Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.14 n.1 Lima ago. 2007
recursos costeros y su manejo
Marc H. Taylor 1 and Matthias Wolff 1
1 Zentrum für Marine Tropenökologie, Bremen, Germany.
Abstract
Eastern Boundary Current systems (EBCSs) are among the most productive fishing areas in the world. High primary and secondary productivity supports a large biomass of small planktivorous pelagic fish, "small pelagics", which are important drivers of production to the entire system whereby they can influence both higher and lower trophic levels. Environmental variability causes changes in plankton (food) quality and quantity, which can affect population sizes, distribution and dominance among small pelagics. This variability combined with impacts from the fishery complicate the development of management strategies. Consequently, much recent work has been in the development of multispecies trophic models to better understand interdependencies and system dynamics. Despite similarities in extent, structure and primary productivity between EBCSs, the Peruvian system greatly differs from the others in the magnitude of fish catches, due mainly to the incredible production of the anchovy Engraulis ringens. This paper reviews literature concerning EBCSs dynamics and the state-of-the-art in the trophic modeling of EBCSs. The objective is to critically analyze the potential of this approach for system understanding and management and to adapt existing steady-state models of the Peruvian system for use in (future) dynamic simulations. A guideline for the construction of trophodynamic models is presented taking into account the important trophic and environmental interactions. In consideration of the importance of small pelagics for the system dynamics, emphasis is placed on developing appropriate model compartmentalization and spatial delineation that facilitates dynamic simulations. Methods of model validation to historical changes are presented to support hypotheses concerning EBCS dynamics and as a critical step to the development of predictive models. Finally, the identification of direct model links to easily obtainable abiotic parameters is emphasized to add practicality to the model as a predictive tool.
Keywords: Trophic modeling; Eastern Boundary Current Systems; Upwelling; Small pelagic fish; Perú
Resumen
Los sistemas de corrientes limítrofes orientales (EBCSs) están considerados las zonas pesqueras más productivas del mundo. La alta productividad primaria y secundaria soporta una gran biomasa de pequeños peces pelágicos planctivoros, "pelágicos pequeños". Los mismos, son importantes en la conducción de la producción a la totalidad del sistema, adquiriendo influencia sobre los niveles tróficos altos y bajos. Cambios ambientales causan cambios en calidad y cantidad del plancton (alimento), y pueden afectar los tamaños de poblaciones, distribución espacial, y dominancia de los pelágicos pequeños. Esta variabilidad combinada con los impactos de la pesquería complica el desarrollo de las estrategias para el manejo del sistema. Consecuentemente, los trabajos desarrollados recientemente han sido enfocados al desarrollo de modelos tróficos multiespecificos que permitan entender mejor las interdependencias y la dinámica del sistema. A pesar de las semejanzas en el grado, estructura y productividad primaria entre las EBCSs, el sistema peruano tiene una magnitud de capturas mucho más alta, principalmente debido a la gran producción de la anchoveta Engraulis ringens. En el presente trabajo revisamos la literatura concerniente a la dinámica de las EBCSs y el estado del arte en el modelamiento trófico de las mismas. El objetivo fue analizar críticamente el potencial del modelamiento trófico para la comprensión y manejo del sistema y la adecuación de los modelos estacionarios al sistema peruano, para un uso (futuro) en simulaciones dinámicas. Se presenta una guía para la construcción de modelos trófo-dinámicos, tomando en cuenta las interacciones tróficas y ambientales más importantes. En consideración de la importancia de los pelágicos pequeños en las dinámicas del sistema, se ha hecho énfasis en desarrollar una compartimentalización apropiada y una delineación espacial que facilite simulaciones dinámicas. Métodos de validación del modelo con respecto a los cambios históricos se presentan para apoyar las hipótesis sobre la dinámica de EBCSs y se consideran un paso crítico para el desarrollo de modelos de predicción. Finalmente, la identificación de conexiones entre modelos y parámetros abióticos fácilmente obtenibles se acentúa para agregar sentido práctico a los modelos como herramientas de predicción.
Palabras claves: Modelaje trófico; Sistemas de Corrientes de Borde Este; Peces pelágicos pequeños; Perú
1. Introduction
Eastern Boundary Current Systems (EBCSs) are among the most productive fishing areas in the world and the Humboldt Current Large Marine Ecosystem (HCLME) alone comprised between 6-13% of the world's annual catch between 1994-2003 (FAO, 2003). This productivity is due to the upwelling of cold, nutrient rich waters to the photic zone where it is taken up by primary producers _ particularly, large diatoms. This high level of primary production forms the base of the food web, which is remarkably similar in all four of the main EBCSs (Humboldt Current, Canary Current, Benguela Current, and California Current). Species compositions are often different, but the general trophic organization is similar and includes numerous species of phytoplankton and zooplankton, relatively few species of small pelagic fish feeding directly on the plankton, higher carnivorous species of fish, and top predators such as tuna, birds, and marine mammals. The relatively small number of small pelagic fish species comprises the bulk of fisheries landings and has been proposed to be an important forcing group to both higher and lower trophic levels (i.e. "wasp-waist" control) (Cury et al., 2000). While these generalizations might be true to a certain extent for the aforementioned EBCSs, differences among systems complicate direct comparison (Moloney et al., 2005).
EBCSs differ remarkably in terms of fisheries production, likely due to physical differences in oceanography that affect biological production. Fisheries biologists have grappled to understand why the Peruvian fish catch (total and on a per area basis) is so much higher than that of all other EBCSs. This is mainly due to the huge production of the Peruvian anchovy, Engraulis ringens, which has surpassed 10 million tons in production during several years. The "Peruvian puzzle" to fish production, as coined by Cury et al. (1998), seems to have more to do with prevailing oceanographic conditions than any particular physical attribute such as total area of continental shelf. Bakun (1996) eloquently demonstrated the physical differences between EBCSs in terms of their upwelling potential _ Peru's advantage, due to its proximity to the equator and resulting large Rossby radius, lies in its capacity for strong upwelling under relatively low wind forcing conditions. This creates a "particularly rich, non-turbulent, benign environment" by which coastal plankton communities can develop and be maintained through longer residence times, thus favoring grazing fish populations (Bakun and Weeks, 2006). Production of anchovy, or more specifically their annual recruitment, has been shown to be at a maximum during conditions of intermediate strength of offshore transport (Parrish et al., 1983; Cury and Roy, 1989; Roy et al., 1992; Cury et al., 1998). In particular, a high level of recruitment and subsequent catch appears to occur within a narrow optimal "environmental window" of alongshore wind speeds coupled with a high degree of upwelling. This optimal wind speed falls between 5-6 m/s, a velocity which is at the upper limit of where wind begins to create hydrodynamically "rough" water conditions (Deacon and Webb, 1962). While strong upwelling provides increased nutrients to the photic zone, excess turbulence can be detrimental to primary producers by decreasing light penetration, and to grazers by increased dispersal of available food (Ware, 1992). Furthermore, trade winds set up a basin-wide slope in sea level in the Pacific, whereby Peru is able to maintain a shallow thermocline, leading to enhanced nutrient supply and productivity (Chavez et al., 2003). An interesting byproduct of these oceanographic conditions is that plankton probably become concentrated above the shallow thermocline, thus improving the grazing efficiency of small pelagic fish.
