Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.12 no.3 Lima Oct./dic 2005
Franz Cardoso1, Paul Baltazar2 and Jorge Bautista3
1Laboratorio de Biología y Sistemática de Invertebrados Marinos, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos.
2Gerencia de Acuicultura, Fondo de Desarrollo Pesquero (FONDEPES).
3Area de Cultivos Marinos, Instituto del Mar del Perú.
Presentado: 03/11/05
Aceptado: 06/01/06
Abstract
The early development of the Patagonian squid, Loligo gahi D'Orbigny,1835, was studied in the field and in the laboratory. Egg strands, spawned off San Lorenzo Island, Peru, were collected, carried to the laboratory, and incubated in a closed sea water system. Egg capsules ranged from 88 to169 mm in length, and each capsule contained between 56 and 114 fertilized eggs. Individual eggs ranged from 1,7 to 2,1 mm in length, and the mantle length of hatchlings varied from 1,9 to 2,8 mm. Development took about 20 days at a mean temperature of 19 ºC. The pattern of embryonic development is similar to that previously observed in other species of Loligo. Following hatching, paralarvaes survived for 45 days with a diet of zooplankton (copepods, mysids and polychaete larvaes).
Keywords: Loligo gahi , early development, hatching, paralarvae, Peru.
Resumen
El desarrollo temprano del calamar patagónico, Loligo gahi D'Orbigny, 1835 fue estudiado en el campo y en el laboratorio. Las puestas colectadas en la Isla San Lorenzo, Perú, fueron transferidas al laboratorio, e incubadas en un sistema cerrado de agua marina. Las cápsulas midieron de 88 a 169 mm de longitud y cada cápsula contenía entre 56 y 114 huevos fertilizados. Los huevos midieron de 1,7 a 2,1 mm de longitud y la longitud del manto de los individuos eclosionados varió de 1,9 a 2,8 mm. El desarrollo de las paralarvas se logró a los 20 días, a una temperatura promedio de 19 ºC. El patrón de desarrollo embrionario es similar al observado en otras especies de Loligo. Las paralarvas sobrevivieron 45 días con una dieta de zooplancton (copepódos, micidáceos y larvas de poliquetos).
Palabras clave: Loligo gahi, desarrollo temprano, eclosión, paralarva, Perú
Introduction
The Patagonian squid, Loligo gahi D'Orbigny, 1835, is a neritic cephalopod reported to range in distribution in the southeastern Pacific Ocean from Puerto Pizarro, Peru (03° 30'S) to southern Chile (56° 30'S) (Roper et al., 1984; Cardoso et al., 1998). In the southwestern Atlantic Ocean, the species is reported to occur in the Falkland Islands (Hatfield and Rodhouse, 1994), and as far north as the coast of Argentina, Gulf of San Matias (Roper et al., 1984). A number of researchers (Nesis, pers. comm. 1985, 1987; Filippova et al., 1997) think that the squid identified as L. gahi in the southwest Atlantic is probably distinct and could be Loligo patagonica Smith, 1881.
Although Loligo gahi is an economically important resource in the artisanal fisheries of Peru (Cardoso, 1991) and Chile (Osorio et al., 1979), it is minimally present in the local commercial fish markets. Previous studies related to this species have focused on taxonomy (Nesis, 1973, 1987; Castellanos & Cazzaniga, 1979; Roper., 1984; Brakoniecki, 1984, 1986) and fishery biology (Arancibia & Robotham, 1984; Cardoso, et al., 1998; Villegas, 2000). Details on early life stages were characterized and illustrated by Guerra et al., 2001.
The present study provides information on the occurrence of egg strings and describes additional details on the early life history of L. gahi, in order to provide the basis for the beginning culture of this species in Peru.
