Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.13 no.1 Lima Oct. 2006
NOTA CIENTÍFICA
1 Departament de Genètica i de Microbiologia Universitat Autònoma de Barcelona, SPAIN.
2 Australian School of Environmental Studies, Griffith University, Nathan, Australia.
Presentado: 28/02/2006
Aceptado: 28/08/2006
Abstract
Adults of the dry area specialist Strymon jacqueline Nicolay & Robbins, 2005 (Lepidoptera: Lycaenidae: Eumaeini) are here recorded feeding on extrafloral nectar of the large cactus Neoraimondia arequipensis var. gigantea (Werdermann & Backeberg) Ritter. The significance of these observations is discussed in relation to lycaenid survival in a xeric environment, pollination and mate location.
Keywords: Ants, butterflies, cactus, desert, Formicidae.
Resumen
Se describe el uso de néctar extrafloral del cactus Neoraimondia arequipensis var. gigantea (Werdermann & Backeberg) Ritter por parte de adultos de la mariposa especialista en zonas áridas Strymon jacqueline Nicolay & Robbins, 2005 (Lepidoptera: Lycaenidae: Eumaeini). Se discuten las implicaciones de estas observaciones en relación a la supervivencia y reproducción en un entorno desértico y a la polinización.
Palabras clave: Hormigas, mariposas, cactus, desierto, Formicidae .
The eumaeine lycaenid Strymon jacqueline was recently described by Nicolay & Robbins (2005) as a dry-area specialist that occurs in the Tumbesian Regions and the Río Marañón Valley (northwestern Peru, Cajamarca) at elevations between 460-1300 m. The type locality is Peru, Cajamarca, kilometre 62 Pacasmayo to Cajamarca, 600 m. This species probably breeds continuously all year round since adults have been collected in March, June, September and November. It belongs to the S. serapio species group, although its precise systematic position within this group remains unclear.
On the afternoon of May 11, 2005 the authors stopped briefly next to the road that runs along the Chilete-Jequetepeque river valley, from Pacasmayo to Cajamarca, kilometre 95, 550 m (07°13'46"S, 79°03'15"W). The site was extremely hot and dry, and the vegetation sparse (Fig. 1).

Surprisingly, about 20 individuals of S. jacqueline, both males and females, were observed flying around and landing on some large Neoraimondia arequipensis var. gigantea (Werdermann & Backeberg) Ritter, dominant cacti in the surrounding landscape. No other butterfly species were seen in the immediate area.
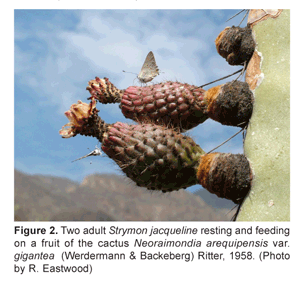
The S. jacqueline appeared to be especially interested in the many fruits that protruded laterally from the cactus (Fig. 2) and a closer investigation revealed that the butterflies were actively searching or feeding on small droplets of extrafloral nectar that the cactus fruits produced (Fig. 3).

Some S. jacqueline were also seen to visit the cactus flowers. In addition to the butterflies, several wasps and flies were also actively feeding on the same exudates; however, no ants were found on the cacti.
Subsequent to the first observations, a second site was investigated in a similar dry habitat some 50 km further down the Chilete-Jequetepeque Valley at kilometre 44, 440 m (07°13'15" S, 79°09'35" W). This second site consisted of a long, narrow valley adjacent to the road where the vegetation was slightly taller and more diverse than the first site and included numerous giant cacti N. arequipensis var. gigantea. However, after about 30 min of exploration, no butterflies were seen, except for several dark tailed hesperiids, probably of the genus Urbanus, which were flying straight down the valley. A prominent N. arequipensis cactus with many fruits was found to be visited by a large number of ants of the genus Crematogaster plus a few Pseudomyrmex workers that were feeding on the extrafloral nectar droplets.