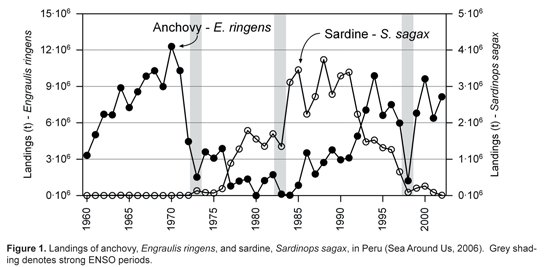
Environmental variability within EBCSs creates large changes in productivity, which is ultimately felt economically through decreased catches. In the case of the Peruvian upwelling system in the Humboldt EBCS, production of small pelagic fish is affected on annual scales (e.g. associated with El Niño-Southern Oscillation _ ENSO, "El Niño") as well as larger decadal scales (Fig. 1). Future management may benefit from a deeper understanding of how this environmental variability affects recruitment and trophodynamics of these economically important species as well as the overall productivity of the ecosystem.
Given the extent of declining fish catch worldwide, there has been significant support to reform the ways in which we manage and assess fishery resources for the purpose of sustained production. Traditional management has focused on the single species and has largely ignored inter-specific trophic interactions, the rationale being that each species occupying a niche in the ecosystem is most affected by its own population dynamics and the basic parameters of growth and mortality. The fishery is perceived as an additional component to the population's mortality, a parameter that fisheries biologists can manage in order to maintain an optimal level of production over time (i.e. Maximum Sustainable Yield, "MSY"). These "traditional" single-species approaches have been criticized in recent years as being overly simplistic given the complexity of ecosystems. However, Mace (2004) states that to "scapegoat" all single-species models would be alarmist given that in many cases the suggested exploitation rates may not have been implemented successfully, and that a reduction in fishing mortality of a single species has actually proved successful in many stock recoveries. On the other hand, the management of small pelagic fish populations is complicated due to their specific characteristics: 1) highly stochastic populations linked to environmental variability, 2) some functional redundancy between species, and 3) a strong interdependence between the target species and the rest of the ecosystem. These factors call for a more holistic "Ecosystem Approach to Fisheries" (see Browman and Stergiou, 2004) and as a result, recent work in EBCSs has attempted to bridge the gap between theoretical and practical uses of multispecies models.
The objective of this paper is to suggest a methodological approach for developing models that will serve as a predictive tool for management in the Peruvian upwelling system. In order to arrive at a framework for such a tool, a literature review was conducted of trophic modeling of EBCSs with the objective of adapting existing steady-state models of the Peruvian system for use in dynamic simulations. Methods of model validation to historical changes are presented as a critical step to the development of predictive models for management. Finally, the identification of direct links of models to easily obtainable abiotic parameters is emphasized for adding practicality to the approach as a predictive tool.
2. Model considerations
One modelling package that has gained popularity in recent years is the use of steady-state trophic models such as Ecopath (Christensen and Pauly, 1992), which allow the user to construct a simplified representation of the ecosystem based on the flows of energy among species or functional groups. With the further development of the accompanying dynamic simulation package, Ecosim, users are now able to explore past and future impacts of fishing and environmental disturbances and explore optimal fishing policies (Walters et al., 2000). Data demands of the program package Ecopath with Ecosim (EwE) are also relatively smaller in comparison to other dynamic models (e.g. Multispecies virtual population models _ MSVPAs, Individual-based models _ IBMs), thus lending itself to wider use as a management tool.
As with any model, the focus and scope of the problem has to be addressed. First, trophic modelling requires that one must simplify the infinitely complex ecosystem into a manageable representation through the identification of key functional species groups of similar life history, dynamics, and diet, and to focus on those relationships important to the problem at hand. Secondly, the definition of an appropriate temporal resolution (e.g. based on a yearly average, decadal average, seasonal, etc.) and spatial boundaries to the model are of utmost importance. In the following sections, considerations for modeling the dynamics of the Peruvian upwelling system are presented alongside a review of EBCS dynamics and previous models.
2.1 Functional groups and dynamics of EBCSs
Due to their ecological and economic importance, previous models have focused on small pelagic fish. Cury et al (2000) highlighted evidence suggesting both bottom-up and top-down control on production to predators and plankton, respectively. This "wasp-waist" forcing may be attributed to their grazing efficiency and large biomass that, in a sense, funnels energy through the relatively few species comprising the group. The importance of anchovy grazing on phytoplankton in coastal waters of Peru has even been compared to copepods, whereby carbon fixed by primary producers was estimated to be channeled through the trophic web equally between the two groups (Walsh, 1981). A further review of these and other findings by Alheit and Niquen (2004) led them to conclude that, "Understanding the trophic interactions between anchovy, sardines and zooplankton might be a key to understanding their dynamics in the [Humboldt Current]."
One of the most pressing questions that exist in EBCSs concerns the dynamics that govern small pelagics, and in particular, the factors responsible in a regime shift. Regime shifts typically involve a change in dominance between anchovy and sardine, yet may also include changes to the biomass of larger mackerels. The "classic" regime shift between sardine and anchovy appears to be a regular part of the dynamics in the Peruvian system as has been verified through the fossil record using fish scale deposits (DeVries and Pearcy, 1982). Recent evidence has linked these shifts with warm and cold temperature periods occurring on decadal scales over entire ocean basins (Alheit and Niquen, 2004).
In Peru, changes in small pelagic fish abundances can also be observed under the inter-annual scale disturbances associated with El Niño. Anchovy tend to occupy the nearshore areas within the first 30 nautical miles where there is colder water due to upwelling while sardines are often located further offshore. During El Niño, the intrusion of warm, equatorial waters and the lowering of the thermocline cause elevated sea surface temperatures and upwelling is restricted to the upper warmer-water layer with a few limited cold-water cells. Anchovy populations retreat to these few remaining upwelling areas and sardines move inshore (Ñiquen and Bouchon, 2004). These dynamics in dominance and spatial distribution have previously been attributed to anchovy being better adapted to cold temperatures and sardines to warmer temperatures; however, Bakun and Broad (2003) point out that temperature may not necessarily be the cause given that in the western Pacific sardines do relatively well during cold periods, and that the two species tend to replace each other over longer (with respect to temperature fluctuations) time scales as the dominant grazer of plankton. These dynamics lend support for a secondary response to environmental forcing and further probing of the species' trophic interactions becomes necessary.
In most EBCSs it has been observed that some degree of functional redundancy or overlap exists among small pelagics feeding on plankton, yet marked differences in feeding preferences have also been observed. Sardines possess a particularly fine-meshed filtering apparatus in their gillrakers allowing for the filtering of smaller-sized particles. Anchovy, on the other hand, are more specialized and efficient at feeding on larger-sized particles (James and Findlay, 1989; Van der Lingen, 1994). The result of these adaptations, at least in the Benguelan populations, is that anchovy seem to have higher clearance rates (per weight) than sardine when particle size is greater than about 500-600 nm (Van der Lingen, 1994). Particles of this size include large mesozooplankton such as calanoid copepods as well as chain-forming diatoms - known to comprise the major part of the Peruvian anchovy's diet.
