Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.14 no.1 Lima Aug. 2007
Aníbal F. Cóndor_Golec
MSc Organic Plant Production, Agronomist Engineer, Peruvian researcher, address: Calle Francisco de Cuellar 451 casa 18, Monterrico Surco, Lima 33, Lima-Perú
Introductión
Azadirachta indica A. Juss (Meliaceae) is an indian tree, commonly called the neem tree. It has many useful compounds that act as insecticide. Neem insecticides have in their composition azadirachtin, a tetranortriterpenoid plant limonoid which can be isolated from the seeds of the neem tree (Mordue & Blackwell, 1993). Azadirachtin is also termed Azadirachtin A (Rembold, 1989).
Several commercial preparations are available including: Azatin, Bioneem, Neemies, Safer's ENI, Wellgro, RD-Repelin, Neemguard, Neemark and Neemazal. Neem seed oil is often a starting material for such insecticides, their biological activity being related to its azadirachtin content (Isman et al., 1990).
Formulations include emulsifiable concentrates (ECs), suspension concentrates (SCs), ultra low volume (ULV) formulations (Feuerhake and Schmutterer, 1985) and granular formulations (Saxena, 1989). Succesful control of pests with neem products can be obtained with the use of solvents for the formulations of field applications (Mordue & Blackwell, 1993).
The effects of azadirachtin on insects include feeding and oviposition deterrence, growth inhibition, and fecundity and fitness reductions (Schmutterer, 1990).
Azadirachtin is a common example of a natural plant defence chemical affecting feeding, through chemoreception (primary antifeedancy), that consists in the blockage of the input from receptors that normally respond to phagostimulants, or from stimulation of specific deterrent cells or both (Chapman, 1974; Dethier, 1982) and through a reduction in food intake due to toxic effects if consumed (secondary antifeedancy), where food intake is reduced after application of azadirachtin in ways which bypass the mouth part chemoreceptors (Mordue & Blackwell, 1993). Antifeedancy can be assessed from crude to refined neem extracts to neem enriched extracts to pure azadirachtin. Lepidoptera show effective sensitivity to azadirachtin with antifeedant effects at concentrations ranging between 1 and 50 ppm. Azadirachtin has also growth regulatory effects on larval insects like disruption of moulting, growth inhibition, malformation, which may contribute to mortality. This is attributed to a disruption of endocrine events as the down-regulation of haemolymph ecdysteroid level through the blockage of release of PTTH, prothoracicotropic hormone, from the brain-corpus cardiacum complex, or to a delay in the appearance of the last ecdysteroid peak showing a complete moult inhibition. There are also effects on allatropin and juvenile hormone titres (Mordue & Blackwell, 1993). Dealing with reproduction, adverse effects on ovarian development, fecundity and fertility have been reported (Karnavar, 1987).
As the neem-based insecticides are not toxic to humans and many beneficial arthropods, and the pests are unlikely to become resistant, these insecticides have been advocated to replace synthetic insecticides become the more sensible to be used in most pest management programs (Feng and Isman, 1995; Immaraju, 1998; Walter, 1999).
Under laboratory conditions, low doses of azadirachtin (10 and 20 ppm) did not harm hymenopteran parasitoid Apanteles glomeratus but its host, final instar Pieris brassicae had reduced feeding and gradually died. But the parasitoid is killed when larval instars are treated in the field. A delay in spraying neem extracts until later larval stages appear would be convenient according to Schmutterer (1992).
Low doses of neem seed extracts (NSE), 10 ppm of active ingredient, allowed hymenopteran parasitoids to emerge unharmed and these were able to mate and seek new fruit fly hosts, enhancing the effectiveness of biological control (Stark et al., a, b, 1990, 1992).
There are also some studies that prove the neem's lack of toxicity against spiders and mites. Like Chiracanthium mildeu (predator of citrus fruit) with its prey Tetranychus cinnabarinus that is highly susceptible to neem (Mansour and Ascher, 1983). Phytoseiulus persimilis is also not harmed by NSE, specially its fecundity while T. cinnabarinnus is up to 58 times more toxic than it (Mansour et al., 1987). Delphastus pusillus a coccinellid predator was not toxic to azadirachtin when applied either the plant or Bemisia tabaci eggs (Hoelmer et al., 1990).