Extrafloral or vegetative nectaries are defined as sugar-producing glands situated outside the flowers that are not directly involved in pollination (Bentley, 1977; Elias, 1983). They occur in more than 90 plant families worldwide (Koptur 1992), particularly among the angiosperms, and their morphology ranges from almost microscopic glands to highly conspicuous specialized structures (Bentley, 1977; Elias, 1983). Extrafloral nectar feeding by adult Lepidoptera is quite common, especially in the Lipteninae and Miletinae (Lycaenidae) (Farquharson, 1921; Jackson, 1937; Atsatt, 1981; Callaghan, 1992; Fiedler, 1996). Adult lycaenids are also known to feed on homopteran honeydew (e.g. Hinton, 1951; Pierce, 1995), and Gilbert (1976) published a photograph of an adult of Megalopalpus Röber feeding from the dorsal nectary secretion of an unidentified lycaenid caterpillar. Adults of the Australian lycaenid Jalmenus evagoras have also been observed feeding from the dorsal nectary secretions of conspecific larvae (A. H. Axén and R. Eastwood, pers. obs.). Some lycaenid and riodinid larvae also feed on plant extrafloral nectaries (DeVries & Baker, 1989; DeVries et al., 1992; Fiedler, 1996). Indeed, many lycaenid and riodinid larvae have unusual feeding habits beyond classical phytophagy, including entomophagy (Hinton, 1951; Cottrell, 1984; Fiedler, 1991, 1996; Pierce 1995; Pierce et al., 2002), trophallaxis with adult ants (Cottrell, 1984; Fiedler, 1991; Pierce et al., 2002), and feeding on homopteran-secreted honeydew (Waterhouse, 1932; Hinton, 1951; Pierce, 1995). Outside of the Lycaenidae and Riodinidae, extrafloral nectar feeding has been suggested for the hesperiid Hesperopsis gracielae (MacNeill, 1970), although direct evidence is still lacking in this case (Wiesenborn, 1997). Several species of adult moths, including many pest species, are nocturnal feeders at extrafloral nectaries of cotton (Gossypium hirsutum L.) (Lukefahr & Rhyne, 1960; Rejesus, 1968; Rogers, 1985; Wiesenborn & Baker, 1990).
Unfortunately, the larval morphology and hostplant for S. jacqueline is still unknown. The species belongs to a Bromeliaceae-feeding lineage (Nicolay & Robbins, 2005) and although some scattered bromeliad plants were present on the ground and rocks at the location where our specimens were collected, no butterfly activity was observed near these plants.
An association between N. arequipensis and ants has been described from the Lima region of Peru for N. arequipensis var. roseiflora (Werdermann & Backeberg) Rauh and Camponotus sp. ants (Ostolaza, 1985; Novoa et al., 2003). Moreover, numerous other studies have documented ant visitation to Cactaceae extrafloral nectaries suggesting it is a frequent phenomenon worldwide (e.g. Lloyd, 1908; Pickett & Clark, 1979; Blom & Clark, 1980; Pemberton, 1988; Oliveira et al., 1999). The benefit of such a relationship for the ants seems evident since cacti extrafloral nectar represents a source of water, sugars and amino acids in xerophytic environments (Pickett & Clark, 1979; Ruffner & Clark, 1986). Several hypothetical benefits for the cacti have been proposed, including defence against herbivores, and enhanced access to soil nutrients due to the presence of ants nesting in the soil at the base of the cactus (Wagner, 1997). Moreover, it has been suggested that extrafloral nectaries may also play an indirect role in pollination, for example by helping to attract potential pollinators (Ford & Forde, 1976; Wäckers & Bonifay, 2004). Indeed, adults of S. jacqueline were observed feeding on cactus flowers, so the possibility exists that this species could serve N. arequipensis as a pollinator. Furthermore, simultaneous concentrations of male and female S. jacqueline on fruiting plants of N. arequipensis may facilitate mate location; however, although all specimens appeared freshly eclosed, we did not observe any mating activity or male territorial behavior.
The locality where we collected S. jacqueline is situated about 30 km from the type locality where Gerardo Lamas collected a series of four specimens in 1981. One of these specimens was collected on a cactus, and in the species description of S. jacqueline, Nicolay and Robbins state that adults have been observed on cactus plants `in the middle of the desert'. It is possible that these individuals were also searching for or imbibing cactus extrafloral nectar. The poor environment where S. jacqueline flies seems unlikely to be able to sustain large populations of butterflies, and Nicolay and Robbins (2005) note that S. jacqueline appears to be exceedingly rare. However, Nicolay and Robbins also recorded `ten individuals in one day at two localities' and R. Robbins (pers. comm.) has informed us that these individuals were observed as aggregations on flowers. Thus, localised nectar sources, either flowers or extra floral nectaries may stimulate aggregating behaviour in S. jacqueline and could account for occasional observations of relatively large numbers of individuals.
The substantial variability that this species displays in wing pattern (Nicolay & Robbins 2005) was also observed in the series of eight specimens collected on this occasion. However, the wide variation in colour among individuals found at a single site does not support the hypothesis of Nicolay & Robbins (2005) that the intensity of the female orange scaling may be correlated with altitude. The amount of orange on the submarginal area of the dorsal forewings of females varied widely and was even absent in some individuals. The presence of the two ventral hindwing basal spots was also variable in both sexes.