In general, larger chain-forming diatoms are associated more with upwelling areas of higher nutrient concentrations while more oligotrophic environments tend to be dominated by smaller phytoplankton, and bacteria cycles become more important (Rodriguez et al., 2001). The warm phase during the 1970's and 80's in Peru resulted in a regime shift to sardine dominance with declining zooplankton concentrations in comparison to the 1960's and earlier, when anchovy was dominant (Alheit and Niquen, 2004; Ayon et al., 2004). Similarly, a reduction in the upwelling of cool, nutrient-rich waters during an El Niño event has also been linked to changes in the phytoplankton community in northern Chile, from the typical diatom-dominated phytoplankton to pico- (0,7_2,0 μm) and nanoplankton (2,0_23,0 μnm) dominated (Iriarte and Gonzalez, 2004). During the El Niño in Peru in 1997-1998, anchovy presumably responded to these changes by retreating to the remaining centers of upwelling at about 16° S where diatom abundances were still relatively high (Ñiquen and Bouchon, 2004). However, in general diatom biomasses were greatly reduced and anchovy were forced to feed on larger relative quantities of zooplankton throughout the Peruvian coast (Fig. 2).
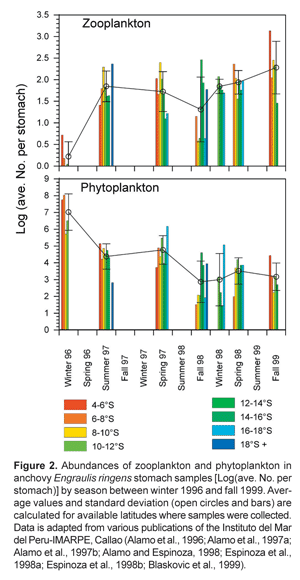
Small pelagics will probably have very different diet matrices in Peru than in other EBCSs. Both sardine and anchovy are omnivores and likely opportunistic feeders that commonly switch to consume whatever the plankton composition happens to be. In the Benguelan system, anchovy are much more of a zooplankton feeder than in the Humboldt, feeding mainly on large calanoid copepods and euphausiids, ingested through particulate feeding (James, 1987; Van der Lingen, 2002). During normal upwelling periods, stomach contents of anchovy sampled off the Peruvian coast contained >95% diatoms (numbers) (Alamo et al., 1996; Alamo et al., 1997a; Alamo et al., 1997b; Alamo and Espinoza, 1998; Espinoza et al., 1998a; Espinoza et al., 1998b; Blaskovic et al., 1999). Similar estimates have been used for trophic models in Peruvian and Chilean systems (Jarre et al., 1991; Neira et al., 2004). The more than ten-fold higher production of small pelagic fish in the Humboldt compared to other EBCSs has been attributed to anchovy feeding directly on phytoplankton (Walsh, 1981). This is logical given that feeding on one lower trophic level would provide about 10 times more food if we assume a mean transfer efficiency between trophic levels in aquatic systems of about 10% (Christensen and Pauly, 1993; Pauly and Christensen, 1995).
2.2. Compartmentalization
recent models have paid much more attention to the compartmentalization of plankton and small pelagics; particularly, those models constructed for later use in dynamic simulations have focused on plankton divisions by cell size. The basic constructions of the four models reviewed (Jarre et al., 1991; Shannon and Jarre-Teichmann, 1999; Shannon et al., 2003; Neira et al., 2004) are shown in Table 1.
For the Benguelan models, zooplankton was split into three size groups: i. Microzooplankton (<200 nm), ii. Mesozooplankton (200_2000 nm), iii. Macrozooplankton (2-20 mm). Gelatinous zooplankton (jellyfish and salps) was compartmentalized separately. The Chilean model separates the group into compartments of Copepods and Euphasiids. While this provides an important separation between the zooplankton consumed by anchovy and sardine (principally copepods) versus that of chub and horse mackerels (principally euphausiids), additional compartmentalization is advised to account for the previously mentioned differences in particle size feeding preferences for anchovy and sardine. In particular, sardines are known to feed more heavily on cyclopoid copepods (usually <200 nm) while anchovy feed more on larger calanoid copepods and euphausiids (James, 1987; Konchina, 1991; Van der Lingen, 2002). In the past model for Peru, zooplankton was not divided into size-specific groups, however, Jarre-Teichmann and Christensen (1998) recommended that a closer look at plankton compartments was needed to obtain a more detailed understanding of the system.
Small pelagic fish are compartmentalized fairly similarly for the main species of anchovy, sardine, chub mackerel (not in the Chilean model), and horse mackerel. As recommended for the modeling of life history dynamics (Christensen and Walters, 2004), separation of a single species into several functional groups, by size or life history classes, has been made in the S. Benguelan and Chilean models. In this way, differences in food intake, vulnerability to predation, and recruitment constraints related to juvenile size and fecundity can be accounted for. In the case of the S. Benguela model, horse mackerel is split into juvenile and adult groups due to differences in biomass, catch and diet (juveniles are strictly zooplanktivorous while adults eat zooplankton and fish). Other small pelagic fish are included in the Benguelan models such as Mesopelagics, Redeye, and Other small pelagic fish. The absence of mesopelagic fish in the other models may represent a significant shortcoming, as this group represents a large amount of biomass and is a potentially important food item to other species. The S. Benguela model estimates suggest that hake consumed 1.1 million tons of mesopelagic fish during the 1990's (Shannon et al., 2003). Mesopelagic fish have also been seen to venture further inshore during El Niño years in Peru, observable through acoustic surveys, catches, and in the stomach contents of coastal marine mammals (Arias Schreiber, 2003).
Demersal fish are given more attention in the Chilean model due to their importance to the region and possibly better data. These groups, especially hake, are often lacking sufficient data relating to life history, yet the two most recent models have incorporated separate stages for hake given the important predatory relationship described between adult hake on anchovy. In Peru, hake populations have suffered severely _ to the point where the fishery was eventually closed in September 2002 and now operates at a much smaller scale. From diet studies, hake is observed to have undergone a severe change in diet; from adults feeding on other abundant demersals and sardine in a survey from 1985 to intense cannibalism among individuals of 4-5 years and older in 2001 (Ballón, 2005). Hake has important connections to the pelagic system as well, especially for small juveniles that feed more pelagically on euphausiids mainly (Shannon et al., 2004a; Ballón, 2005; Tam et al., 2006). The Peruvian hake population was thought to have increased during the 1980's due in part to an increase in sardine abundance, which comprised a large portion of the adult hake's diet (Ballón, 2005).Besides hake (being the principle demersal species), Other demersal fish are either simply labeled as such (Jarre et al., 1991; Shannon and Jarre-Teichmann, 1999), further divided into Pelagic- and Benthic-feeding compartments (Shannon et al., 2003), or divided into individual species as is the case of Central Chilean model (Neira et al., 2004).
Cephalopods were considered to be an important functional group in the S. Benguela model (Shannon et al., 2003). Moloney et al. (2005) have standardized this group's production to consumption ratio, P/Q at 0.3 in models of EBCSs. Production estimates vary; however, the group is notoriously productive and a voracious consumer, making it an important compartment to future models. The Humboldt squid, Dosidicus gigas, has gained importance in recent years in Peru as its biomass increased dramatically after the last El Niño in 1997-98. It has remained at high levels ever since despite the development of a large industrial fishery. Its distribution is more limited to the north of Peru, and is observed to feed opportunistically with a high degree of cannibalism _ especially among larger size classes nearing the end of their lifecycle. Given its large consumption (estimated consumption to biomass ratio, Q/B for summer 2005 in Peru was 8.91), the group has been thought to have an important impact on hake populations (specifically, the more pelagic-feeding juveniles) and was estimated to account for as much as 21% of the total mortality of hake. Furthermore, a 14% similarity in prey between the jumbo squid and hake may also indicate an important competitive relationship between the two species (Alegre et al., 2005).