There are also adverse effects of neem against beneficials. There was suppression of ecdysis and 100% mortality of Cotesia congregata, hymenopteran parasitoid of Manduca sexta. This was seen with injections of 10 µg azadirachtin into newly ecdysed fourth or fifth instar host larvae (first larval ecdysis of the wasps) (Beckage et al., 1988). There were also moulting delays and deformities in Perillus bioculatus when Leptinotarsa decemlineata was treated with NSE (Hough-Goldstein and Keil, 1991). Also NSE reduced the population of Encarsia ssp. and Aleurodiphilus spp., parasitoids of Bemisia tabaci (Price and Schuster, 1991). Bees can also be harmed if spraying is done before flowering (Schmutterer and Holst, 1987).
This short background of neem insecticides will let us understand and study more in detail the present applied case.
The objective of the present essay was to study recent cases of the effect of neem products on parasitoids. Experiments done under laboratory and field conditions were discussed. The main aspects to describe and analyse were formulation and concentration of neem products, mode of application, timing of application and parameters asessed (mortality, adult emergence, antifeedancy, as the most important).
Description of relevant methodologies
Lyons et al., (2003) carried out a bioassay where eggs of the Mediterranean flour moth, Ephestia kuehniella, treated with an acetone solution containing an azadirachtin-based formulation (Azatin EC, 3% azadirachtin; 100% Azadirachtin standard and Neem EC, 4.6% azadirachtin). They were presented to individual Trichogramma minutum females. Survival of T. minutum females 1 day after treatment, number of eggs parasitized, proportion of parasitized eggs from which adults emerged and sex ratios of emerging adult parasitoids. Two formulations of neem-seed extracts containing azadirachtin and a purified azadirachtin standard were tested at an operational dose (50g azadirachtin/ha or 0.00375%) for conifer-feeding sawflies application rate in Canada, and at 10 times the operational dose (500g azadirachtin/ha or 0.0375%). No significant effects were observed on survival of parasitoid females with the operational dose but at 500 g azadirachtin/ha female survival was significantly reduced by Azatin EC and Neem EC and no reduction was seen with the 100% azadirachtin treatment, being other components responsible for the toxicity of females. At 500 g azadirachtin the number of eggs parasitized was greatly reduced by Azatin EC and slightly reduced by Neem EC but was not reduced by an azadirachtin standard. However, sex ratio of emerging adults was not affected.
Tang et al., (2002) developed a bioassay where 10 pairs of adults of Lysiphlebus testaceipes (parasitoid of Toxoptera citricida) were confined in a glass vial containing leaf dipped in neemix 4.5 (4.5% azadirachtin), at 11, 45 and 180 ppm of azadirachtin. Mortality was evaluated. Emergence rate of adult parasitoid was also tested, from 50-60 aphid mummies located in sour orange foliage and treated in Neemix suspension. Survival was not significantly reduced with the first two neem concentrations as well as the percentage of adult emergence. However, only a small significant reduction in survival and emergence rate of parasitoids was obtained with 180 ppm of azadirachtin.
Akol et al., (2002), evaluated two neem-based insecticides, Neemroc EC (neem seed oil (NSO) and Neemros (neem kernel cake powder (NKCP), and the synthetic pyrethroid Karate, to prove safety of sprays to Diadegma mollipla (parasitoid of Plutella xylostella). Sprays were applied at the recommended dose field rate of 15ml/l of water (300ml/ha) and 25g/l of water (500g/ha) for each neem product mentioned, respectively. Toxicity to neem (mortality and longevity), foraging behaviour and parasitation ability of Diadegma mollipla (female adults) in second instar of P. xyllostella (infested over cabbage leaves), were tested. There were no mortality with NKCP-sprayed treatment and insignificant mortality in the NSO treatment compared to Karate with 100% of mortality. There were no significant differences in the mean longevity of the wasps between the neem insecticides. Searching, ovipositing and grooming were the predominant activities spending more time on infested plants with second instar diamondback moth (DBM). There were no differences in parasitism rates, with a mean of 52% among treatments, with a water-sprayed treatment included.