Two males and one female S. jacqueline are deposited in the collections of the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM); one male and one female in the National Museum of Natural History, Smithsonian Institution, Washington, DC, (USNM); and three females, with bodies preserved in 100% ethanol and wings in separate glassine envelopes, are deposited at the Museum of Comparative Zoology (MCZ) DNA and Tissue Collection, Harvard University, Cambridge, Massachusetts (sample codes: RV-05-M335 to RV-05-M337). Alcoholic samples of the ants collected on the cacti are deposited in the DNA and Tissue Collection at the MCZ (sample codes: RV-05-J845 and RV-05-M338).
The concentration of insects on fruiting cacti in such a harsh environment contrasted generally with the lack of insect activity in the surrounding area and on other nearby cacti of the same species with fewer or no developed fruits. This leads us to conclude that the extrafloral nectar of N. arequipensis var. gigantea is an important source of nutrients and/or water in this xeric environment and acts as a powerful attractant for several species of insects.
Acknowledgements
Support for the expedition that led to the observations reported here came from National Science Foundation grant DEB 0447242 to N.E. Pierce. We warmly thank Gerardo Lamas (MUSM), Naomi E. Pierce (MCZ) and Robert K. Robbins (USNM) for their encouragement and comments on the present paper. Many thanks to Carlos Ostolaza (Sociedad Peruana de Cactus y Suculentas) for identifying the cactus and to Corrie Saux Moreau (MCZ) for determination of the ant genera.
Literature cited
1. Atsatt P. R. 1981. Lycaenid butterflies and ants: selection for enemy-free space. American Naturalist 118: 638-54. [ Links ]
2. Bentley, B. L. 1977. Extrafloral nectaries and protection by pugnacious bodyguards. Annual Review of Ecology and Systematics 8: 407-428. [ Links ]
3. Blom P. E. & W. H. Clark. 1980. Observations of ants (Hymenoptera: Formicidae) visiting extrafloral nectaries of the barrel cactus, Ferocactus gracilis Gates (Cactaceae), in Baja California, Mexico. Soutwestern Naturalist 25: 181-196. [ Links ]
4. Callaghan C. J. 1992. Biology of epiphyll feeding butterflies in a Nigerian Cola forest (Lycaenidae: Lipteninae). Journal of the Lepidopterists' Society 46(3): 203-214. [ Links ]
5. Cottrell C. B. 1984. Aphytophagy in butterflies: its relationship to myrmecophily. Zoological Journal of the Linnean Society 79: 1-57. [ Links ]
6. DeVries P. J. & I. Baker. 1989. Butterfly exploitation of an ant-plant mutualism: Adding insult to herbivory. Journal of the New York Entomological Society 97(3): 332-340. [ Links ]
7. DeVries P. J., Chacon, I. A. & D. Murray. 1992. Toward a better understanding of host use and biodiversity in riodinid butterflies (Lepidoptera). Journal of Research on the Lepidoptera 31: 103-26. [ Links ]
8. Elias T. S. 1983. Extrafloral nectaries: their structure and functions. In: B. Bentley & T. S. Elias (eds), The Biology of Nectaries. New York, Columbia University Press. Pp. 147-203. [ Links ]
9. Farquharson C. O. 1921. Five years' observations (19141918) on the bionomics of Southern Nigerian insects, chiefly directed to the investigation of lycaenid life histories and to the relation of Lycaenidae, Diptera and other insects to ants. Transactions of the Entomological Society of London 1921: 319-448. [ Links ]
10. Fiedler K. 1991. Systematic, evolutionary, and ecological implications of myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papilionoidea). Bonner Zoologische Monographien 31: 5-157. [ Links ]
11. Fiedler K. 1996. Interactions between lycaenid butterflies and ants in Peninsular Malaysia. In: D. S. Edwards et al. (eds), Tropical Rainforest Research - Current Issues. Amsterdam, Kluwer Academic Publishers. Pp. 291-296. [ Links ]
12. Ford H. A. & N. Forde. 1976. Birds as possible pollinators of Acacia pycnantha. Australian Journal of Botany 24: 793-795. [ Links ]
