Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.12 n.2 Lima ago./set 2005
Reynaldo Linares-Palomino*
*Albrecht-von-Haller-Institut for Plant Sciences
Department of Systematic Botany, University of Göttingen, Germany.
Número especial: Bosques relictos del NO de Perú y SO de Ecuador
Weigend, Rodríguez y Arana (Comps.)
Publicado online: 08/12/05
Abstract
A study to reveal spatial distribution patterns in four characteristic dry forest tree species was undertaken in six one-hectare plots in the Cerros de Amotape National Park, northwestern Peru. The modified Ripley's K statistic was used. Eriotheca ruizii (K. Schum.) A. Robyns (Bombacaceae), Bursera graveolens (Kunth) Triana & Planch. (Burseraceae), Caesalpinia glabrata Kunth (Leguminosae) and Cochlospermum vitifolium (Willd.) Spreng. (Cochlospermaceae) present in 11 out of 17 cases, patterns that are not significantly different from a completely random pattern. At the analysed spatial scale, this disagrees with the widely held notion that tropical tree species present clumped patterns. The different factors that may contribute to the observed patterns are discussed.
Keywords: dispersal, dry forests, regeneration, Ripley's K, Peru, Cerros de Amotape National Park.
Resumen
Se realizó un estudio de los patrones de distribución espacial de cuatro especies de árboles características de los bosques secos del Parque Nacional Cerros de Amotape en el noroeste peruano, inventariando seis parcelas de una hectárea cada una. Para ello se utilizó la versión modificada de la estadística K de Ripley. Eriotheca ruizii (K. Schum.) A. Robyns (Bombacaceae), Bursera graveolens (Kunth) Triana & Planch. (Burseraceae), Caesalpinia glabrata Kunth (Leguminosae) y Cochlospermum vitifolium (Willd.) Spreng. (Cochlospermaceae) presentan patrones que no son significantemente diferentes de un patrón completamente al azar en 11 de los 17 casos analizados. Al nivel de la escala espacial analizada, esto está en desacuerdo con el postulado general para bosques tropicales de que las especies vegetales tienden a encontrarse agrupadas. Estos resultados se analizan y discuten a la luz de los diversos factores que influyen en producirlos.
Palabras Clave: dispersión, bosques secos, K de Ripley, regeneración, Perú, Parque Nacional Cerros de Amotape.
Introduction
An early account by A. R. Wallace (1895) suggested that trees in tropical forests were uniformly dispersed and at very low densities. It is noteworthy that the first contradicting evidence against this statement came from studies in a tropical dry forest (Hubbell, 1979). He found that 44 out of 61 studied species showed statistically significant clumping among adults. The remaining 17 species had patterns that could not be distinguished from random. Since then, several other studies confirmed the predominance of clumped or random dispersion patterns, the latter with much lower frequency (e.g. Batista & Maguire, 1998).
The analysis of spatial pattern in biology, and specifically of vegetation, is an important tool to help in the understanding of the arrangement of the system. Spatial pattern determines the local environment of each individual and thus, through competition processes, its ability to develop and grow or its probability of dying. It also determines the chance of establishing seedlings and the renewal capacity of the stand. But this relation between spatial pattern and forest dynamics is two-sided: the demographic processes modify the stand pattern retroactively through the replacement of trees (Goreaud et al., 1999).
Following Skarpe (1991), regular patterns have been interpreted as a result of density-dependent mortality caused by intraspecific competition for an evenly distributed resource. Aggregated patterns have been explained in terms of regeneration ecology and disturbances. Random distributions may result either from processes directly producing such a distribution, or from the combined effects of agents tending to turn aggregated patterns into regular patterns or vice versa.
Spatial Pattern has been analysed quite extensively in humid and wet tropical forests (Sterner et al., 1986; Wong et al., 1990; Batista & Maguire, 1998; Pellisier, 1998; Pelissier & Goreaud, 2001) and African savannas (Skarpe, 1991; Caylor et al., 2003), but has received little attention in seasonally dry tropical forests (SDTF). In fact, an extensive literature search yielded only two publications, apart from reports of studies in deciduous forests in India (Sagar et al., 2003). The first and best known one is by Hubbell (1979) in the Mesoamerican dry forests of Guanacaste, Costa Rica (13,44 ha). The only other one of which I am aware is a study by Josse & Balslev (1994) in the Parque Nacional Machalilla, Ecuador on a heavily degraded Neotropical dry forest (1 ha).
The equatorial pacific SDTFs, comprising the forests in SW Ecuador, NW and Interandean Peru, have been identified as a floristically and structurally different phytogeographic unit of the SDTFs in the Neotropical Region (Gentry, 1995; Linares-Palomino et al., 2003) and are subject to heavy deforestation processes (Linares-Palomino, 2004). Only few intact SDTFs remain in southwestern Ecuador (Dodson & Gentry, 1991; Best & Kessler, 1995).
The aim of this paper is to examine the spatial structure of characteristic tree species of a seasonally dry forest and to discuss the results in the light of the biological and anthropogenic factors that could produce them.
Study area
The study was conducted in the Cerros de Amotape National Park, departments of Tumbes and Piura, Peru (Fig. 1). The topography of the area is hilly, due to the presence of the Cordillera de los Amotapes, an extension of the Andes and altitude ranges from 120m to 1500m towards the southern area of the Park. The soils are predominantly vertisols and lithosols-vertisols (CDC-UNALM, 1992; AUGE-COBOL, 1998), usually considered as soils with high fertility (Brown, 1982).
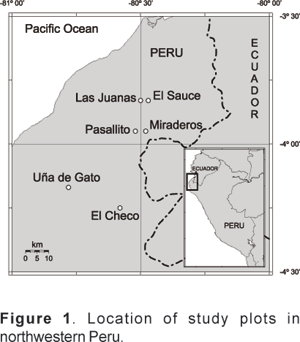
The climate in the area is classified as a transition zone between the desert climate of the Peruvian coast and the tropical sub-humid regions in Ecuador (CDC-UNALM, 1992). It is seasonal with rains falling usually between December and April. The annual amount of precipitation varies widely and is particularly influenced by the cyclic occurrence of El Niño.
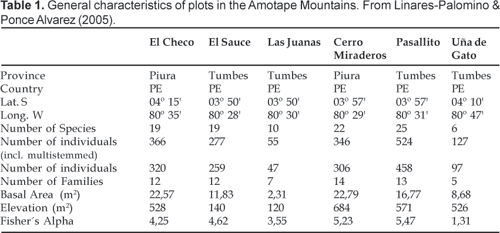
Plots have species richness values between six and 25, with densities per hectare from 55 to 524 individuals. These densities result from measuring multi-stemmed trees as separate individuals. The percentage of multi-stemmed individuals is variable, ranging from 3,2% for Bursera graveolens (Kunth) Triana & Planch. at El Sauce to 60% for Caesalpinia glabrata Kunth at Miraderos. Densities for individual plants only are in the range of 47 458 individuals. The most important tree families are the Bombacaceae, Fabaceae, Bignoniaceae, and Combretaceae. Caesalpinea glabrata, Tabebuia spp., Terminalia valverdeae A. H. Gentry, Eriotheca ruizii (K. Schum.) A. Robyns and Ceiba trichistandra (A. Gray) Bakh. are the most widespread and abundant species. Further ecological and floristic characteristics of each plot were reported elsewhere (Linares-Palomino & Ponce Alvarez, 2005).
Methods
Fieldwork was carried out between May and November 2000. Six one-hectare plots (100 x 100 m) were established in different areas of the Cerros de Amotape National Park following the methodology of Alder & Synnot (1992) (Table 1). All woody species with diameter at breast height (DBH) > 10 cm were identified in situ if possible, and coded. Each individual was mapped onto a co-ordinate system with the origin at the SW corner, showing its position within the plot (Fig. 2). For the purpose of the spatial pattern analysis multi-stemmed trees were considered as one individual. For the present study four species where chosen based on their occurrence in differentially located areas of the cordillera. Their distribution implies different microclimatic preferences, most importantly with regard to humidity and exposure to orographic fog. Eriotheca ruizii (Bombacaceae) and Bursera graveolens (Burseraceae) are the only species that are present in all six plots. However, E. ruizi presents only one individual at Las Juanas, and the latter was not included in the analysis. Caesalpinia glabrata (Leguminosae) is present in three plots located on western, eastern and ridge top plots. Additionally, Cochlospermum vitifolium (Willd.) Spreng. (Cochlospermaceae), present in three plots, was also analysed in order to compare the results with those obtained by Hubbell (1979). Thus, 17 cases were analysed in the six plots.
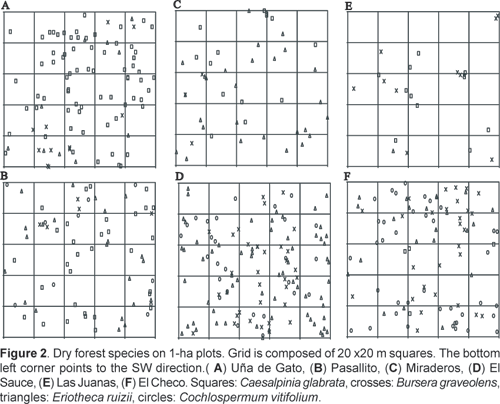
Spatial distribution patterns were analysed with SpPack version 1.3 (Perry, 2004) using Ripley's K(t) function (Ripley, 1977). Ripley's K(t) function is useful because it gives a description of spatial structure at different scales in one graphic (Cressie, 1993). Data setup was set on as follows (default values): minimum and maximum x and y values (i.e. length of the sides of the plots): 0 and 100; t value: 1-50 (as suggested by the software, it should not exceed ½ the length of the shortest dimension of the plot); method of edge correction: Weighted Edge Points. The 99% confidence intervals for the statistic were estimated by performing a Monte Carlo procedure with 499 replicates for a = 0,01. To simplify display and interpretation of the results, SpPack presents K(t) and also a transformed version of K(t) to stabilize variance: L(t) = (K(t)/p)0,5-t (Besag, 1977; Haase, 1995; Martens et al., 1997), together with their respective confidence intervals. Random patterns have a theoretical value of L(t)=0, clumped patterns L(t)>0 and regular patterns L(t)<0. If for a given distance t the measured value of L(t) is higher than the upper value of the confidence interval, we can conclude that the spatial structure is significantly clumped at distance t. On the opposite, if the value below the lower value of the confidence interval, we can conclude that the spatial structure is significantly regular at distance t. If the value is inside the confidence interval, we cannot reject the hypothesis of complete spatial randomness (CSR).
Results
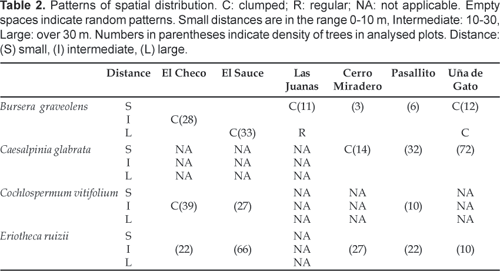
A spatial structure not significantly different from CSR could be observed in 11 out of the 17 cases analysed (Fig. 3).
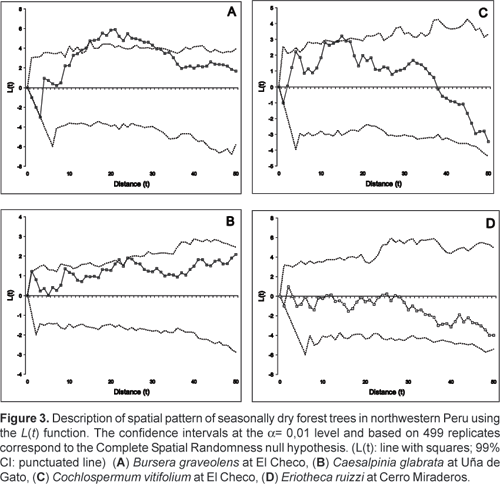
C. glabrata showed a spatial structure not significantly different from randomness at all distances at Uña de Gato and Pasallito. At Cerro Miraderos it showed clumping over short distances (56 m and 910 m). E. ruizii showed a spatial structure not significantly different from randomness at all distances in all plots. C. vitifolium showed a spatial structure not significantly different from randomness at all distances at Pasallito and El Sauce. At El Checo it showed clumping over intermediate distances (2526 m). Only B. graveolens showed a spatial structure with clumped, regular and random patterns. In two plots (Cerro Miraderos and Pasallito), it showed a spatial structure not significantly different from random at all distances. In three plots it showed a spatial structure that was significantly clumped, either at small (18 m, at Uña de Gato), intermediate (1139 m and 1631 m, at Uña de Gato and El Checo) or large distances (1950 m, at El Sauce). There was only one plot in which it showed all three kinds of patterns. At Las Juanas it showed significantly clumped spatial structure at small distances (37 m) and significantly regular spatial structure at large distances (3843 m).
Discussion
The causes for the observed patterns can be manifold. Highly local soil conditions (such as variable pH, fertility or structure over short distances), microclimatic factors (exposed ridges vs. fissures in the area, small seasonal water courses), anthropogenic disturbance (wood extraction and land conversion by locals), herbivory (naturally occurring forests animals, as well as free roaming cattle and goats,) are some of the factors that can contribute to it.
The most frequent result, a pattern not significantly different from random, might have been influenced by the low density of some of the analysed species. Especially for those below 30 trees/ha it is difficult to truly assess clustering. This is because the power of the test for L(t) depends on tree density of the analysed species in the plot (F. Goreaud, pers. comm.). This can lead to the fact that species like B. graveolens in Cerro Miraderos and Pasallito, for example, might actually have some kind of clumping. We need larger areas to confirm that the obtained patterns are truly not significantly different from random.
Caesalpinia glabrata
The random patterns shown by C. glabrata in the plots analysed may be the result of its dispersal properties. The species, and its close relatives in the Libidivia group of the Caesalpinoideae, all have indehiscent fleshy or woody pods rich in tannins (Lewis & Klitgaard, 2002). No detailed phenological studies exist for the area, but preliminary observations reported almost continuous fruiting from January to September during 1999 (unpublished data). It is believed that the seeds are released only after the pods rot on the ground or by gnawing of small rodents or after being eaten by larger herbivores (G. Lewis, pers. comm.). In the past, these pods may have been eaten, and their seeds dispersed, by a now extinct megafauna. There are faunal paleoecological records dated at ca. 13,000 years before present from the nearby Talara area, which confirm the presence of numerous, large herbivores (such as mastodons, horses, deer, camelids) who would have lived in a dry, open savanna-like habitat (Campbell, 1982). Nowadays there are free-roaming herds of livestock (mainly goats, cattle and sheep) in much of the dry forests of northwestern Peru. Local inhabitants reported that livestock are fed with every available food source from the forest, and preferentially, with C. glabrata pods, especially in times of severe drought. These animals, and probably ungulates like the relatively rare white-tailed deer (Odocoileus virginianus var. peruvianus (Gray 1874)), perform the role of seed dispersers. If rodents were involved in the seed dispersal, the seeds would typically be transported to their nests or burrows, and may germinate there. This condition can lead to both clumped and random spatial patterns as has been shown for rodent-dispersed rainforest tree species (Forget et al., 1999). Another dispersal possibility for C. glabrata may be the decomposition of the barochorous pods, and subsequent seed release. Fruits are heavy enough not to be transported by wind or surface flowing water. The first hypothesis is probably the most important mechanism for the patterns reported, however, a combination of different dispersal mechanisms cannot be ruled out.
Eriotheca ruizii and Cochlospermum vitifolium
The random patterns found in E. ruizii and C. vitifolium can also be explained by their dispersal properties. During 1999 E. ruizii flowered from May to August during the dry season. The fruits are dispersed from June to November, shortly before the onset of rains. The coriaceous obovoid capsules contain the ellipsoid seeds embedded in abundant kapok (Robyns, 1963). Thus, wind dispersal (anemochory) is likely the primary dispersal mechanism, although parakeets and squirrels have been seen carrying its fruits, mainly for building their nests with the kapok. In the Brazilian Cerrado, the nutritious oily seeds of E. gracilipes (K. Schum.) A. Robyns have been suggested as food for rodents and birds (Gottsberger & Silberbauer-Gottsberger, 1983). However, I have always observed big clumps of kapok and seeds, and even whole fruits, being released from the tree. I suspect these are too heavy to be transported effectively by wind over longer distances. Once they reach the ground they are ripped apart by the same wind and form a carpet of kapok, still holding seeds. These events, usually shortly before the rains, might contribute to the observed random pattern. Additionally, Fischer (1997) on a study of E. pentaphylla (Vell.) A. Robyns, observed a higher germination rate when the kapok was still adhered to the seeds as compared to seeds without kapok. This would suggest that seeds carried away from the mother trees by animal dispersers, without the kapok, or seeds left without the kapok, the latter having been removed for nest building, would have much lower survival and germination possibilities.
A similar case could be made for C. vitifolium, «one of the most characteristic trees of the coastal deciduous forests and savannas» as stated by Molau (1983). Its capsules are 510 cm long, globose or obovate and contain several seeds embedded in kapok (Molau, 1983; Vázquez-Yanes et al., 1999). The capsules are dehiscent and open usually during the dry season. Frankie et al., (1974) reported the fruiting of C. vitifolium in a Costa Rican dry forest at the end of a long and severe dry season, and just before the onset of the rainy season. Preliminary data from the study area reported fruiting trees between July and November during 1999. This suggests that the main dispersal mechanism is anemochory, as has already been reported for C. regium (Schrank) Pilg. in the Brazilian Cerrado (Gottsberger & Silberbauer-Gottsberger, 1983). However, INRENA (2002) in a report on the trees of the study area mentions for C. vitifolium that «its fruits are a food source for birds and squirrels», which could be also zoochorous dispersal agents. Hubbell (1979), however, reported significant clumping in juveniles, adults and total population of C. vitifolium, over a much wider range (rectangles with side lengths of 196 m). In out study area statistically significant clumping could only be detected in one of the three plots where C. vitifolium was present, and only over short intermediate distances.
Bursera graveolens
B. graveolens produces fruits that are small, very tardily dehiscent, one-seeded, and usually more or less trigonal (Gentry, 1993). The fruiting season has been reported to begin after the end of the dry season in southwestern Ecuador, extending from April to June (Aguirre, 2002). Preliminary data from the study area reported trees fruiting from March to August during 1999 (unpublished data). Seeds are arillate and probably consumed by several species of birds, iguanas and rodents on Santa Fe Island (Galapagos) (Clark & Clark, 1981). The same study found that 86% of B. graveolens juveniles were 3 m or more from the closest branch of an adult plant. This result was explained by two mechanisms: 1) since Bursera seeds have no structures to facilitate wind dispersal, a biotic dispersal agent was suggested and 2) due to some unknown factor(s), regeneration of Bursera was inhibited under adult trees. The apparent dispersal distance of B. graveolens seeds was relatively short. Mean distance from juveniles to nearest adult was 7 m and 90% of young B. graveolens were within 20 m of a Bursera tree. However, real dispersal distances may be greater since the closest adult is not necessarily the parent. The results of the present study show clumped, random and regular patterns for B. graveolens. In two of the plots random patterns were dominant over the whole distance analysed (Table 2). In another two plots clumped patterns over short distances were observed. In the latter case, if we assume that the nearest tree is the mother tree, it might be possible that the seeds were dispersed effectively to nearby areas only and not affected by seed predation, herbivory or interference from adult trees. Little natural regeneration of this tree has been observed in dry forests in Loja, Ecuador (Aguirre, 2002), perhaps as a result of intensive browsing by livestock (feral goats and cattle) (cf. Clark & Clark, 1981; Hamann, 2001).
The present study shows a static picture of the studied species' pattern. The clumped species patterns postulated by Hubbell (1979) could not be confirmed for four important dry forest species. However, since this ecosystem, is subjected to severe cyclic phenomena such as El Niño, drastic changes in the vegetation may occur over short periods of time (Bendix et al., 2000, Block & Richter, 2000). Consequently the present picture is far from complete. There is an urgent need for long-term studies on the phenology, breeding systems, regeneration and dynamics of dry forest species in Peru. The present study has mentioned the probable influence of livestock on the dynamics of some of the studied species. Several studies in the Galapagos Islands have demonstrated the detrimental effects feral goats had on plant life (e.g Hamann, 2001). Moreover, a study in the Venezuelan dry forest reported that 74% of the plant species consumed by goats were trees or shrubs (Hernández Acosta, 1986). No quantitative data exist for Peruvian dry forests. However Rodríguez & Uhlenbrock (2002) argued that goats are not to be blamed for deforestation processes in the dry forests of northwestern Peru. Based on household surveys they suggested that selective wood extraction was more important. Exclosure studies are underway in the dry forests at the El Angolo Game Reserve in Piura, Peru (P. Vásquez, pers. comm.) and will undoubtedly contribute to our understanding of the influence of livestock on the dynamics of the forests, as has been shown already for the dry forest in Hawaii (Cabin et al., 2000).
Acknowledgements
This study was made while the author worked at the Cerros de Amotape National Park. I thank the Instituto Nacional de Recursos Naturales (INRENA), Peru, for providing facilities and financial support. Thanks to F. Goreaud and two anonymous reviewers for their comments and suggestions, which greatly improved the paper. I thank G. Lewis for sharing his thoughts on Pleistocenic herbivors of C. glabrata, I. Sánchez for contributing with local knowledge of the forests in northwestern Peru, the park rangers of the Cerros de Amotape National Park for their assistance during fieldwork, A G. Perry for help with statistics and M. Richter and M. Kessler for providing literature.
Literature Cited
Aguirre, Z. 2002. Árboles austroecuatorianos útiles poco conocidos. In: Z. Aguirre, J. E. Madsen, E. Cotton & H. Balslev (eds.). Botánica Austroecuatoriana: 351-374. Quito, Editorial Abya Yala. [ Links ]
Alder, D. & Synnot, T. J. 1992. Permanent Sample Plot Techniques for Mixed Tropical Forest. Tropical Forestry Papers Nº 25. Oxford University Press.
AUGE-COBOL. 1998. El Perú y sus Recursos. AUGE S.A. Editores - COBOL S.R.L.
Batista, J. L. F. & Maguire, D. A. 1998. Modeling the spatial structure of tropical forests. Forest Ecology and Management. 110: 293-314.
Bendix, J., Bendix, A. & Richter, M. 2000. El Niño 1997/1998 in Nordperu: Anzeichen eines Ökosystem-Wandels?. Petermanns Geographische Mitteilungen. 144: 20-31.
Besag, L. 1977. Contributions to the discussion of Dr. Ripley's paper. Journal of the Royal Statistical Society B. 39: 193-195.
Best, B. J. & Kessler, M. 1995. Biodiversity and conservation in Tumbesian Ecuador and Peru. BirdLife International, UK.
Block, M & Richter, M. 2000. Impacts of heavy rainfalls in El Niño 1997/1998 on the vegetation of Sechura Desert in Northern Peru. Phytocoenologia. 30: 491-517.
Brown, K. S. 1982. Paleoecology and regional patterns of evolution in Neotropical Forest Butterflies. In: G. T. Prance (ed.), Biological diversification in the Tropics: 245-308. New York, Columbia University Press.
Cabin, R. J., Weller, S. G., Lorence, D. H., Flynn, T. W., Sakai, A. K., Sandquist, D. & Hadway, L. J. 2000. Effects of long-term ungulate exclusion and recent alien species control on the preservation and restoration of a Hawaiian tropical dry forest. Conservation Biology. 14: 439-453.
Campbell, K. E. 1982. Late Pleistocene events along the coastal plain of northwestern South America. In: G. T. Prance (ed.), Biological diversification in the Tropics: 423-440. New York, Columbia University Press.
Caylor, K. K., Shugart, H. H., Dowty, P. R. & Smith, T. M. 2003. Tree spacing along the Kalahari transect in southern Africa. Journal of Arid Environments. 54: 281-296.
CDC-UNALM. 1992. Estado de la conservación de la diversidad natural de la región noroeste del Perú. Centro de Datos para la Conservación - Universidad Nacional Agraria La Molina. Lima, Perú.
Clark, D. A. & D. B. Clark. 1981. Effects of seed dispersal by animals on the regeneration of Bursera graveolens (Burseraceae) on Santa Fe Island, Galapagos. Oecologia. 49: 73-75.
Cressie, N. A. C. 1993. Statistics for spatial data. Wiley Series in Probability and Mathematical Statistics.
Dodson, C. & Gentry, A. H. 1991. Biological extinction in western Ecuador. Annals of the Missouri Botanical Garden. 78: 273-295.
Fischer, E. A. 1997. The role of plumes in Eriotheca pentaphylla (Bombacaceae) seed survival in south-eastern Brazil. Journal of Tropical Ecology. 13: 133-138
Forget P.-M., Mercier F. and Collinet F. 1999. Spatial patterns of two rodent-dispersed rain forest trees Carapa procera (Meliaceae) and Vouacapoua americana (Caesalpiniaceae) at Paracou, French Guiana. Journal of Tropical Ecology. 15: 301-313.
Frankie, G. W., Baker, H. G. & Opler, P. A. 1974. Comparative phonological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. Journal of Ecology. 62: 881-919.
Gentry, A. H. 1993. A Field Guide to the families and genera of woody plants of northwestern South America (Colombia, Ecuador, Peru) - with supplementary notes on herbaceous taxa. Conservation International, Washington, DC.
Gentry, A. H. 1995. Diversity and floristic composition of Neotropical dry forests. In: S. H. Bullock, H. A. Mooney & E. Medina (eds.), Seasonally Dry Tropical Forests: 146-194. Cambridge, Cambridge University Press.
Goreaud, F., Courbaud, B. & Collinet, F. 1999. Spatial structure analysis applied to modelling of forest dynamics: a few examples. In: A. Amaro & M. Tomé (eds.), Proceedings of the IUFRO workshop, Empirical and process based models for forest tree and stand growth simulation, 20-26 September 1997, Oeiras, Portugal. Novas Tecnologias. 155-172.
Gottsberger, G. & Silberbauer-Gottsberger, I. 1983. Dispersal and distribution in the Cerrado vegetation of Brazil. In: K. Kubitzki (ed.), Dispersal and Distribution, An International Symposium: 315-352. Hamburg, Paul Parey Verlag.
Haase, P. 1995. Spatial pattern analysis in ecology based on Ripley's K-function: Introduction and methods of edge correction. Journal of Vegetation Science. 6: 575-582.
Hamann, O. 2001. Demographic studies of three indigenous stand-forming plant taxa (Scalesia, Opuntia, and Bursera) in the Galápagos Islands, Ecuador. Biodiversity and Conservation. 10(2): 223-250.
Hernández Acosta, I. 1986. Ramoneo de las cabras en un bosque seco tropical: Especies consumidas y su valor nutritivo. Revista de la Facultad de Agronomía, LUZ. 7: 64-71. Universidad del Zulia, Maracaibo, Venezuela.
Hubbell, S. P. 1979. Tree dispersion, abundance, and diversity in a tropical dry forest. Science. 203: 1299-1309.
INRENA (Instituto Nacional de Recursos Naturales). 2002. Manual divulgativo de especies forestales de la Reserva de Biosfera del Noroeste. Tumbes.
Josse, C. & Balslev, H. 1994. The composition and structure of a dry, semideciduous forest in western Ecuador. Nordic Journal of Botany. 14: 425-434.
Lewis, G. P. & Klitgaard, B. B. 2002. Leguminosas del Sur de Ecuador. In: Z. Aguirre, J. E. Madsen, E. Cotton & H. Balslev (eds.). Botánica Austroecuatoriana: 185-224. Quito, Editorial Abya Yala.
Linares-Palomino, R. 2004. Los Bosques Tropicales Estacionalmente Secos: II. Fitogeografía y Composición Florística. Arnaldoa. 11(1): 103-138.
Linares-Palomino, R & S. I. Ponce-Alvarez. 2005. Tree community patterns in seasonally dry tropical forests in the Cerros de Amotape Cordillera, Tumbes, Peru. Forest Ecology and Management. 209: 261-272.
Linares-Palomino, R., Pennington, R. T. & Bridgewater, S. 2003. The phytogeography of the seasonally dry tropical forests in Equatorial Pacific South America. Candollea. 58: 473-499.
Martens, S. N., Breshears, D. D., Meyer, C. W. & Barnes, F. J. 1997. Scales of above-ground and below-ground competition in a semi-arid woodland detected from spatial pattern. Journal of Vegetation Science. 8: 655-664.
Molau, U. 1983. Cochlospermaceae. In: G. Harling & B. Sparre (eds.). Flora of Ecuador, no. 20: 11-15. Stockholm, Department of Systematic Botany, University of Göteborg.
Pelissier, R. 1998. Tree spatial patterns in three contrasting plots of a southern Indian tropical moist evergreen forest. Journal of Tropical Ecology. 14: 1-16.
Pelissier, R.& Goreaud, F. 2001. A practical approach to the study of spatial structure in simple cases of heterogeneous vegetation. Journal of Vegetation Science. 12: 99-108.
Perry, G. L. W. 2004. SpPack: spatial point pattern analysis in Excel using Visual Basic for Applications (VBA). Environmental Modelling & Software. 19: 559-569.
Ripley, B. D. 1977. Modelling spatial patterns. Journal of the Royal Statistical Society B. 39: 172-212.
Robyns, A. 1963. Essai de Monographie du genre Bombax L. s.l. (Bombacaceae). Bulletin du Jardin Botanique de l'État à Bruxelles. 33: 1-315.
Rodríguez, A & Uhlenbrock, M. 2002. Goats, or Scapegoats for Desertification: Time to Take another Look at Firewood Demand in Peru. ICARDA Caravan 16. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria.
Sagar, R., Raghubanshi, A. S. & Singh, J. S. 2003. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. Forest Ecology and Management. 186: 61-71.
Skarpe, C. 1991. Spatial patterns and dynamics of woody vegetation in an arid savanna. Journal of Vegetation Science. 2: 565-572.
Sterner, R. W., Ribic, C. A. & Schatz, G. E. 1986. Testing for life historical changes in spatial patterns of four tropical tree species. Journal of Ecology. 74: 621-633.
Vázquez-Yanes, C., Batis Muñoz, A. I., Alcocer Silva, M. I., Gual Díaz, M. & Sánchez Dirzo, C. 1999. Árboles y arbustos potencialmente valiosos para la restauración ecológica y la reforestación. Reporte técnico del proyecto J084. CONABIO - Instituto de Ecología, UNAM.
Wallace, A. R. 1895. Natural selection and tropical nature. London, Macmillan.
Wong, M. S. J., Wright, S. P. H. & Foster, R. B. 1990. The spatial pattern and reproductive consequence of outbreak defoliation in Quararibea asterolepis, a tropical tree. Journal of Ecology. 78: 579-588.
Correspondencia
*Albrecht-von-Haller-Institut for Plant Sciences
Department of Systematic Botany, University of Göttingen, Untere Karspuele 2, 37073 Goettingen - Germany
E-mail:pseudobombax@yahoo.co.uk