2.3. Spatial boundaries
Steady-state trophic modeling requires that the user defines boundaries to the ecosystem under study. One can imagine the difficulties involved with an open marine system, such as a pelagic environment, where species are constantly on the move and in flux with prevailing oceanographic conditions. Connections to the coastal environment are also important, and for this reason there has been an attempt to delineate Large Marine Ecosystems (LMEs) that conceivably contain a high degree of interconnectedness, having important implications for management. The US National Oceanic and Atmospheric Administration (NOAA) have provided the following definition of a LME:
"Large Marine Ecosystems are regions of ocean space encompassing coastal areas from river basins and estuaries to the seaward boundaries of continental shelves and the outer margins of the major current systems. They are relatively large regions on the order of 200 000 km2 or greater, characterized by distinct: (1) bathymetry, (2) hydrography, (3) productivity, and (4) trophically dependent populations". (www.lme.noaa.gov)
Presently, 64 LMEs have been described and represent about 95% of the world's annual marine fishery yields. The Humboldt Current, Canary Current, Benguela Current, and California Current EBCSs are also considered to be individual LMEs. Despite this delineation, trophic modeling efforts of EBCSs rarely focused on the entire LME. Is this significant or does it represent a shortcoming in our acceptance of defined LMEs as a useful concept for management?
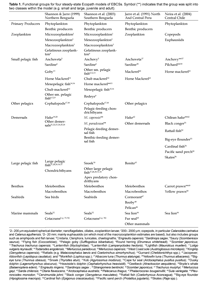
In EBCSs, the width of the upwelling zone is a function of water depth, stratification, and latitude (ca. 10-30 km wider near the equator due to Rossby radius). However, the productive band of high biomass and associated fisheries is often wider and can extend to over 100 km (Ware, 1992). Nixon and Thomas (2001) provide a review of previous delineations of the Peruvian upwelling system and find that estimations of area range over ten-fold, from less than 40·103 km2 to over 500·103 km2. Most of the uncertainty arises from three main factors: 1) estimation based on the actual physical upwelling of water versus inclusion of a larger area of significant biological impact, 2) differing lengths of coastline used in the calculation, and 3) large seasonal and inter-annual variability in the extent of upwelling off Peru. The authors go on to provide their own estimates of size based on remote sensing estimates of surface water chlorophyll a concentrations; specifically the area of "productive habitat" where concentrations exceed 1.0 mg·m-3 was considered. Using this criteria, the size of the productive habitat was observed to vary 10-fold (including the ENSO event of 1997/98), presumably in relation to the degree of upwelling and, subsequently, nutrient concentrations in the photic zone. The use of remote sensing has undoubtedly shed a great deal of light on the variability in primary production for EBCSs, however the application of such a mobile boundary complicates the efforts of the modeler, especially if one is to attempt dynamic modeling over longer periods of time. In the case of modeling small pelagic fish populations, their distribution often occurs within a known range of the coast due to other limiting factors besides food, such as prevailing currents, which play important roles in their lifecycle. It is thus recommended that a defined spatial border be based on the life histories of the main functional groups influencing system dynamics.
In the cases of the Humboldt and Benguelan systems, trophic models have defined boundaries around particular stocks of small pelagic fish that often correspond with centers of upwelling. In the Humboldt, stocks of sardine and anchovy overlap in latitudinal distribution in northern to central Peru, southern Peru to northern Chile, and off Talcuano in Chile (Serra, 1983), with an additional sardine stock off Coquimbo in Chile (Serra and Tsukayama, 1988) (Fig. 3).
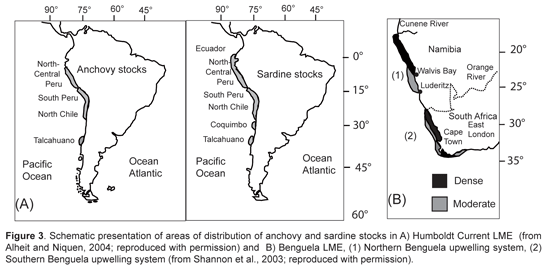
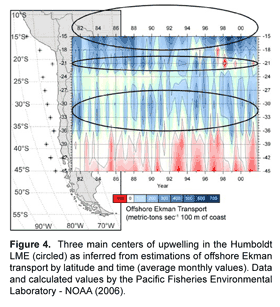
Jarre-Teichmann and others (Jarre et al., 1991; Jarre-Teichmann and Pauly, 1993; Jarre-Teichmann and Christensen, 1998; Jarre-Teichmann et al., 1998) have focused primarily on the northernmost stocks of Peru while Neira et al. (Neira and Arancibia, 2004; Neira et al., 2004) have modeled the zone of the southern stocks of central Chile (33°-39° S). The Benguelan EBCS has also been modeled separately for the different principle stocks of small pelagic fish in the northern (Shannon and Jarre-Teichmann, 1999) and southern regions (Shannon et al., 2000; Shannon et al., 2003; Shannon et al., 2004b) (Fig. 3). While some connectivity may exist between stocks, they are essentially separately functioning populations and the size of the population, or absolute abundance, is related to the area in which there is closure of the life cycle (Sinclair, 1988; Sinclair and Iles, 1988, 1989). A nice example of this is given by Gutierrez and Herrera (Gutierrez Torero and Herrera Almirón, 2002) for the Peruvian anchovy showing that the species' biomass and distribution are correlated (Fig. 5) and are influenced by the strength of upwelling. Thus, as upwelling increases (cold periods), the size of the closure of the lifecycle also increases.
In general, when the focus of a modeling exercise is on the description of a particular resource, it makes sense to define borders that will encompass the species' lifecycles under most conditions rather than to constantly reformulate borders based on a changing area of suitable habitat. While some connection between these small pelagic populations may exist, it is likely of less importance to within population dynamics. LME definitions may thus have practicality as a policy tool, yet modeling of the entire LME will likely benefit from the separation of these stocks and the corresponding lower trophic levels.
3. System characteristics
Carr (2002) compared potential productivity in the four main EBCSs using remote sensing between September 1997 and August 1999. Results indicated that in terms of primary productivity (extrapolated from chl a concentrations), the Humboldt system ranks third after the Benguelan and Canary systems. The Peruvian coastline, in particular, had by far the greatest productivity in the Humboldt Current, yet the entire Humboldt Current system's productivity was not considered to be exceptional. The robustness of the method of calculating primary productivity is difficult to assess without a crosscheck from field data, yet even a direct sample of chl a concentration would not give sufficient descriptive information concerning pico-, nano-, micro-, and chain-forming plankton proportions and their overall productivities. It seems likely that differences in plankton quality rather than quantity may be a key factor in explaining the exceptional anchovy production in Peru. Additionally, Peru seems to reflect an optimal situation for production with relatively constant upwelling year-round under wind speeds of intermediate strength, possibly providing an optimal situation for both adult feeding and recruitment.
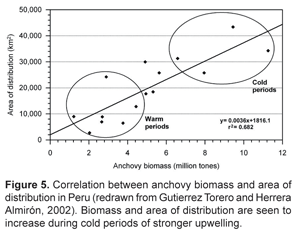
The first comprehensive comparison between EBCS steady-state models was done by Jarre-Teichmann and Christensen (1998), wherein they compared subregions of the four main EBCSs: Peru 4-14° S, "Namibia" (Benguela) 15-35° S, Canary 12-25° N, and California 28-43° N. These models were compared under similar model constructions and scale (70 km from the coast), thus representing an ideal situation for comparison. The authors focused on the main fish species: anchovy, sardine, horse-mackerel, mackerel, and hake, and in describing and discerning local and global characteristics. The general structure of the trophic flow diagrams is similar for all four systems with functional groups located at similar trophic levels. "Size" (total biomass) and total system throughput (T) varied greatly between the systems primarily due to differences in entered values for primary production. The Peruvian system ranked highest in both categories; however, as previously mentioned, remote sensing estimates of primary production presented by Carr (2002) indicate that these values may be too high or at least not higher than the Benguelan system. Anchovy productivities were similar between systems and the fact that their natural mortality was always substantially higher than fishing mortality, even in the heavily-fished Peruvian system, points to their importance as a food source to the rest of the system (Jarre-Teichmann and Christensen, 1998). Sardine production was highest in the Namibian (Benguelan) system, where plankton composition may favor its feeding strategy.
Models presented by Jarre-Teichmann and Christensen (1998) are compared to the other previously mentioned large upwelling systems in Figure 6. Differences in the spatial delineation of the systems may affect many of the summary statistics provided by Ecopath, making comparisons difficult. Specifically, the inclusion of a larger proportion of oligotrophic oceanic waters further from the productive upwelling coast will dilute key descriptors such as biomass and total system throughput. The outer boundaries to the modeled areas were as follows: 70 km - North and Central Peru (Jarre-Teichmann and Christensen, 1998), 60 km - Central Chile (Neira et al., 2004), and 500m depth isocline - Northern and Southern Benguela (Shannon and Jarre-Teichmann, 1999; Shannon et al., 2003). These extensions from the coast are not largely different, but will have an affect on size-specific statistics in Fig. 6, all except Mean trophic level of the fishery).
One of the more revealing statistics is that of transfer efficiency (TE), which describes the proportion of energy entering a trophic level that is transferred to the next trophic level. It is calculated in Ecopath as the ratio between the summed exports and predation, and the energy throughput (total consumption). High gross food conversion efficiencies (GE) correspond to high production/consumption ratios, and lead to high transfer efficiencies (Christensen and Pauly, 1993). TE is therefore restricted to describing consumer trophic efficiencies due to the fact that the present models do not quantify solar energy input to producer compartments. Previously, upwelling systems were thought to have a relatively low mean TE in comparison to the average of about 10% in other aquatic systems (Jarre-Teichmann, 1992; Christensen and Pauly, 1993; Jarre-Teichmann and Pauly, 1993; Jarre-Teichmann and Christensen, 1998) due to the relatively short food chain length from primary producers or detritus to top predators in upwelling systems (Ryther, 1969). Jarre and Christensen (1998) observed TE's below 10% for their models, yet more recent models of the Southern Benguela system have much higher efficiency to trophic level V. The authors explain that this may be a result of the model's construction wherein the splitting of the zooplankton group caused a shift in their observed trophic level. In the models by Jarre and Christensen (1998), the trophic level of zooplankton is slightly above 2, while Shannon et al. (2003) have the following trophic level assignments: Microzooplankton 2,3; Mesozooplankton 2,6; Macrozooplankton 2,7, which shifts all subsequent consumer compartments to higher trophic levels. Christensen and Pauly (1993) also showed a tendency of increasing trophic levels to "appear" as one describes diet compositions in greater detail. This seems to be supported by the newer models of the Southern Benguela in which 31 compartments were used and subsequently have closer to 5 versus 4 trophic levels in the Jarre and Christensen (1998) models.
The higher TE of the N. Benguelan observed by Shannon et al. (2003) seems to be more of the exception than the norm among EBCSs. In particular, plankton available to small pelagic fish are of very different quality resulting in increased carnivory by anchovy, more similar to the Peruvian situation under El Niño conditions. High TE values may indicate a "bottle-neck" of flows between zooplankton (TL II and III) and small pelagic fish (TL III and IV), pointing to their importance in the overall trophic structure of the ecosystem and possibly food limitation to small pelagics (Shannon et al., 2003). Not mentioned by the authors, but likely an important factor is that zooplankton biomass was not known, and thus was back-calculated assuming an ecotrophic efficiency (EE) of 0,95. In other words, the model assumes that 95% of the group's production will be consumed by higher predators, and it is possible that this assumption has elevated the TE values of TLs II and III.
High non-predatory losses (defined by low EE) are typical between producer and 1st consumer (herbivore) levels in EBCSs. This results in a large portion of primary production going directly to detritus where it is remineralized. In the case of the S. Benguela, it was estimated that between 55-60% of net primary production is consumed by herbivores with losses being attributed to a possible "match-mismatch" between zooplankton and phytoplankton blooms (Shannon et al., 2003). This parameter is however notoriously difficult to estimate and therefore is often left open for Ecopath to calculate. Nevertheless, in upwelling areas EE is assumed to be typically low for phytoplankton (~0,5) and in some cases for zooplankton compartments as well.
4. Validation of the model - linking cause and effect
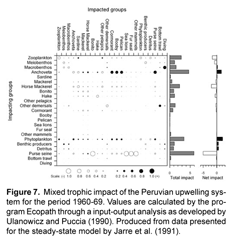
The linking of cause and effect, or the creation of models that can at least reproduce observed historical responses to disturbances such as fishing, has been described as a critical step for applying trophic modeling to policy analysis (Shannon et al., 2004a). Within the Ecopath package, users can determine interactions within the steady-state model through the "mixed trophic impact" operation. This feature offers a test to the sensitivity of the model by changing the biomass of one particular functional group and viewing the impact of this change to all other compartments' biomasses. Direct (e.g. negative impact predator-prey) and indirect (e.g. competition, positive impact predator-prey) effects can be identified, helping one visualize new equilibriums under disturbances to individual groups. An example of this routine is shown in Figure 7, generated from data presented in Jarre et al.'s (1991) model of the Peruvian system between 1960-69. We can immediately see that anchovy influences many other groups, in most cases these are easily interpretable; negative impacts to its competitors (sardine and other pelagics) and prey (zooplankton), and positive affects on its predators (mackerel, horse mackerel, hake, and seabirds). Anchovy are also seen to have a net positive effect on the system, a feature that is primarily shared by groups at the base of the food chain - primary producers and detritus.
Recent models of the southern Benguela system have used routines in Ecosim for assessing the relative importance of human vs. environmental impacts on system dynamics. Within Ecosim, a user can view how a system is reacting along each time step (iterative), allowing for a more realistic impression of the intensity and duration of change before equilibrium is achieved. Using this tool, users can better describe the processes of cause and effect through time - an important dimension needed in validating a model's outcome to observed historic changes. Dynamic simulations have compared these factors through the linking of steady-state models (Shannon et al., 2004b) and through fitting to time-series data (Shannon et al., 2004a). In both cases, a time period encompassing the observed shift in small pelagic dominance was used. These examples are presented as valuable proxies for future model validation in the Peruvian system in the following sections:
In the linking of two steady-states (Shannon et al., 2004b), models were constructed on decadal scales: the anchovy-dominant 1980's, and the 1990's when sardines increased in importance and anchovy populations declined. Dynamic simulations involved subjecting one of the models to conditions of the alternate period. These alternate state conditions forced either the rate of fishing mortality (F) to small pelagics (anchovy, sardine, adult horse mackerel, and juvenile horse mackerel) or "environmental forcing" by affecting the vulnerability rates (the instantaneous density of a prey vulnerable to a particular predator, Christensen et al., 2000) of zooplankton to sardines and anchovy. The resulting biomasses of key groups at equilibrium to the alternate state's values provided a measure of comparison for the different forcing.