13. Gilbert L. E. 1976. Adult resource in butterflies: African lycaenid Megalopalpus feeds on larval nectary. Biotropica 8(4): 282-283. [ Links ]
14. Hinton H. E. 1951. Myrmecophilous Lycaenidae and other Lepidoptera - a summary. Proceedings of the South London Entomological and Natural History Society 1949-50: 111-175. [ Links ]
15. Jackson T. H. E. 1937. The early stages of some African Lycaenidae (Lepidoptera), with an account of the larval habits. Transactions of the Royal Entomological Society of London 86: 201-238. [ Links ]
16. Koptur S. 1992. Extrafloral nectary-mediated interactions between insects and plants. In: E. Bernays (ed.), InsectPlant Interactions, vol. 4. CRC Press, Boca Raton. Pp. 81129. [ Links ]
17. Lukefahr M. J. & C. L. Rhyne. 1960. Effect of nectariless cotton on populations of three lepidopterous insects. Journal of Economical Entomology 53: 242-243. [ Links ]
18. Nicolay S. S. & R. K. Robbins. 2005. Five new dry-area South American Strymon species (Lycaenidae: Theclinae) and their biogeographic significance. Journal of Research on the Lepidoptera 38: 35-49. [ Links ]
19. Novoa S., V.Castro, A. Ceroni & I. Redolfi. 2003. Relación entre la hormiga Camponotus sp. (Hymenoptera: Formicidae) y una comunidad de cactus (Cactaceae) en el Valle del Río Chillón. Ecología Aplicada 2(1): 69-73. [ Links ]
20. Oliveira P. S., V. Rico-Gray, C. Díaz-Castelazo & C. Castillo-Guevara. 1999. Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae). Functional Ecology 13: 623-631. [ Links ]
21. Ostolaza C., W. L. Mitich & J. M. King. 1985. Neoraimondia arequipensis var. roseiflora (Werd & Backbg) Rauh. Cactus and Succulent Journal 57: 60-64. [ Links ]
22. Pemberton R. W. 1988. The abundance of plants bearing extrafloral nectaries in Colorado and Mojave desert communities of southern California. Madroño 35: 238-246. [ Links ]
23. Pickett C. H. & W. D. Clark. 1979. The function of extrafloral nectaries in Opuntia acanthocarpa (Cactaceae). American Journal of Botany 66: 618-625. [ Links ]
24. Pierce N. E. 1995. Predatory and parasitic Lepidoptera: Carnivores living on plants. Journal of the Lepidopterists' Society 49(4): 412-453. [ Links ]
25. Pierce N. E., M. F. Braby, A. Heath, D. J. Lohman, J. Mathew, D. B. Rand & M. A. Travassos. 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annual Review of Entomology 47: 733-771. [ Links ]
26. Rejesus R. S. 1968. Bio-ecological studies on the cotton leaf perforator, Bucculatrix thurberiella Busck (Lepidoptera: Lyonetiidae). PhD Thesis, University of California, Riverside, California. [ Links ]
27. Rogers C. E. 1985. Extrafloral nectar: Entomological implications. Bulletin of the Entomological Society of America 31: 15-20. [ Links ]
28. Ruffner G. A. & W. D. Clark. 1986. Extrafloral nectar of Ferocactus acanthodes (Cactaceae): composition and its importance to ants. American Journal of Botany 73: 185-189. [ Links ]
29. Wäckers F. L. & C. Bonifay. 2004. How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85(6): 1512-1518. [ Links ]
30. Wagner, D. 1997. The influence of ant nests on Acacia seed production, herbivory and soil nutrients. Journal of Ecology 85: 83-93. [ Links ]
31. Waterhouse G. A. 1932. What Butterfly is That? A Guide to the Butterflies of Australia. Angus and Robertson, Sydney. 291pp. [ Links ]
32. Wiesenborn W. D. 1997. Hesperopsis graciliae (MacNeill) (Lepidoptera: Hesperiidae) flight between hostplants and Prosopis glandulosa Torrey. Pan-Pacific Entomologist 73(3): 186-189. [ Links ]
33. Wiesenborn W. D. & T. C. Baker. 1990. Upwind flight to cotton flowers by Pectinophora gossypiella (Lepidoptera: Gelechiidae). Environmental Entomology 19(3): 490-493. [ Links ]
Correspondencia:
1 ICREA researcher at Departament de Genètica i de Microbiologia Universitat Autònoma de Barcelona Edifici C, Campus de la UAB. 08193 Cerdanyola del Vallès, SPAIN.
E-mail: Roger Vila rogervilau@yahoo.es
2 Australian School of Environmental Studies, Griffith University, Nathan, Qld 4111, Australia.
E-mail: Rod Eastwood R.Eastwood@griffith.edu.au