Saber et al., (2004) found that there were adverse effects of Neemazal 1% on Trichogramma cacoeciae. Egg discs of hosts (Cydia pomonella and Sitrotoga cerealella) were dipped in 3000 ppm solution of the formulated insecticide at different preimaginal stages. Adult emergence (wasps) from eggs was affected more in S. cerealella than C. pomonella, as its egg chorion may prevent insecticide penetration. Parasitized host eggs exposed to insecticide at pupal stage yielded the lowest percentage of emergence followed by eggs treated at larval and prepupal stages, especially on C. pomonella. More damage is seen as the parasitoid advance in development on C. pomonella eggs.
Mitchell et al., (2004) made a trial with aqueous suspension from neem seed kernel. A 50% honey solution was mixed using the neem suspension at 0, 0.625, and 5%. Antifeedant effects and oviposition behaviour was evaluated on adults of Gryon fulviventre, egg parasitoid of Clavigralla scutellaris, a coreid pest of pigeonpea Cajanus cajan. Repellency of neem was seen on adults of G. fulviventre, being the response concentration-dependent. Duration of antennation (washing the antennae) was significantly affected by neem as the time spent was extended compared to a 0% neem concentration. However, success rate of wasps that parasitized neem-dipped Clavigralla eggs did not differ from the wasps offered control eggs dipped in distilled water. Neem also did not interfere with the ability of G. fulviventre females to gauge the suitability of the host egg, to determine whether it had been marked or to locate an appropriate are to commence drilling.
Exposure of parasitized eggs to 5% neem suspension did not reduce emergence success of resulting adult wasps (progeny). Also longevity of males and female wasps was not affected by preimaginal exposure (larval or prepupal stage) to 5% neem.
Raguraman & Singh (1999), tested neem seed oil at concentration of 5, 2,5, 1,2, 0,6 and 0,3% on egg parasitoid Trichogramma chilonis for biological effects, namely oviposition and feeding deterrency, toxicity, sterility and IGR activity. The host egg used was Corcyra cephalonica (rice moth). In the choice test done NSO at all concentrations drastically reduced egg laying by T chilonis. While in a no choice condition, neem seed oil at or above the 0,6% concentration caused more than 50% reduction in parasitization. Feeding deterrence was also seen at or above 1,2% concentration in both tests mentioned above. In feeding toxicity tests, NSO (treated with honey ) at 5% concentration caused less than 50% mortality to both males and females but in contact toxicity (host eggs treated with neem seed oil), females were affected sparing males. No sterility effect (parasitism, adult emergence, longevity) was seen when the parasitoid was fed with neem seed oil-treated honey. Pretreatment of host (before parasitism) with neem seed oil at 5 and 2,5% affected the T. chilonis adult emergence. On the other hand, posttreatment (after parasitism) did not influence the developing stages of this parasitoid and adult emergence as this is well- protected by the chorion of the host egg.
Simmonds et al., (2002), used two neem formulations (a crude ethanol extract from A. indica, 1% azadiractin and Azatin EC, 3% azadirachtin), over a range of 10000 ppm to 1 ppm. Mortality was calculated at 100 ppm. The parasitoid Encarsia formosa and its host the glasshouse whitefly Trialeurodes vaporariorum (dwarf french bean pest) were tested. There were no toxic effects (significant mortality) on adults of E. formosa with the use of the two neem products, obtaining a LD50 of 1000 ppm A. indica derived treatments did not decrease the proportion of E. formosa emerging from parasitized whitefly pupae, in a cage experiment. About the stabbing behaviour (brown wound marks that reflects oviposition) tested, E. formosa does not discriminate between untreated whitefly nymphs and those treated with A. indica-derived botanicals, as more than 1000 ppm of the insecticide is needed to elicit a 50% discrimination index. Leaves treated with A. indica-derived botanicals at 100 ppm, resulted in more parasitoids emergence from whitefly pupae than the control and other botanicals tested (naphtoquinones from Calceolaria andina and pyretrum).
Stansly & Liu (1997), made a comparison with neem seed extract (Margosan-O, 2,5 g/l azadirachtin) at 0,06 g of active ingredient, insecticidal soap, sugar esters from Nicotiana gossei, the pyrethroid Capture and Sunspray oil to show selectivity of insecticides to Encarsia pergandiella. The first three insecticides showed little effect or no effect on E. pergandiella, an endoparasitoid of Bemisia argentifolii (sweet potato whitefly). A significant reduction in parasitoid emergence from second and third instar nymphs of B. argentifolii was obtained proving neem's toxicity. No significant effect was observed from the soap and Margosan-O applied 7 days after parasitization on survival and emergence of young larval parasitoids. More than 50% of parasitoid pupae treated with neem insecticide, the soap and N. gossei sugar ester, survived to emerge compared to the chemical insecticides where most parasitoid were killed. Mortality of wasp adults was also relatively slight, with similar results in glass and leaf surfaces. Neem insecticide residues on tomato plants reduced parasitization of B. argentifolii statistically as much as the pyrethroid.
Lowery & Isman (1995), compared NSO applied in three concentrations, 0,5%, 1% and 2% (aqueous emulsions) to see the effect of neem on parasitism of Myzus persicae (green peach aphid) by Diaeretiella rapae, on mustard cabbage sprayed with neem. Also emergence of adult parasitoid was evaluated, dipping mummies of M. persicae into the emulsion previously. Also a field study was carried out with different crops where NSO (1%) and NSE (neem seed extract) (1%) (containing 20 ppm azadirachtin each) were applied, parasitized aphids were counted. Foliar sprays of NSO reduced the absolute number of parasitized M. persicae in dose-dependent manner. Emergence of adult D. rapae decreased with increasing concentrations of NSO but no significant differences were seen among rates of NSO. Dipping mummies in solutions of NSO affected parasitoid survival (emergence of D. rapae adults) adversely, especially from young mummies. About the field trial, absolute counts of parasitized aphis did not differ from any treatment including the control. Number of mummies per 1000 aphids in the two neem treatments (NSO and NSE) were 3,8 and 3,5 times higher than controls.
Ahmad et al., (2003), carried out an experiment with neem kernel water extract (NKWE) at 5 concentrations of 30, 60, 125, and 250 mg azadirachtin/l. In order to see the parasitoid effect of D. rapae on aphids, placed on potted cabbage plants. NKWE was applied to soil and leaves. Parasitization, weight of mummies and rate of emergence in two aphid generations were evaluated. Application to the soil, produced negative reactions, when aphids on the plants were parasitized, showing low percentage parasitization, lowered mummy weight, low emergence rate of adults of F1 and F2. On the other hand, foliar sprays of NKWE have less severe effects in D. rapae.
Goudegnon et al., (2000), developed a field experiment (cabbage cultivation) where a neem kernel solution was used, that contained 0,61% azadirachtin (w/w) and was compared with deltamethrin to see the effect on diamondback moth and Cotesia plutellae. Concluding that apparently the neem extract does not affect the C. plutellae populations. Despite that the ratio C. plutellae to P. xylostella after treatments was significantly different from the deltamethrin treatment.
Raguraman & Singh (1998), tested an aqueous suspension and an ethanolic extract of neem seed kernel (NSK) at 0,3, 0,6, 1,2, 2,5 and 5% concentrations for ovipositional deterrency, feeding deterrency, toxicity, sterility and insect growth regulatory effects on larval parasitoid Bracon hebetor. Bioassays were carried out with choice and no-choice tests and treated raisins. There was no significant effect on oviposition and sterilizing effects on B. hebetor when using the two NSK extracts. B. hebetor adults that settled on raisins treated with neem extracts showed abnormal behavioural responses and did not prefer to feed. Parasitoid eggs and pupae were unaffected by the extracts tested. Pretreatment of host larvae before parasitizing with aqueous suspension above 0,6% concentration, affected the development of the parasitoid severely, also the adult emergence.
A similar pattern was seen with the ethanolic extract of NSK, but less adult emergence was obtained. The parasitoid larvae were killed by feeding contaminated host larvae and also through contact (posttreatment) with neem extracts at all concentrations. There were malformations seen on unemerged parasitoid pupae in the neem treatments.