Fitting to time-series (Shannon et al., 2004a) routines used annual estimates of catch and biomass for species or species groups for the period 1978-2002 as a baseline by which to gauge the fit of simulations through a comparison of sum of squares (SS). The effect of fishing on dynamics was forced with independent estimates of changing yearly fishing rates (fishing mortality or fishing effort). Again, environmental effects focused on vulnerability rates for the predator-prey interactions most sensitive to change (with emphasis on interactions of small pelagics) and for primary production (impacting phytoplankton P/B). A "fit to time-series" search routine for "best-fit" values were performed for vulnerabilities and turnover rates (P/B) for primary producers.
4.1. Fishing impacts
Changing fishing rate was shown to have a relatively smaller impact on system dynamics than environmental factors in the southern Benguela. In the case of the influence on a regime shift, as was tested with the linking of two steady-state models, the application of the alternate state's rate of fishing mortality did cause many biomasses to change in the correct direction but not of the same magnitude (Shannon et al., 2004b). Using a time series for changing fishing rates also only slightly improved the fit of the model's prediction of catch and biomass (2-3% reduction in SS) over a constant value of fishing mortality taken from the 1978 steady-state model. Moloney et al. (2005) point out that the South Benguelan fishery operates on a higher trophic level than in other EBCSs due to the differing diet of small pelagics, and composition of the catch. This resulted in a more than doubled estimate over the Peruvian fishery in Flows required per unit of catch ([t 1°prod] [t catch]-1 km-2 y-1). Despite this energetically more costly target species, the fishery in the southern Benguela was determined to require a smaller proportion of primary production to sustain it when compared to the Peruvian system (4% and 10%, respectively), reflecting the lower fishing rates in the Benguela. There may also be a larger impact from the fishery on the Peruvian system's dynamics due both to a larger total flow required to sustain the fishery as well as it being more focused on the dynamically important small pelagics. The mixed trophic impact analysis of the Peruvian system from 1960-69 also shows the fishery to be the highest-impacting group with a largely negative net effect overall (Fig.7)
4.2. Predator/prey impacts
The models of the Southern Benguela have focused on flows between zooplankton abundances as the primary driver to small pelagics and higher trophic levels. The strength of this forcing has been addressed through search routines within Ecosim for best-fit estimations of vulnerability. The estimated vulnerabilities that best fit the stock dynamics in the southern Benguela resembled wasp-waist forcing, thus, supporting the findings of Cury et al. (2000). This resulted in zooplankton being top-down controlled by anchovy, sardine, round herring and juvenile horse mackerel (high vulnerability), and with anchovy, sardine, round herring, and small hake exerting bottom-up control over their predators (low vulnerability). The adjustments of these vulnerabilities produced the most significant improvements to the fit of the simulation (40% reduction in SS over fitting with fishing rates alone), thus stressing the importance of the parameter in modeling trophic dynamics and, in particular, the role of small pelagics in upwelling systems (Shannon et al., 2004a). Further searches for a "best fit" of primary production improved SS by 4-6% and 12%, when applied after and before vulnerability searches respectively.
Shannon et al. (2004b) adjusted vulnerabilities between sardine and anchovy and their prey in order to simulate changing plankton fraction abundances (and hence their vulnerabilities) during the observed regime change from the 1980's to 1990's steady state models. In the application of the alternate state's vulnerability values between these groups (phyto- and zooplankton), the "opposite" regime was obtained, and changes to biomasses of many groups were in the same direction and of a similar order of magnitude. Furthermore, changes to the vulnerability of mesozooplankton alone were found to have similar effects (although of a smaller magnitude) to those of changes to both meso- and macrozooplankton. This led the authors to conclude that, "Model shifts between an anchovy `regime' (1980's) and the possible move towards a sardine `regime' (1990's) in the southern Benguela ecosyst7777777m are likely to have been caused by changes in the availability of mesoplankton to anchovy and sardine" (Shannon et al., 2004b).
In both examples, environmental forcing was considered more important than the effects of fishing in driving small pelagic dynamics. Furthermore, these examples help to add support for two main hypotheses concerning EBCS dynamics: 1) Wasp-waist forcing, and 2) importance of plankton quality ("environmental forcing").
The authors were able to explain about half the variance in the time-series based on a combination of fishing, vulnerability settings and productivity patterns. Whether this reduction is "significant" is unclear and difficult to assess. Even though some time series are well reproduced by the simulation (e.g. sardine and anchovy), the authors point out that many other time series do not show much of a trend and thus their validity must be questioned. Overall, the authors stress that such simulations are meaningful as a first step towards ecosystem modeling as well as a tool in evaluating ambiguity in trends from the more traditional stock assessment and survey series.
5. Prospective for real-time prediction
The dynamic models presented suggest and illustrate the importance of the links between plankton and small pelagics in driving EBCS dynamics. Unfortunately, periodic sampling of the plankton over such large spatial scales is difficult and so creates problems when trying to apply forcing functions to trophic models for the purpose of real-time prediction. On the other hand, plankton changes (especially phytoplankton) may be linked more easily to abiotic indices available through remote sensing (e.g. sea surface temperature - SST, upwelling indices, ENSO indices _ ex. "NIÑO3") in an attempt to create predictors of changing plankton biomass. The Peruvian system may represent an ideal situation for such an exercise given evidence of a strong direct trophic link between phytoplankton production and small pelagics. As mentioned before, plankton quality is possibly at least as important as quantity to small pelagic dynamics and so estimation of chl a pigment concentrations alone through remote sensing may prove insufficient in estimating changes among different phytoplankton size fractions. Fortunately, historical plankton sampling data exists for the Peruvian coastal waters from previous research cruises (IMARPE, performed seasonally), and may allow for the calculation of biomass for different size fractions of phytoplankton either by flow cytometry or through biovolume calculation from cell counts (see Edler, 1979; Hillebrand et al., 1999; Sun and Liu, 2003).
An initial exercise to convert abundance values derived from published IMARPE cruise data into biovolume reveals the importance of large celled diatoms in the coastal zone (ca. 85% of total phytoplankton volume), coinciding with the anchovy's principle habitat. Other phytoplankton taxonomic groups appear to be less affected by their proximity to the nearshore upwelling zone (Fig. 8). While the tendency of decreasing volume offshore is consistent with remote sensing data (SeaWifs), calculated biovolume values are much higher for the nearshore zone. Several factors could explain such a discrepancy: 1) Conversion factors for chl a to wet weight provide only a rough estimate; 2) Limited in situ sample size (N= 39); 3) Cloud cover typical of the Peruvian coast during strong upwelling periods may result in underestimations of chl a concentrations of nearshore waters. The conversion of historical data into volume is a major objective of IMARPE and should shed light on the dynamics of the plankton community in the coming years.

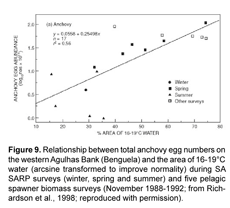
Highly productive phytoplankton assemblages, dominated by chain-forming diatoms, have been shown to occur in the Humboldt Current during the upwelling of cold, nutrient-rich waters to the photic zone. Comparing the degree of upwelling of this water mass (e.g. area in km2 where SST <20°C) to the biomass of different phytoplankton size fractions may provide a simple empirical relationship. The likelihood of a strong correlation existing between upwelling strength and increased food supply to anchovy is high given that both adult populations as well as fecundity (egg production) have been observed to increase during periods of increased upwelling (Fig. 5 and Fig. 9). Watters et al. (2003) made use of a similar empirical model to force primary production in their trophic model of the eastern tropical Pacific; SST anomalies were correlated to surface chlorophyll concentrations and a forcing function was applied only to the biomass of large phytoplankton due to observations that the biomass of diatoms varies substantially during warm and cold events, whereas picoplankton (i.e. small phytoplankton) biomass is relatively stable (Bidigare and Ondrusek, 1996; Landry et al., 1996).
At present, we are unaware of any attempts to use trophodynamic models for real-time prediction. The reason is obvious enough - prediction requires: 1) reliable trophic models based on empirical evidence, 2) model tuning to historical changes, and 3) links between easily observable indices and change to key drivers of the system. Fortunately, the creation of predictive models for the Peruvian upwelling system has many previous examples of work in EBCSs from which to draw from. Within the Peruvian system itself, fish stock dynamics observed over different time scales (decadal and El Niño) also provide valuable information on the Peruvian system's response to perturbation, useful in model tuning. Recent years have seen a much more advanced and thorough collection of data (e.g. satellite imagery, oceanographic measurements, hydroacoustical fish stock assessments), especially during the last large El Niño of 1997/98, which provide an opportune data set for fisheries ecologists to address the "Peruvian puzzle".
6. Summary: The role of trophic modeling in solving the puzzle
Our understanding of the functioning of EBCSs has advanced greatly in the past 60 years since large-scale fisheries began to exploit their enormous capacity for fish production. Our knowledge of the Peruvian upwelling system has benefited from several disciplines _ from underlying physical and geochemical processes to predator-prey dynamics and the impacts of man. This review has attempted to illustrate the continuing role that trophodynamic modeling can play in exploring past and future change as well as bridging the gap between theory and management. In summary, several considerations were highlighted for the future construction of trophodynamic models for the Peruvian system:
1) Compartmentalization
a. Plankton _ Size-fractionated compartments of zooplankton and phytoplankton to take into account the feeding differences between small pelagic fish species (e.g. diatoms, micro- meso- and macrozooplankton).
b. Life-history _ Intra-species separations by size or other life-history classification taking into account differences in food intake, vulnerability to predation, and recruitment (e.g. Peruvian hake).
c. Additional functional groups _ Several new groups should be added given new insight into their trophic importance (e.g. mesopelagics, cephalopods, benthic vs. pelagic feeding demersals).
2) Spatial
a. Latitude _ Definition of boundaries that allow for the closure of the life cycle for key functional groups. In the Humboldt LME, anchovy and sardine stock delineations appear to be correlated to upwelling centers (e.g. north and central stocks from about 4°-15°S).
b. Extension from the coast _ Due to the highly variable upwelling changes in Peru, a stationary boundary is recommended that encompasses the spatial dynamics of the key populations and/or biologically productive zone (ca. 100km, Ware, 1992; Nixon and Thomas, 2001).
3) Temporal _ Steady-state models based on yearly averages are the most feasible given the sampling frequency in Peru. Focusing on the data-rich years since 1996, an immediate exploration of the dynamics surrounding the last El Niño of 1997/98 seems possible. Adaptation of past trophic models of the 1960's would benefit from an exploration of the impressive longer time-series data presented in the book edited by Pauly and Tsukayama (1987).
In conclusion, the complex dynamics of EBCSs and their connections to environmental variability present an ideal situation for the application of multispecies models for management. This is one of many examples of marine resources along the Chilean and Peruvian coastline affected by environmental variability, which are being addressed under the EU-project, CENSOR _ "Climate variability and El Niño Southern Oscillation: Impacts for natural resources and management." The ability of models to predict some of these changes has thus been a focus of the project due to the connection of these resources to that of resource users' livelihoods.
Acknowledgements
This study was financed and conducted in the frame of the EU-project CENSOR (Climate variability and El Niño Southern Oscillation: Impacts for natural resources and management, contract No. 511071) and is CENSOR publication No. 0049.
Literature cited
Alamo, A., I. Navarro, P. Espinoza, et al., 1996. Alimentary spectrum and food ration of Engraulis ringens and of Sardinops sagax sagax, and mortality of Peruvian anchoveta eggs by predation, Informe. Instituto del Mar del Peru, Lima (Peru): 34-42. [ Links ]
Alamo, A., J. Espinoza, P. Zubiate, et al., 1997a. Feeding behaviour of the most important pelagic Peruvian resources during summer and early autumn 1997. Cruise 9702-04, Informe. Instituto del Mar del Peru, Callao: 82-89.
Alamo, A., P. Espinoza, P. Zubiate, et al., 1997b. Alimentary behaviour of Peruvian anchovy Engraulis ringens during winter 1996. Cruise RV Humboldt 9608-09, Informe. Instituto del Mar del Péru, Lima, Péru: 38-46.
Alamo, A. and J. Espinoza, 1998. Alimentary variations in Engraulis ringens and other pelagic resources during 1997 winter-spring, Informe. Instituto del Mar del Péru, Callao, Péru: 45-52.
Alegre, A., V. Blaskovic, R. Castillo, et al., 2005. Comportamiento alimentario del calamar gigante (Dosidicus gigas), enfatizando la depredacion ejercida sobre le merluza (Merluccius gayi peruanus). Instituto del Mar del Peru, Callao, Peru: 36.
Alheit, J. and M. Niquen, 2004. Regime shifts in the Humboldt Current ecosystem. Prog. Oceanogr. 60: 201-222.
Arias Schreiber, M., 2003. Prey spectrum and feeding behaviour of two sympatric pinnipeds (Arctocephalus australis and Otaria flavencens) in relation to the 1997-98 ENSO in southern Peru. MSc., University of Bremen, Bremen, Germany, 60 pp.
Ayon, P., S. Purca and R. Guevara-Carrasco, 2004. Zooplankton volume trends off Peru between 1964 and 2001. Ices J Mar Sci 61 (4): 478-484.
Bakun, A., 1996. Patterns in the ocean. Ocean processes and marine population dynamics. University of California Sea Grant, California, USA, in cooperation with Centro de Investigaciones Biologicas de Noroeste, La Paz, Baja California Sur, Mexico, 323 pp.
Bakun, A. and K. Broad, 2003. Environmental `loopholes' and fish population dynamics: comparative pattern recognition with focus on El Nino effects in the Pacific. Fisheries Oceanography 12 (4-5): 458-473.
Bakun, A. and S. J. Weeks, 2006. The Marine Ecosystem off Peru: What are the secrets of its fishery productivity and what might its future hold? The Humboldt Current System: climate, ocean dynamics, ecosystem processes, and fisheries.
Ballón, R. M., 2005. Comparative analysis of the community structure and trophic relations of the Peruvian hake Merluccius gayi peruanus and its by-catch of the years 1985 and 2001. MSc., University of Bremen, Bremen, Germany, 62 pp.
Bidigare, R. R. and M. E. Ondrusek, 1996. Spatial and temporal variability of phytoplankton pigment distributions in the Central Equatorial Pacific Ocean. Deep-Sea Res. (II Top. Stud. Oceanogr.) 43 (4-6): 809-833.
Blaskovic, V., P. Espinoza, F. Torriani, et al., 1999. Feeding behaviour of anchovy off Peruvian coast during spring 1998. Cruise R/V Jose Olaya Balandra 9811-12. Inf. Inst. Mar Peru (146): 77-84.
Browman, H. I. and K. I. Stergiou, 2004. Perspectives on ecosystem-based approaches to the management of marine resources. Marine ecology progress series 274: 269-303.
Brown, P. C., S. J. Painting and K. L. Cochrane, 1991. Estimates of phytoplankton and bacterial biomass production in the northern and southern Benguela ecosystems. South Africa Journal of Marine Science 11: 537-564.
Brush, M. J., J. W. Brawley, S. W. Nixon, et al., 2002. Modeling phytoplankton production: Problems with the Eppley curve and an empirical alternative. Marine Ecology Progress Series 238: 31-45.
Carr, M. E., 2002. Estimation of potential productivity in eastern boundary currents using remote sensing. Deep-Sea Res. (II Top. Stud. Oceanogr.) 49 (1-3): 59-80.
Chavez, F. P., J. Ryan, S. E. Lluch-Cota, et al., 2003. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science 299 (5604): 217-221.
Christensen, V. and D. Pauly, 1992. ECOPATH II - a software for balancing steady-state models and calculating network characteristics. Ecol Model 61: 169-185.
Christensen, V. and D. Pauly, 1993. Flow Characteristics of Aquatic Systems. In: Christensen, V. and D. Pauly (Eds.), Trophic models of aquatic systems. ICLARM Conference Proceedings: 338-352.
Christensen, V., C. J. Walters and D. Pauly, 2000. Ecopath with Ecosim Version 4, Help system©.
Christensen, V. and C. J. Walters, 2004. Ecopath with Ecosim: methods, capabilities and limitations. Ecol. Model. 172: 109-139.
Cury, P. and C. Roy, 1989. Optimal environmental window and pelagic fish recruitment success in upwelling areas. Canadian Journal of Fisheries and Aquatic Sciences 46 (4): 670-680.
Cury, P., C. Roy and V. Faure, 1998. Environmental constraints and pelagic fisheries in upwelling areas: the Peruvian puzzle. South African Journal of Marine Science/Suid-Afrikaanse Tydskrif vir Seewetenskap 19: 159-167.
Cury, P., A. Bakun, R. J. Crawford, et al., 2000. Small pelagics in upwelling systems: patterns of interaction and structural changes in «wasp-waist» ecosystems. ICES Journal of Marine Science 57 (3): 603-618.
Deacon, E. L. and E. K. Webb, 1962. Small-scale interactions. In: Hill, M. N. (Ed.), The sea: ideas and observations on progress in the study of the seas. Interscience, New York
Delgado, E., P. Villanueva, F. Chang, et al., 2001. The Peruvian marine phytoplankton during Summer 2000. Instituto del Mar del Peru, Lima (Peru): 85-98.
DeVries, T. J. and W. G. Pearcy, 1982. Fish debris in sediments of the upwelling zone off central Peru: A late Quaternary record. Deep-Sea Research 29 (1A): 87-109.
Edler, L., 1979. Phytoplankton and Chlorophyll: Recommendations on Methods for Marine Biological Studies in the Baltic Sea. Baltic Marine Biologists Publication, 1-38 pp.
Espinoza, J., I. Navarro and F. Torriani, 1998a. Variations in the alimentary spectrum of the main pelagic resources during autumn 1998. Cruise RV Humboldt 9803-05 from Tumbes to Tacna. Inf. Inst. Mar Peru (135): 134-142.
Espinoza, P., V. Blaskovic and I. Navarro, 1998b. Comportamiento alimentario de Engraulis ringens, a finales del invierno 1998. Crucero de evaluacion idroacustica de recursos pelagicos 9808-09. Inf. Inst. Mar Peru 141: 67-71.
FAO, 2003. Yearbook of Fishery Statistics
Gutierrez Torero, M. and N. Herrera Almirón, 2002. An approach based on acoustic data to study the variability in distribution and abundance of small pelagics in the Humboldt ecosystem. In: Van der Lingen, C. D., C. Roy, P. Fréon, et al. (Eds.), GLOBEC-SPACC/ IDYLE/ ENVIFISH Workshop on Spatial Approaches to the Dynamics of Coastal Pelagic Resources and their Environment in Upwelling Areas. Instituto del Mar del Peru, Cape Town, South Africa: 27-30.
Hillebrand, H., C. D. Duerselen, D. Kirschtel, et al., 1999. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 35 (2): 403-424.
Iriarte, J. L. and H. E. Gonzalez, 2004. Phytoplankton size structure during and after the 1997/98 El Nino in a coastal upwelling area of the northern Humboldt Current System. Marine ecology progress series 269: 83-90.
James, A. G., 1987. Feeding ecology, diet and field-based studies on feeding selectivity of the Cape anchovy Engraulis capensis Gilcrist. In: Payne, A. I. L., J. A. Gulland and K. H. Brink (Eds.), The Benguela and comparable ecosystems. South African Journal of Marine Science: 161-177.
James, A. G. and K. P. Findlay, 1989. Effect of particle size and concentration on feeding behaviour, selectivity and rates of food ingestion by the Cape Anchovy Engraulis capensis. Mar. Ecol. Prog. Ser. 50 (3): 275-294.
Jarre-Teichmann, A., 1992. Steady-state modelling of the Peruvian upwelling ecosystem. PhD thesis, University of Bremen, Bremen, Germany, 153 pp.
Jarre-Teichmann, A. and D. Pauly, 1993. Seasonal changes in the Peruvian upwelling ecosystem. In: Christensen, V. and D. Pauly (Eds.). ICLARM, Manila (Philippines): 307-314.
Jarre-Teichmann, A. and V. Christensen, 1998. Comparative modelling of trophic flows in four large upwelling ecosystems: global versus local effects. ORSTOM, Paris (France)
Jarre-Teichmann, A., L. J. Shannon, C. L. Moloney, et al., 1998. Comparing trophic flows in the southern Benguela to those in other upwelling ecosystems. South African Journal of Marine Science/Suid-Afrikaanse Tydskrif vir Seewetenskap 19: 391-414.
Jarre, A., P. Muck and D. Pauly, 1991. Two approaches for modelling fish stock interactions in the Peruvian upwelling ecosystem. Multispecies Models Relevant to Management of Living Resources. 171-184.
Konchina, Y., 1991. Trophic status of the Peruvian anchovy and sardine. Journal of Ichthyology 31 (4): 59-72.
Landry, M. R., J. Kirshtein and J. Constantinou, 1996. Abundances and distributions of picoplankton populations in the Central Equatorial Pacific from 12 degree N to 12 degree S, 140 degree W. Deep-Sea Res. (II Top. Stud. Oceanogr.) 43 (4-6): 871-890.
Mace, P. M., 2004. In defence of fisheries scientists, single-species models and other scapegoats: Confronting the real problems. Marine Ecology Progress Series 274: 285-291.
Moloney, C. L., A. Jarre, H. Arancibia, et al., 2005. Comparing the Benguela and Humboldt marine upwelling ecosystems with indicators derived from inter-calibrated models. Ices J Mar Sci 62: 493-502.
Neira, S. and H. Arancibia, 2004. Trophic interactions and community structure in the upwelling system off Central Chile (33-39 degrees S). Journal of Experimental Marine Biology and Ecology 312 (2): 349-366.
Neira, S., H. Arancibia and L. Cubillos, 2004. Comparative analysis of trophic structure of commercial fishery species off Central Chile in 1992 and 1998. Ecol Model 172 (2-4): 233-248.
Ñiquen, M. and M. Bouchon, 2004. Impact of El Nino events on pelagic fisheries in Peruvian waters. Deep-Sea Res. 51: 563-574.
Nixon, S. and A. Thomas, 2001. On the size of the Peru upwelling ecosystem. Deep-Sea Res. (I Oceanogr. Res. