process at embolic base, median apophysis deeply excavated (fig. 17B-C); embolus (fig. 17A-B) coniform-like, arising prolateral, embolic base (fig. 17B, D) projecting into ventral and dorsal processes; conductor (fig. 17A-B, D) hyaline, short, originating from narrow base at mesoapex of tegulum, embraces embolic tip.
Female (syntype from Colonia Tovar). Total length 6,16. Markings typical, abdomen yellowish gray with 2 anterior bands, posterior transversal band split into gray spots. Carapace 2,52 long, 2,12 wide. Ocular area 0,60 long, 0,99 wide; diameter of eyes AM:AL:PM:PL, 0,13:0,11:0,22:0,24; AME-AME nearly AME diameter; PME separation 2 times PME diameter; clypeal height 0,18. Endites 0,86 long, 0,51 wide; labium 0,36 long, 0,42 wide; sternum 1,21 long, 1,26 wide. Femur I 1,3 times carapace width. Spination: palpus -femur d0-0-1-1, p0-0-0-1, r0-0-0-1, patella d0-0-1, p1-0-0-0, tibia d1-0-0-1, p2-0-0-0, tarsus p2-1-0-0, r1-1-0-0; leg I -femur d0-1-1-1, p0-0-2-1, r1-1-1-1, patella d0-0-1, tibia r0-1-1-0-0, v2-2-2-2-2, metatarsus r0-0-1-0-0-0, v2-2-2-2-2-2; leg II -femur d0-1-1-1, p1-1-0-1, r1-1-1-1, patella d0-0-1, tibia p1-0-1-0, r1-0-1-0, v2-2-2-2-2, metatarsus p1-0-0-1-0-0, r1-0-0-1-0, v2-2-2-2-2-2. Leg measurements:
| I | II | II | IV | Palp | |
| Femur | 2,76 | 2,35 | 2,52 | 3,03 | 1,08 |
| Patella | 1,06 | 0,80 | 0,84 | 0,84 | 0,51 |
| Tibia | 2,74 | 2,52 | 2,30 | 2,88 | 0,66 |
| Metatarsus | 2,74 | 2,63 | 2,63 | 3,47 | — |
| Tarsus | 0,80 | 0,88 | 0,95 | 1,28 | 1,06 |
| Total | 10,10 | 9,18 | 9,24 | 11,50 | 3,31 |
Epigynum (fig. 18A) with median sector and lateral lobes fused near the middle; median sector, in most specimens, with shallow and hairy central fossa; lateral lobes convex and slightly sclerotized; broad copulatory openings. Vulva (fig. 18B) with spermathecae diverging posteriorly, head recognized by few large pores; fertilization ducts open postero-mesal.
Variation (5 females, 5 males). Total length 4,03- 6,46; carapace length 1,18-1,23 times width; PER 1,23-1,32 times AER; clypeal height 1,00-1,44 times AM diameter; femur I 1,22-1,34 carapace width; dorsum of abdomen slightly marked or with a continuous transverse posterior band; ventral spines on tibiae I-II vary from 5 to 6, spination pattern on legs III and IV quite variable. Epigynum may lack a well defined central fossa.
Material examined. Venezuela: The types cited above; Colonia Tovar [ca. 10° 25' N, 67 °16' W; 1886 m] (MNHN 11028), 2 males, 4 females; Corozal, nr. Caracas [ca. 10°18'N, 67°20'W; 971m] (MNHN 10976), 2 males; El Junquito [ca. 10° 28' N, 67° 04' W; 1825 m], viii.1948 (Marcuzzi, UCV no. xii-886), 1 male, 2 juveniles; El Junquito, 16.x.1949 (Marcuzzi, LS), 1 female, 2 juveniles;.
Distribution. Northeastern Venezuela (Map 1).
Caloctenus carbonera new species
Type. Holotype female from La Carbonera [ca. 08° 38' N, 71° 21' W], NW of Merida on the road to La Azulita, Merida, Venezuela, 2200 m, in cloud forest (11.i.1985; J. Palmer), deposited in MCZ.
Etymology. The specific name is a noun in apposition from the type locality.
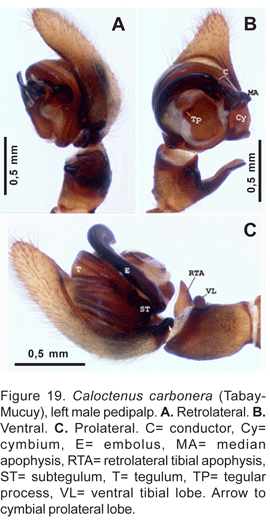
Diagnosis. Individuals of both sexes have 3 stout teeth on the retromargin of the fang furrow. Males are distinguished by the massive embolus and a nearly square sclerotized tegular section between the embolus and median apophysis base (figs. 19B, 20A). Females have a lightly sclerotized epigynum (fig. 21A), with median sector and lateral lobes fused anteriorly into a broad pocket.
Male description (Tabay-Mucuy). Total length 4,64. Carapace gray brown, markings typical. Abdomen light gray brown, darker anteriorly, an anterior yellowish gray band widens into a triangle at the middle. Carapace 2,45 long, 1,86 wide, 0,68 high; ocular area 0,42 long, 0,92 wide; diameter of eyes AM:AL:PM:PL, 0,16:0,07:0,22:0,24; AME-AME nearly AME diameter; PME separation 0,41 times PME diameter. Clypeal height 0,16; sternum 1,10 long, 1,10 wide; labium 0,27 long, 0,37 wide; endites 0,70 long, 0,37 wide. Femur I length 1,38 times carapace width. Spination: palpus -femur d0-1-1, p0-0-1, patella d0-0-1, p1-0-0, tibia p2-0-0; leg I -femur d1-0-1-1, p0-2-1-1, patella d0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2; leg II -femur d1-0-1-1, p1-0-1-0, r1-0-0-1, patella d0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2. Leg measurements:
| I | II | II | IV | Palp | |
| Femur | 3,03 | 2,66 | 2,56 | 3,18 | 0,84 |
| Patella | 0,88 | 0,84 | 0,69 | 0,73 | 0,44 |
| Tibia | 3,54 | 3,07 | 2,37 | 2,96 | 0,62 |
| Metatarsus | 3,54 | 3,03 | 2,74 | 3,76 | — |
| Tarsus | 1,31 | 1,13 | 1,02 | 1,28 | 0,84 |
| Total | 12,30 | 10,73 | 9,38 | 11,91 | 2,74 |
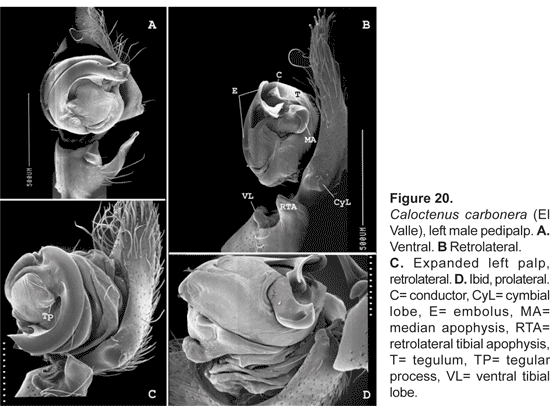
Palpal tibia shorter than cymbium (figs. 19A-C), length 0,21 times femur I; RTA, almost as long as tibia length (figs. 19B-C, 20A), blunt-tipped (fig. 20B-C). Cymbium with large retrolateral projection (figs. 19B, 20A), small probasal projection (fig. 19C); tegulum with small process at embolus base (figs. 19A, 20A); median apophysis (figs. 19A-B, 20B, D) small, infolding margins meeting apically becoming an acute tip; embolus stout,apex bifid (fig. 20C), conductor does not embrace embolic tip (figs. 20A-B, D).
Female (holotype).- Total length 4,10. Carapace gray brown, markings typical; abdomen with stripes joining into a trapezoid pattern. Carapace 2,01 long, 1,62 wide, 0,66 high; ocular area 0,51 long, 0,85 wide, diameter of eyes AM:AL:PM:PL, 0,13:0,04:0,22:0,22; AM-AM nearly AM diameter; PME separation 0,41 times PME diameter; clypeal height 0,13. Sternum 0,93 long, 0,95 wide; endites 0,48 long, 0,29 wide. Femur I 1,10 times carapace width. Spination: palpus -femur d0-1-1, p0-0-0-1, patella d0-0-1, p1-0-0, tibia p2-0-0-0, r1-0-0-1, tarsus p2-1-0-0, r1-1-0-0; leg I -femur d0-1-1-0, p0-2-1-0, r1-0-0-0. v0-1-2-2, patella d-0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2-1; leg II -femur d0-1-1-1, p1-1-1-0, r1-0-1-0, patella d0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 1,79 | 1,70 | 1,64 | 2,04 | 0,69 |
| Patella | 0,69 | 0,66 | 0,58 | 0,62 | 0,37 |
| Tibia | 1,86 | 1,64 | 1,35 | 1,83 | 0,42 |
| Metatarsus | 1,75 | 1,61 | 1,61 | 2,19 | — |
| Tarsus | 0,47 | 0,47 | 0,58 | 0,73 | 0,58 |
| Total | 6,56 | 6,08 | 5,76 | 7,41 | 2,06 |
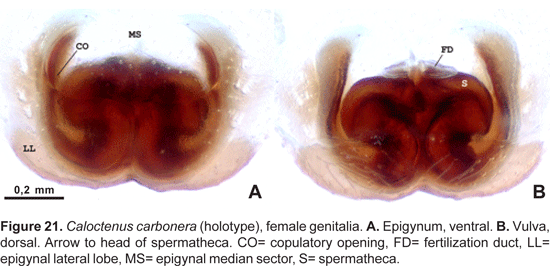
Epigynum (fig. 21A) lightly sclerotized, with epigynal median sector and lateral lobes fused anteriorly; median sector broad, lateral lobes pocket-like; copulatory ducts open anteriorly on sides of the pocket, posterior margins of LL strongly convex. Vulva (fig. 21B) with heavily sclerotized spermathecae, copulatory ducts long, convoluted; fertilization ducts moved to an anterior position.
Variation (5 males). Total length 4,10-4,67, carapace length 1,24-1,32 times width; PER 1,26-1,31 times AER; clypeal height 0,87-1,07 times AME diameter; cheliceral length 0,66-0,75; femur I 1,23-1,40 times carapace width; spination pattern on legs III-IV variable.
Additional Material Examined. Venezuela: Merida: Tabay-Mucuy, Send. Lag. Suero [ca. 08° 38' N, 71° 04' W], cloud forest, 2250 m, 17.vi-2.viii.1989, flight interception trap (S. & J. Peck 89-219, AMNH), 2 males; El Valle, 15 km NE Merida [ca. 08° 35' N, 71° 08' W], cloud forest, 2400 m, 24-vi.-2.viii.1989, flight interception trap (S. & J. Peck 89-230, AMNH), 3 males; Tachira: Pregonero [ca. 08° 01' N, 71° 45' W], Camp Siberia, La Idea, rainforest, 1200 m, 10-31.vii.1989, flight interception trap (S. & J. Peck 89-258, AMNH), 1 male.
Distribution.- Northwestern Venezuela (Map 1).
Caloctenus oxapampa new species
Type. Holotype female and paratype male from Oxapampa, Cerro El Mirador [ 10° 34' 56" S, 75° 23' 72" W], Pasco, Peru, 1859 m (19.vi.1986, D. Silva D.), deposited in MUSM.
Etymology.- The specific name is a noun in apposition from the type locality.
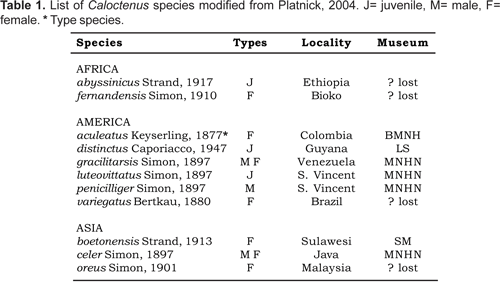
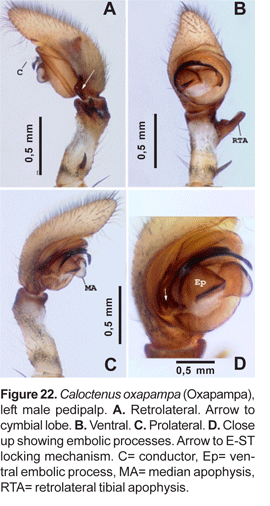
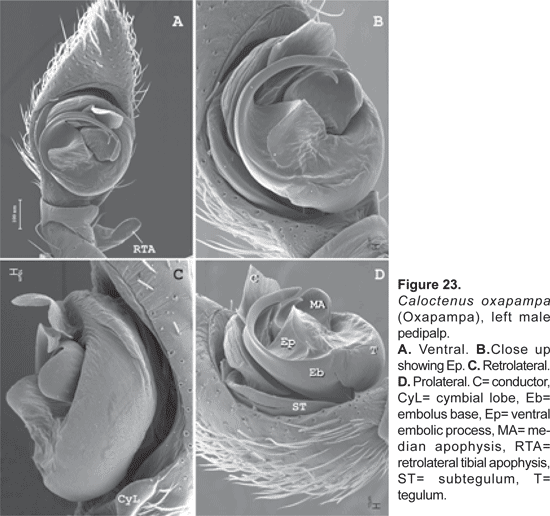
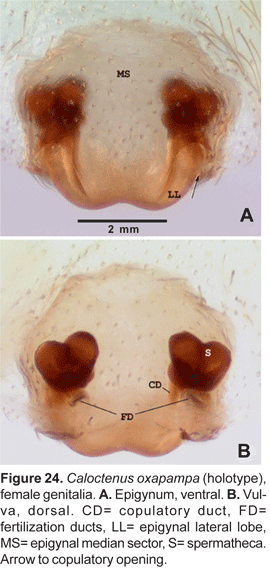
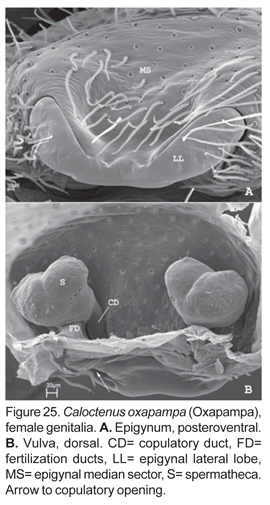
Diagnosis. Males of C. oxapampa differ from all other species in the large sclerotized process at the embolus base (figs. 22B-D, 23A-D); thin and broad median apophysis (fig. 22D, 23A-B); and palpal tibia with retrobasal thorn (fig. 22A-B). Females have epigynal median sector and lateral lobes fused posteriorly (fig. 24A-B, 25A); epigynal median sector broad, translucent; and lateral lobes narrow, projecting upwards (fig. 25A).
Male description (paratype). Total length 3,95. Carapace dark orange brown, markings typical. Abdomen gray darker at both sides, dorsum speckled with whitish spots (fig. 1C) or with large guanine marking posterior (fig. 2B). Carapace 2,01 long, 1,70 wide; ocular area 0,40 long, 0,81 wide; diameter of eyes AM:AL:PM:PL, 0,15: 0,07: 0,20: 0,22; AME-AME 0,85 times AME diameter; PME separation 0,32 times PME diameter; clypeal height 0,09. Sternum 0,99 long, 1,06 wide; labium 0,26 long, 0,33 wide; endites 0,64 long, 0,31 wide. Femur I length 1,78 times carapace width. Spination: palpus -femur d0-1-1, p0-0-1, r0-0-1, patella d0-0-1, p1-0-0, tibia p2-0-0, r1-0-1, tarsus p2-1-0, r1-1-0; leg I -femur d0-1-1-0, p0-2-1-0, r1-0-0-0, v0-1-2-2, patella d0-0-1, tibia v2-2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2; leg II -femur d0-1-1-1, p1-1-1-0, r1-0-1-0, patella d0-0-1, tibia v2-2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 3,03 | 2,66 | 2,56 | 3,18 | 0,84 |
| Patella | 0,88 | 0,84 | 0,69 | 0,73 | 0,44 |
| Tibia | 3,54 | 3,07 | 2,37 | 2,96 | 0,62 |
| Metatarsus | 3,54 | 3,03 | 2,74 | 3,76 | — |
| Tarsus | 1,31 | 1,13 | 1,02 | 1,28 | 0,84 |
| Total | 12,30 | 10,73 | 9,38 | 11,91 | 2,74 |
Palpal tibia (fig. 22A-C) relatively long, length nearly three times width, 0,23 times femur I, concave at base of RTA; RTA (figs. 22A-B, 23A) slightly subapical, base swollen and concave with rounded apex. Cymbium tapering gradually to base, weak probasal and retrobasal projections (figs. 22A, C; 23C); embolus tapering to apex, broad base projecting ventrally into a large sclerotized process (figs. 22B-D, 23A-D), and prolaterally into a small lobe that fits in a subtegular notch (figs. 22D; 23B, D); median apophysis with broad wings and large beak (fig.22D, 23A-C); conductor with short flange, originating from wide base at apex of tegulum and partly embracing embolic tip (fig. 23A-D).
Female (holotype). Total length 5,17. Carapace brown, markings typical (figs. 1A-B). Carapace 1,75 long, 2,15 wide; ocular area 0,37 long, 0,88 wide, diameter of eyes AM:AL:PM:PL, 0,13: 0,07: 0,22: 0,22; AME-AME 0,85 times AME diameter; PME separation 0,32 times PME diameter; clypeal height 0,09. Sternum 1,02 long, 1,06 wide; endites 0,71 long, 0,26 wide. Femur I 1,02 times carapace width. Spination: palpus -femur d0-1-1, p0-0-1, patella d0-0-1, p1-0-0, tibia p2-0-0, r1-0-0, tarsus p2-1-0, r1-1-0; leg I -femur d0-1-1-0, p0-2-1-0, r1-0-0-0, v0-1-2-2, patella d0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2-1; leg II -femur d0-1-1-1, p1-1-1-0, r1-0-1-0, patella d0-0-1, tibia v2-2-2-2-2-2, metatarsus v2-2-2-2-2-2-2. Leg measurements:
| I | II | III | IV | Palp | |
| Femur | 2,19 | 2,15 | 2,04 | 2,48 | 0,73 |
| Patella | 0,80 | 0,77 | 0,62 | 0,69 | 0,40 |
| Tibia | 2,37 | 2,04 | 1,72 | 2,04 | 0,51 |
| Metatarsus | 2,19 | 2,04 | 2,04 | 2,74 | —- |
| Tarsus | 0,66 | 0,66 | 0,77 | 0,95 | 0,66 |
| Total | 8,21 | 7,66 | 7,19 | 8,90 | 2,30 |
Epigynum slightly sclerotized (fig. 24A), median sector and lateral lobes fused posteriorly (figs. 24A-B, 25A); copulatory openings postero-lateral. Vulva with small, bilobate spermathecae (fig. 24A, 25A), copulatory ducts entering spermathecae postero-mesally (fig. 25B); fertilization ducts posterior (fig. 24B, 25B).
Additional material examined. Peru: Pasco, Oxapampa, tanque de agua, rio San Alberto, 10° 34' 51" S, 75° 23' 47" W, 1909 m, 13-15.i.2004 (J. Böttger, J. Grados, D. Silva, MUSM), 2 females, 2 males, 6 juveniles; Ibid (J. Böttger, J. Grados, D. Silva, CAS), 2 females, 1 male, 2 juveniles; Oxapampa surroundings, aprox. 1800 m, 21.vi.1986 (D. Silva, AMNH), 1 juvenile male.
Distribution. Known only from type locality in central Peru (Map 1).
Acknowledgments
I am deeply indebted to the various curators that provided specimens for this project. I am most grateful to F. Coyle and J. Coddington for their continuous assistance to my studies at Western Carolina University, where this project started as a MS thesis. C. Griswold is much acknowledged for his continuous support to my research work and time for reviewing drafts of this paper. Thanks to R. Jocqué for his comments on the last draft.
Undetermined ctenid spiders in various museums were examined through financial aid from the joint AMNH-Cornell University Program and the Ernst Mayr Grant of the MCZ at Harvard University. A postdoctoral fellowship from the California Academy of Sciences and the Schlinger Foundation greatly contributed to complete this work.
Illustrations are by J. Speckels. J. Bötgger kindly provided photos of the habitat and living spiders. Fieldwork in Peru was made possible through a Research Permit from The Instituto Nacional de Recursos Naturales (INRENA). I am very grateful to B. León, J. Grados and J. Bötgger for making my fieldwork in Peru possible.
Literature cited
Bertkau, P. 1880. Verzeichniss der von Prof. Ed. van Beneden auf seiner im Auftrage der Belgischen Regierung unternommen wissenschaftlichen Reise nach Brasilien und La Plata in Jahren 1872—73 gesammelten Arachniden. Mém. cour. Acad. Belg 43: 1—120. [ Links ]
Benoit, P.L.G. 1974. Contribution à l'étude du genre Africactenus Hyatt avec une clé des espèces (Araneae - Ctenidae). Revue Zool. afr. 88: 131—142. [ Links ]
Bonnet, P. 1955. Bibliographia Araneorum. Toulouse, 2 (2): 919—1925. [ Links ]
Brignoli, P. M. 1972. Sur quelques araignées cavernicoles d'Argentine, Uruguay, Brésil et Venezuela récoltées par le Dr. P. Strinati (Arachnida, Araneae). Revue suisse Zool. 79: 361—385. [ Links ]
Caporiacco, L. di. 1947. Diagnosi preliminari de specie nuove di aracnidi della Guiana Brittanica raccolte dai professori Beccari e Romiti. Monitore zool. ital. 56: 20—34. [ Links ]
Caporiacco, L. di. 1955. Estudios sobre los aracnidos de Venezuela. 2a parte: Araneae. Acta biol. venez. 1: 265—448. [ Links ]
Griswold, C. E. 1991. A revision and phylogenetic analysis of the African spider genus Machadonia Lehtinen (Araneae: Lycosoidea). Ent. Scand. 22: 305—351. [ Links ]
Hyatt, K. H. 1954. The African spiders of the family Ctenidae in the collections of the British Museum (Natural History). Ann. Mag. nat. Hist. (12) 7: 877—894. [ Links ]
Keyserling, E. 1877. Uber amerikanische Spinnenarten der Unterordnung Citigradae. Verh. zool-bot Gesell. Wien 26: 609—708. [ Links ]
Mello-Leitão, C. F. 1936. Contribution a l'étude des Cténides du Brésil. Festschr. Strand 1: 1-31, 598-601. [ Links ]
Pickard-Cambridge, F. O. 1897a. On cteniform spiders from the lower Amazons and other regions of North and South America, with a list of all known species of these groups hitherto recorded from the New World. Ann. Mag. nat. Hist. 19: 52—106. [ Links ]
Pickard-Cambridge, F. O. 1897b. On the cteniform spiders of Ceylon, Burmah, and the Indian Archipelago, west and north Wallace's line: with bibliography and list of those from Australasia, south and east Wallace's line. Ann. Mag. nat. Hist. 20: 329—356. [ Links ]
Platnick, N. I. 2004. The world spider catalog, version 4.5. American Museum of Natural History, online at http://research.amnh.org/entomology/spiders/catalog/index.html [ Links ]
Roewer, C. F. 1954. Katalog der Araneae von 1758 bis 1940. Brussels, 2a: 1-923. [ Links ]
Silva Dávila, D. 1994. Taxonomic revision of the Neotropical spider genus Caloctenus Keyserling (Araneae, Ctenidae). MS Thesis, Western Carolina University, North Carolina, USA. [ Links ]
Silva Dávila, D. 2003. Higher-level relationships of the spider family Ctenidae (Araneae: Ctenoidea). Bull. Am. Mus. nat. Hist. 274: 1—86. [ Links ]
Simon, E. 1897a. Histoire naturelle des araignées. Paris, 2 (1): 1—192. [ Links ]
Simon, E. 1897b. On the spiders of the island of St. Vincent. Part III. Proc. Zool. Soc. London 1897: 860—890. [ Links ]
Simon, E. 1901. On the Arachnida collected during the Skeat expedition to the Malay Peninsula. Proc. zool. Soc. Lond. 1901(2): 45-84. [ Links ]
Simon, E. 1909. Araneae. 2e partie. In Michaelsen & Hartmeyer, Die Fauna Sudwest-Australiens. Jena, 2(13): 152—212. [ Links ]
Simon, E. 1910. Arachnides recueillis par L. Fea sur la côte occidentale d'Afrique. 2e partie. Ann. Mus. civ. stor. nat. Genova 44: 335—449. [ Links ]
Strand, E. 1913. Neue indoaustralische und polynesische Spinnen des Senckenbergischen Museums. Arch. Naturg. 79(A6): 113—123. [ Links ]
Strand, E. 1917. Arachnologica varia XXI-XXIV. Arch. Naturg. 82(A3): 39—44. [ Links ]
Walckenaer, C. A. 1805. Tableau des aranéides ou caractères essentiels des tribus, genres, familles et races que renferme le genre Aranea de Linné, avec la désignation des espèces comprises dans chacune de ces divisions. Paris: 1—88. [ Links ]
Correspondencia
Postal adress: Diana Silva, Dpto. Entomología, Museo de Historia Natural, Apartado postal 14-0434, Lima 14- Perú.
email: Silva, Diana [dsilva@calacademy.org]