Perera et al. (2000), made experiments on cabbage plants with neem seed oil preparation (NSO), and Azatin EC with an azadirachtin content of 1 g/l and 30g/l, respectively. To see the effect of diamondback moth and its parasitoid Cotesia plutellae. The concentrations used were: NSO, 0,1 ml/l, 1 ml/l, and 2 ml/l; Azatin, 0,01 ml/l, 0,1 ml/l and 1 ml/l. Second-instar P. xylostella larvae was enclosed with female C. plutellae, and then P. xylostella larvae were exposed to the antifeedant treatments at the fourth instar. There was no effect of the antifeedants on the formation of cocoon by C. plutella as the host larvae was at the fourth instar. Some of the C. plutellae which had been exposed to antifeedant survived to emerge as adults. There was no emergence seen with concentrations at or above 1 ml/l of the neem products. The number of adult C. plutella emerging as percentages of the numbers of cocoon formed, were significantly lower than for the control treatment, showing the detrimental effect of neem.
Discussion
From the literature studied, neem seems not to be toxic to parasitoids for the ones that belong to the Hymenoptera order.
The parameters usually measured to quantify the effect of neem on parasitoids are: level of parasitization, survival of adults (mortality), parasitoid development, adult emergence, longevity and antifeedancy.
High doses of neem insecticides used in experiments as expected are harmful for parasitoids. For instance, like the dose of 20 ppm azadirachtin used in the experiments of Feldhege & Schmutterer (1993) and Lower & Isman (1995). A high dose of azadirachtin (500g/ha) like the one used in the bioassay of Lyons et al. (2003) indicated a reduction in egg parasitism due to a decrease in the survival of parasitoid females of T. minutum and also their developmental success. Higher concentrations of neem (500 ppm) deterred adult wasps E. formosa from stabbing in a leaf-disc choice bioassay done by Simmonds et al., (2002).
There are many cases where neem treatments do not affect parasitoids and can be compatible with Integrated Pest Management programmes (application of neem insecticides and parasitoid releases). This is because either the concentration is low or operational doses (commonly field dose) are used. This may vary according to the species and exposure to the product and the product itself. As demonstrated by Tang et al., (2002) with Neemix applied at 11 and 45 ppm of azadirachtin to control brown citrus aphid where there was no significant reduction on the survival rates of L. testaceipes. Lyons et al., (2003) demonstrated that there is a minimal impact on T. minutum when using the operational concentration (0,00375%) of products that contain azadirachtin to control E. kuehniella.
No adverse effects (acute toxicity) with the direct exposure of neem formulations were seen on longevity, foraging behaviour and ability to parasitize hosts (P. xyllostella) of D. mollipla adults in laboratory tests (Akol et al., 2002).This is due to rapid excretion of azadirachtin below levels affecting the hormonal titres that are critical in developing insects (Rembold et al., 1984). D. mollipla perceived and responded to the foraging cues from the host plant and/or host more than 24 h after treatment (Akol et al., 2002).
Despite of the strong effect on G. fulviventre of aqueous neem extract, neither survivorship of adults nor egg parasitization rates were affected. There was also no effect of neem at preimaginal stages of the egg parasitoid (Mitchell et al., 2004).
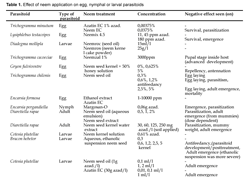
Cage experiments done by Simmonds et al., (2002) demonstrated that A. indica-derived botanicals did not decrease the number of emerging adults E. formosa when applied to parasitized whitefly pupae. This resulted into a high level of control of whitefly, compared to the residual effects and deterrent activity of naphtoquinones and pyretrum, other plant-based insecticides. There are also trials where unavoidably, the negative effect of neem is seen (see Table 1), for example in the study of Saber et al., (2004), Trichogramma cacociae is affected by Neemazal application under laboratory conditions.
According to Lowery & Isman (1995), neem seed oil is toxic to D. rapae both treatment of plants and dipping of aphid mummies under laboratory conditions. However, in field studies neem treatments were not detrimental. This is because of the degradation of azadirachtin by ultraviolet light, avoidance of treated plant parts, or quick colonization of treated plots by aphid natural enemies.
In the trial of Stansly & Liu (1997), Margosan-O reduced parasitization of whiteflies due to repellency. However, concentrations used were below what would normally be sprayed to control whitefly and as a result below what its parasitoid might experience at certain times and places.
Ahmad et al., (2003) give no recommendation for the use of parasitoids in combination with neem preparations as application to the soil had a long-lasting effect on the rate of parasitization and on survival (as demonstrated with the parasitoid D. rapae).
Antifeedancy of neem on parasitoids is clearly seen when a substrate (honey or raisins) are treated with the insecticide (Raguraman & Singh ,1998; 1999).
It has been proved that alcoholic extracts of neem seed are more toxic to than aquoeus neem suspension and neem oil on the parasitoid Bracon brevecornis and B. hebetor (Raguraman & Singh, 1999; Srivastava et al., 1997).
It is important to know the timing of application of the neem insecticides, especially dealing with host stage. Treatment of crops with neem products after parasitization will conserve the parasitoid T. chilonis according to the results carried out by Raguraman & Singh, (2004). T. chilonis may be sensitive to some neem volatile substances like salannin, explaining why it showed diminished oviposition deterrence and feeding deterrence. In the field T. chilonis (host eggs, adults) might be affected by neem extracts through contact or feeding toxicity. This is why neem pretreament of crops should be avoided in case of inundative release of this wasp.
Selectivity of insecticides (emphasizing neem products) should be defined with respect to life stages of the beneficial (Stansly & Liu, 1997). Host egg plays an important role on the effect of insecticide on preimaginal stages (pupal stage) (Saber et al., 2004). In their trial, the more developed the parasitoid is the more damages is seen. The chorion of host eggs also plays a role in neem insecticide penetration (Saber et al., 2004; Raguraman & Singh, 2004).
In order to avoid contamination of host and parasitoid larvae a minimum safety period should be followed after spraying NSK extract in the field as suggested by (Raguraman & Singh, 1999).
Some pests that received neem treatments can have been already parasitized and their parasitoids might still be expected to emerge but in reduced number. This is seen if the treatment was received at late stages of the larval development like in P. xylostella (Perera et al., 2000).
A delay on spraying neem extracts until later larval stages appear would be convenient according to Schmutterer (1992).
Results obtained under laboratory and field conditions differ. It is suggested to carry out further research in the field or more laboratory trials that simulate real conditions. For example, leaf bioassays to test the effects of neem insecticides were used to mimic closely the wasps's normal experience by Stansly & Liu (1997).
Some authors do not explain clearly or the set up of the experiment is not suitable to test the effect of neem on parasitoids, like in the article written by Goudegnon et al. (2000) who concluded that there was no negative effect seen on C. plutellae.
Conclusions
Adult emergence, parasitization, mortality and antifeedancy are the most important parameters when assesing the effects of neem on parasitoids. There is a minimal impact of neem on parasitoids if applied in low doses or commonly field applied doses. Aqueous-neem suspensions are less toxic than neem oil extracts. The timing of application (host stage) plays an important role in order not to affect the parasitoids. Field experiments are more relevant for assessing the effect of neem.
Acknowledgments
I am grateful to Dr. Joop van Loon, staff member of the Laboratory of Entomology from Wageningen University-The Netherlands who checked the present topic.
Literature cited
Ahmad, M., OBiewatsch, H.R. and Basedow, T. 2003. Effects of neem-treated aphids as food/hosts on their predators and parasitoids. Journal of Applied Entomology 127: 458-464.
Akol, A.M., Sithanantham, S., Njagi, P.G.N., Varela, A., and Mueke, J.M. 2002. Relative safety of sprays of two neem insecticides to Diadegma mollipla (Holgren), a parasitoid of the diamondback moth: effects on adult longevity and foraging behaviour. Crop protection 21: 853-859.
Beckage, N. E., Metcalf J. S., Nielson, B. D. and Nesbit, D. J.1988.Disruptive effects of azadirachtin on development of Cotesia congregata in host tobacco hornworm larvae. Archs Insect Biochem. Physiol. 9: 47-65.
Chapman, R. F. 1974. Chemical inhibition of feeding by phytophagus insects-a revie. Bull ent. Res. 64: 339-363.
Dethier, V.G. 1982. Mechanisms of host plant recognition. Entomologia exp. Appl. 31: 49-56.
Feldhege, M., & Schmutterer, H. 1993. Investigations on side-effects of Margosan-O on Encarsia formosa Gah. (Hym., Aphelinidae), parasitoid of the greenhouse whitefly, Trialeurodes vaporariorum Westw. (Hom., Aleyrodidae). Journal of Applied Entomology 115: 37-42.
Feng, R., & Isman, M.B., 1995. Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 51: 831-833.
Feuerhake, K & Schmutterer,H. 1985. Development of a standardized and formulated insecticide from a crude neem kernel extract. Zeitsch. Pflanzenkrankheiten Pflanzenschutz 92: 643-649.
Goudegnon, A.E., Kirk, A.A., Schiffers, B., Bordat, D. 2000. Comparative effects of deltamethrin and neem kernel solution treatments on diamondback moth and Cotesia plutellae (Hym., Braconidae) parasitoid populations in the Cotonou peri-urban area in Benin. Journal of Applied Entomology 124: 141-144.
Isman, M.B., Koul, O., Luczynski, A. and Kaminski, J. 1990. Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 38: 1406-1411.
Hoelmer, K. A., Osborne,L. S. and Yokomi, R. K. 1990. Effects of neem extracts on beneficial insects in greenhouse culture. In Neem's Potential in Pest Management Programs, Proc. USDA Workshop, USDA, ARS, ARS-86 (Eds Locke J. C. and Lawson R. H.), pp. 100-105. Beltsville, MD.
Hough-Goldstein, J. and Keil, C. B.1991. Prospects for integrated control of the Colorado potato beetle (Coleoptera: Chrysomelidae) using Perillus bioculatus (Hemiptera: Pentatomidae) and various pesticides. J. Econ. Ent. 84, 1645-1651.
Karnavar, G.K. 1987. Influence of azadirachtin on insect nutrition and reproduction. Proc . Indian Acad. Sci (Anim. Sci.) 96: 341-347.
Lowery, D.T. & Isman, M.B. 1995. Toxicity of neem to natural enemies of aphids. Phytoparasitica 23(4): 297-306.
Lyons, D.B., Helson, B.V. Bourchier, R.S. Jones, G.C. and McFarlane, J.W. 2003. Effects of azadirachtin-based insecticides on the egg parasitoid Trichogramma minutum (Hymenoptera: Trichogrammatidae). The Canadian Entomologist 135: 685-695.
Mansour, F. A., Ascher, K. R. S. and Omari N. 1986. Toxicity of neem (Azadirachta indica) seed kernel extracts prepared with different solvents, on the spider Chiracanthum mildei. Phytoparasitica 14, 73-76.
Mansour F. A., Ascher K. R. S. and Omari N.1987. Effects of neem (Azadirachta indica) seed kernel extracts from different solvents on the predacious mite Phytoseiulus persimilis and the phytophagous mite Tetranychus cinnabarinus. Phytoparasitica 15, 125-130.
Mitchell, P.L., Gupta, R., Singh, A. and Kumar, P. 2004. Behavioral and developmental effects of neem extracts on Clavigralla scutellaris (Hemiptera: Coreidae) and its eggs parasitoid, Gryon fulviventre (Hymenoptera: Scelionidae). Journal of Economic Entomology 97 (3): 916-923.
Mordue, A.J. & Blackwell, A. 1993. Azadirachtin : an update. Journal of Insect Physiology 39: 903-924.
Perera, D.R., Armstrong, G., Senanayake, N. 2000. Effect of antifeedants on the diamondback moth (Plutella xylostella) and its parasitoid Cotesia plutellae. Pest Management Science 56: 486-490.
Price, J. F. and Schuster, D. J.1991. Effects of natural and synthetic insecticides on sweetpotato whitefly Bemisia tabaci (Homoptera: Aleyrodidae) and its hymenopterous parasitoids. Flua Ent. 74: 60-68.
Raguraman, S & Singh, R.P. 1999. Biological effects of neem (Azadirachta indica) seed oil on eggs parasitoid, Trichogramma chilonis. Journal of Economic Entomology 92 (6): 1274-1280.
Raguraman,S. & Singh, R.P. 1998. Behavioural and physiological effects of neem (Azadirachta indica) seed kernel extracts on larval parasitoid, Bracon hebetor. Journal of Chemical Ecology 24(7): 1241-1250.
Rembold, H., Subrahmanyam, B., & Muller, T. 1989. Corpus cardiacum a target for azadirachtin. Experientia 45: 361-363.
Rembold, H., Forster, H. , Czoppelt, C.H. Rao, P.J., Sieber, K.P., 1984. The azadirachtins, a group of insect growth regulators from the neem tree. In: Schmutterer, H., Ascher K.R.S. (Eds.), Natural Pesticides from the Neem tree and other Tropical Plants Proceedings of the Second International Neem Conference, 25-28 ay 1983, Rauischholzhausen, Germany, pp 153-162.
Srivastava, M., Paul, A.V. N., Rengaswamy, S., Kumar, J., and Parma, R.B.S. 1997. Effect of neem (Azadirachta indica A. Juss.) seed kernel extracts on the larval parasitoid, Bracon brevicornis Wesm. (Hym., Braconidae). Journal of Applied Entomology 121; 51-57.
Saber, M., Hejazi, M.J., and Hassan, S.A. 2004. Effects of Azadirachtin/Neemazal on different stgaes and adult life table parameters of Trichogramma cacoeciae. Journal of Economic Entomology 97(3): 905-910.
Saxena, R. C. 1989. Insecticides from Neem. ACS Symp. Ser 387 (Eds Arnason J. T., Philogene B. J. R. and Morand O.),pp 110-135. American Chemical Society, Washington, D.C.
Schmutterer H. and Hoist H.1987. On the effects of the enriched and formulated neem seed kernel extract AZT-VR-K on Apis mellifera L. J. app. Ent. 103: 208-213.
Schmutterer, H. 1990. Properties and potential of natural pesticides from the neem tree, Azadirachta indica. A. Rev. Ent. 35: 271-297.
Schmutterer H.,1992. EinfluB von azadirachtin. einer azadirachtinfreien fraktion eines alkoholischen niemsamenextraktes und von formulierten extrakten auf verpuppung, schlupf und imaginesder kohlweiglingsbrackwespe Apanteles glomeratus (L.) (Hym., Braconidae). J. appl. Ent. 113, 79-87.
Simmonds, M.S.J., Manlove, J.D., Blaney, W.M., and Khambay, B.P.S. 2002. Effects of selected botanical insectcides on the behaviour and moratlity of the glasshouse whitefly Trialeurodes vaporarium and the parasitoid Encarsia formosa. Entomologia Experimentalis et Applicata 102: 39-47.
Stansly, P.A. & Liu, T.-X. 1997. Selectivity of insecticides to Encarsia pergandiella (Hymenoptera: Aphelinidae), an endoparasitoid of Bemisia argentifolii (Hemiptera: Aleyrodidae). Bulletin of Entomological Research 87: 525-531.
Stark J. D., Wong T. T. Y., Vargas R. I. and Thalman R. K. 1992. Survival, longevity and reproduction of tephritid fruit fly parasitoids (Hymenoptera: Braconidae) reared from fruit flies exposed to azadirachtin. J. econ. Ent. 85, 1125-l 129.
Stark J. D., Vargas R. I. and Wong T. Y.1990a. Effects of neem seed extracts on tephritid fruit flies (Diptera: Tephritidae) and their parasitoids in Hawaii. ARSUSDA. Beitsville Md.: The Service 86, 106-112.
Stark, J.D., Vargas R. I., and Thalman, R. K. 1990b. Azadirachtin: effects on metamorphosis. longevity. and reproduction of three tephritid fruit fly species (Diptera: Tephritidae). J. econ. Ent. 83, 21688-2174.
Tang, Y.Q., Weathersbee III, A.A., and Mayer, R.T. 2002.Effect of neem seed extract on the brown citrus aphid (Homoptera: Aphididae) and its parasitoid Lysiphlebus testaceipes (Hymenoptera: Aphididae) Environmental Entomology 31: 172-176.
Walter, J. F. 1999. Commercial experience with neem products. In: Hall, F.R., Menn, J.J. (Eds.), Method in Biotechnology 5: Biopesticides. Human Press, Totowa, NJ, pp 155-170.
Correspondencia
MSc Organic Plant Production, Agronomist Engineer, Peruvian researcher, address: Calle Francisco de Cuellar 451 casa 18, Monterrico Surco, Lima 33, Lima-Perú
Email Anibal Condor: anibalfcg@yahoo.com
Presentado: 24/04/2006
Aceptado: 15/11/2006