Material and methods
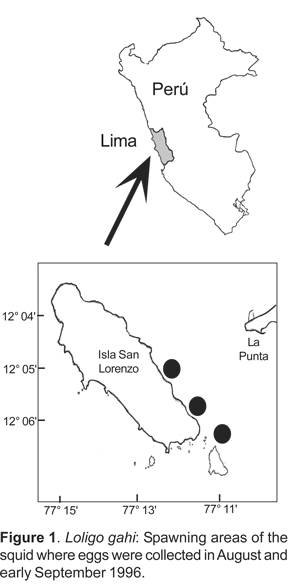
Egg strings of Loligo gahi were collected in the winter of 1996 by SCUBA divers at depths of 5-10 m off San Lorenzo Island (12º05'S, 77º03' W; Fig. 1). A total of 200 egg strings were transported in 10 L plastic containers to the Mariexport Enterprise Laboratory (160 egg strings in August), and Instituto del Mar del Perú Laboratory (40 egg strings in early September). Egg strings that had a thin sandy base were placed at the bottom of the aquarium in order to cover the peduncles. They were maintained in 40 and 90 L aquaria filled with filtered seawater and with moderate aeration, and a daily replacement of one third of water. All eggs were incubated at temperatures ranging from 18-19,2 °C and with a 12 h light/dark photoperiod.
Samples of egg capsules, eggs, and embryos were fixed in 2% formaldehyde neutralized with sodium borate. Fixed samples were measured to the nearest 0,1 mm using calipers. Two capsules were removed daily in order to follow embryonic development. Stages of embryonic development were assigned using the scale described for loliginid squid by Arnold (1965) and Guerra et al. (2001).
Paralarvae of Loligo gahi were characterized according to size (Hatfield and Rodhouse, 1994). Recently hatched paralarvae measure <4,5 mm ML and juveniles are those animals >4,5 mm ML. Paralarvae were fixed in 2% formaldehyde neutralized with sodium borate. A total of 100 recently hatched specimens were fixed for morphometric study. This was repeated at 20 and 45 days post hatching. All measurements were to the nearest 0,1 mm with calipers.
Recent hatchlings were placed in 300 L round tanks with continuous aeration and daily exchange of 100% of the water. The paralarvae were separated into two tanks. In one tank, filtered seawater (1 m m-net) was used and the rotifer, Brachionus plicatilis, was provided as daily food (densities of 128 ind./ml). In the second tank, a biological filter of diatomite was used (100 mm-net) and a diet of concentrated zooplankton (2,5 L/day of copepods, mysid and polychaete larvae) was provided. Dead paralarvae were removed daily using a siphon. Data were statistically analyzed and an analysis of variance was used to compare means (Sokal and Rohlf, 1981).
Results
During the period of observations and collection of egg capsules the temperature of the waters off San Lorenzo Island ranged from 14,9-16,2 ºC (mean 15,4; SD = 0,5 °C) and the oxygen levels from 0,78-4,36 mg/L (3,8; SD = 0,5 mg/L). The inshore currents moved in a north by northeastern direction with a velocity of 17-24 cm/sec.
Egg strings: Loligo gahi spawns in areas where the bottom substrate is either sandy or shelly. The majority of egg clusters are concentrated in deposits on the bottom at depths of 5-7 m. The number of egg capsules spawned in a single cluster ranged 55-349 (mean 329; SD = 0,2). The egg capsules are anchored to the substrate by a thin mucilaginous peduncle that measured 25-35 mm in length. The egg capsules are soft, gelatinous, and finger-like in shape. They vary in lengths from 88-169 mm (n= 72). In each capsule the eggs are arranged in a spiral and between 56-114 eggs (mean 85; SD = 12,7; n=16) were present. There was significant difference in capsule width between laid and hatched eggs (two sample t-tests for equal variances: t= 7, p< 0,05). The biometrics of egg capsules as observed both during laying and hatching is indicated in Table 1.
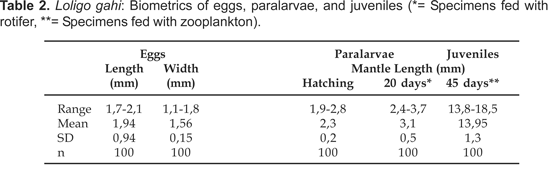

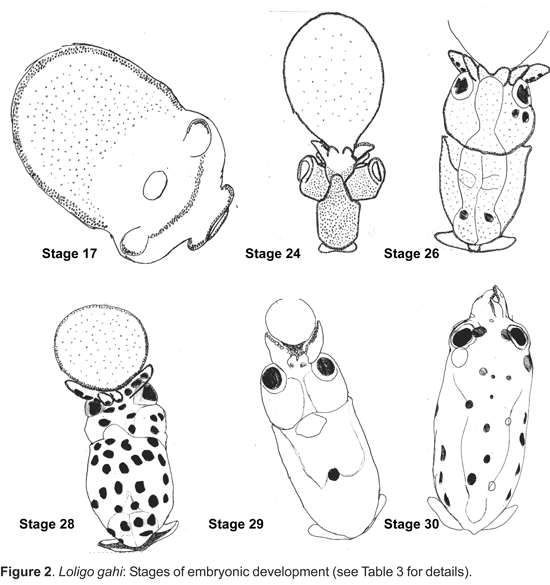
Eggs and embryos: The eggs of Loligo gahi are ovoid; their lengths range from 1,7-2,1 mm and widths from 1,1-1,8 mm (n= 100) (Table 2). Each embryo is enveloped by a chorion membrane and the perivitelline space measured 1,5 mm in diameter. The embryos inside their capsules are separated by dense layers and are arranged at regular intervals. The thirty stages of the embryonic development were observed in egg capsules studied but only were described in Table 3 the organogenesis stages (Fig. 2).
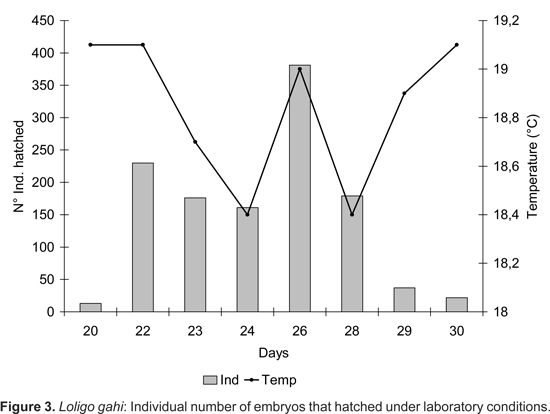
Hatchlings: Prior to hatching, the embryos moved constantly within the chorion capsule, (approximately 48-100 times/min) until breaking through the covering membranes. Hatching began 20 d after the freshly spawned eggs were collected and continued over a 10 d time span. The majority of the embryos hatched during the night and especially on days 22 and 26 (Fig. 3), agreeing with the peaks of greater temperature. The 89% of the embryos hatched at temperatures ranging from 18,5-19,1 ºC.
Paralarvae: Recently hatched Loligo gahi measured about 2,3 mm ML (Fig. 2, Stage 30). Over a period of 45 days the paralarvae increased in length up to 18,5 mm ML in (Table 2). The majority of the paralarvae were clustered near the water surface, aggregated towards the light, and were observed to swim constantly.
Paralarvae fed with the rotifer Brachionus plicatilis lived up to 20 d, reaching mantle lengths of 3,1 mm (= growth rate of 0,04 mm/d). In contrast, paralarvae fed on a diet of mixed zooplankton survived for 45 d and attained mantle lengths of 14 mm (= growth rate of 0,31 mm/d). Two peaks in mortality were observed in both treatments. In the first group 97% of the paralarvae died at 5-7 d and 100% were dead by 20 d. In the second group, only 60% of the paralarvae were dead after 5 d and 100% by 45 d.
Discussion
The presence of egg strings of Loligo gahi in shallow, coastal waters off San Lorenzo Island indicates that this is an area of spawning for the species. Loligo gahi typically deposits strings of eggs in cold water (sea surface temperature 15,4 °C). Where known in other loliginids spawning always occurs in protected, inshore waters: Loligo opalescens (Fields, 1965), L. pealeii (Summers, 1983), L. vulgaris vulgaris (Worms, 1983) and L. vulgaris reynaudii (Sauer et al., 1992).
Most loliginids squid studied lay their egg capsules one by one on a sandy bottom, anchoring them in the sand by the stick basal tip of the capsule (L. opalescens, McGowan, 1954; L. plei, Waller and Wicklung, 1968). The number of egg strings laid by individual females of the loliginid species under consideration appears to be quite variable. Drew (1911) recorded the usual number for a continuous laying period in L. pealeii to range 1 and 6, but one specimen deposited 23 strings. Loligo plei ranges 33 to 63 strings per mass (Roper, 1965). Loligo gahi female squids probably deposited between 14-16 capsules, each containing about 85 eggs. Guerra et al.(2001) stated that L. gahi females in Chile produce 50-60 eggs/capsule. Egg capsules of L. gahi varied considerably in length between Chilean and Peruvian populations; the differences most likely reflect the size of the female very similar to seems for Hanlon et al. (1989) by L. forbesi.
In Loligo gahi found off the coast of Peru, the length of the eggs ranged from 1,7-2,1 mm. This is very similar to L. pealei (Summers, 1983), which has the smallest eggs known of all Loligo species. In comparison to other species, the lengths of the hatchlings of L. gahi are relatively larger (1,9-2,8 mm ML). This size is very similar to the hatchlings of L. bleekeri (Hung-Baeg et al., 1992) and L. gahi from Chile as reported by Guerra et al. (2001).
In order to identify paralarvae species in the family Loliginidae, it is necessary to examine both morphometric characteristics and chromatophore pattern, as were the studies in Lolliguncula brevis, Lolliguncula mercatoris and Loligo vulgaris reynandii (Vecchione, 1982; Vecchione and Lipinski, 1995). Guerra et al. (2001), describes the chromatophore arrangement of newly hatched L. gahi.
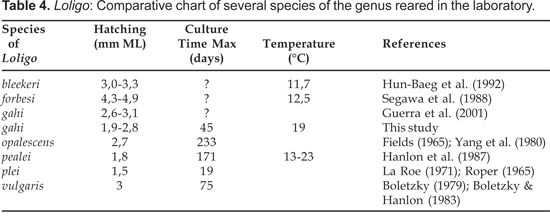
Loligo gahi is a cephalopod species with which we attempt to achieve a culture under laboratory conditions. Boletzky and Hanlon (1983) provide a review on the rearing and culture of cephalopod mollusks in closed seawater system. Hatchlings of L. gahi (2,6-3,1 mm ML) are of medium size, according to Guerra et al. (2001). They are smaller than L. forbesi and L. vulgaris and larger than L. plei and L. pealei (Table 4).
We have obtained two groups of culture for L. gahi, one that survived 20 days and the other one 45 days; and these differences are related to several factors such as water quality (temperature, oxygen concentration, salinity, etc.) and differences in the diet. The best success was obtained in a culture fed with zooplankton and using a biological filter of diatomite. This corroborates the experiences of Hanlon et al. (1979) as to reared hatchlings of L. opalescens with a diet of copepods. The maximum growth rate in our study was double the estimates for L. opalescens (Hurley, 1976), but slower than recorded for L. plei by La Roe (1971). Hanlon et al. (1979) mention that L. opalescens showed a clear preference for copepods, and the growth rates obtained with this diet were slightly higher than reported by Hurley (1976).
Acknowledgments
We thank Dr. Sigurd von Boletzky, Dr. Roger Villanueva, and Mg. Oscar Zuñiga for detailed comments on an earlier version of the manuscript and Dr. Eric Hochberg for several suggestions on how to reorganize the presentation of these ideas.
Literature cited
Arancibia, H. & H. Robothan 1984. Crecimiento y edad del calamar (Loligo gahi, Orbigny) de la Región Austral de Chile. Investigación Pesquera (Chile) 31: 71-79. [ Links ]
Arnold, J.M. 1965. Normal embryonic stages of the squid, Loligo pealii (Lesueur). Biological Bulletin 128: 24-32.
Boletzky, S.V. & R.T. Hanlon.1983. A review of the laboratory maintenance, rearing and culture of cephalopod molluscs. Memoirs of the National Museum Victoria 44: 147-187.
Brakoniecki, T.F. 1984. A full description of Loligo sanpaulensis Brakoniecki, 1984 and a redescription of Loligo gahi d'Orbigny, 1835, two species of squid (Cephalopoda; Myopsida) from the southwest Atlantic. Bulletin of Marine Science 34: 435-448.
Brakoniecki, T.F. 1986. A generic revision of the family Loliginidae (Cephalopoda: Myopsida) based primarily on the comparative morphology of the hectocotylus. Ph.D. Dissertation, University of Miami, Florida, USA. 163 pp.
Cardoso, F. 1991. Los calamares y potas (Cephalopoda: Teuthoidea) del mar peruano. Biota 15: 2-13.
Cardoso, F., J. Tarazona & C. Paredes 1998. Aspectos biológicos del calamar patagónico Loligo gahi (Cephalopoda: Loliginidae) en Huarmey, Perú. Revista Peruana de Biología. 5: 9-14.
Castellanos, Z. J. A. & N.J. Cazzaniga 1979. Aclaraciones acerca de los Loliginidae del Atlántico Sudoccidental (Mollusca: Cephalopoda). Neotropica 25: 59-68
Drew, G.A. 1911. Sexual activities of the squid, Loligo pealei (Les.). I. Copulation, egg laying and fertilization. Journal of Morphology 22: 327-359.
Fields, W. 1965. The structure, development, food relations, reproduction and life history of the squid Loligo opalescens Berry. Fish Bulletin 131: 1-108.
Filippova, J.A., D.O. Alekseev, V.A. Bizikov & D.N. Khromov. 1997. Commercial and mass cephalopods of the World Ocean. A manual for identification. VNIRO: Moscow. 272 pp. [In Russian, English summary].
Guerra, A., F. Rocha, A. F. Gonzalez and L. F. Bückle. 2001. Embryonic stages of the Patagonian squid Loligo gahi (Mollusca: Cephalopoda). The Veliger 44: 109-115.
Hanlon, R.T., R.F. Hixon, W.H. Hulet & W.T. Yang. 1979. Rearing experiments on the California market squid Loligo opalescens Berry, 1911. The Veliger 21: 428-431.
Hanlon, R.T., P.E. Turk, P.G. Lee & W.T. Yang. 1987. Laboratory rearing of the squid Loligo pealei to the juvenile stage: growth comparisons with fishery data. Fishery Bulletin 85: 163-167.
Hanlon, R.T., W.T. Yang, P.E. Turk, P.G. Lee & R.F. Hixon. 1989. Laboratory culture and estimated life span of the eastern Atlantic squid, Loligo forbesi Steenstrup, 1856 (Mollusca: Cephalopoda). Aquaculture and Fisheries Management 20: 15-34.
Hatfield, E.M.C. & P.G. Rodhouse. 1994. Distribution and abundance of juvenile Loligo gahi in Falkland Island waters. Marine Biology 121: 267-272.
Hun-Baeg, G., Y. Sakurai & K. Shimazaki. 1992. Embryonic stages of Loligo bleekeri Keferstein (Mollusca: Cephalopoda). The Veliger 35: 234-241.
Hurley, A.C. 1976. Feeding behavior, food consumption, growth, and respiration of the squid Loligo opalescens raised in the laboratory. Fishery Bulletin 74: 176-182.
La Roe, A.M. 1971. The culture and maintenance of the loliginid squids Sepioteuthis sepioidea and Doryteuthis pleii. Marine Biology 9: 9-25.
Mcgowan, J.A. 1954. Observation on the sexual behaviour and spawning of the squid Loligo opalescens at La Jolla, California. Californian Fish and Game 40: 47-54.
Nesis, K.N. 1973. Cephalopods of the Eastern Equatorial and Southeastern Pacific. Trudy Instituta Okenologii AN SSSR 94: 188-242. [In Russian, English summary]
Nesis, K.N. 1987. Cephalopods of the world. T.F.H. Publications: Neptune City, New Jersey. 351 pp. [translated into English by B.S. Levitov from the Russian versión, 1982. L.A. Burges (ed.)].
Osorio, C., C. Atria & F.S. Mann. 1979. Moluscos marinos de importancia económica en Chile. Biología Pesquera (Chile) 11: 3-47.
Roper, C.F.E. 1965. A note on egg deposition by Doryteuthis plei (Blainville, 1823) and its comparison with other North American loliginid squids. Bulletin of Marine Science 15: 589-598.
Roper, C.F.E, M.J. Sweeney & C.E. Nauen 1984. FAO Species Catalogue. Vol. 3, Cephalopods of the world, and species of interest to fisheries. FAO Fisheries Synopsis 3: 1-277.
Sauer, W.H.H., M.J. Smale & M.R. Lipinski. 1992. The location of spawning grounds, spawning and schooling behaviour of the squid Loligo vulgaris reynaudii (Cephalopoda: Myopsida) off the Eastern Cape Coast, South Africa. Marine Biology 114: 97-107.
Segawa, S., W.T. Yang, H.J. Marthy & R.T. Hanlon 1988. Illustrated embryonic stages of the Eastern Atlantic squid Loligo forbesi. The Veliger 30: 230-243.
Sokal, R.R. & F.J. Rohlf. 1981. Biometry. 2nd ed. W.H. Freeman & Co., New York. 859 pp.
Summers, W.C. 1983. Loligo pealei. Pp 115-142. In: Boyle, P.R. (ed). Cephalopod life cycles. Vol. I. Species accounts. Academic Press: London.
Vecchione, M. 1982. Morphology and development of planktonic Lolliguncula brevis (Cephalopoda: Myopsida). Proceedings of the Biological Society of Washington 95: 602-609.
Vecchione, M. & M.R. Lipinski. 1995. Descriptions of the paralarva of two loliginid squids in South African waters. South African Journal of Marine Science 15: 1-7.
Villegas, P. 2000. Aspectos de la biología y pesquería de Loligo gahi del área del Callao, Perú (Enero 1996 a Mayo 1997). Tesis, Título Profesional de Biólogo, Hidrobiología y Pesquería. Universidad Nacional Mayor de San Marcos. Lima, Perú.
Waller, R.A. & R.I. Wicklung. 1968. Observations from a research submersible mating and spawning of the squid, Doriteuthis plei. BioScience, (February 1968): 110-111.
Worms, J. 1983. Loligo vulgaris. Pp 143-157. In: Boyle, P.R. (ed). Cephalopod life cycles. Vol. I. Species accounts. Academic Press: London.
Yang, W.T., R.T. Hanlon, M.E. Krecjci, R.F. Hixon & W.H. Hulet. 1980. Culture of California market squid from hatching-first rearing of Loligo to subadult stage. Aquabiology 2: 412-418.
Correspondencia:
1Laboratorio de Biología y Sistemática de Invertebrados Marinos, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Apdo. 11-0058, Lima 11, Perú
2Gerencia de Acuicultura, Fondo de Desarrollo Pesquero (FONDEPES), Av. Petit Thouars 115, Lima 1, Perú.
3Area de Cultivos Marinos, Instituto del Mar del Perú, Apdo. 22, Callao, Perú
E mail Franz Cardoso:fcardosop@unmsm.edu.pe













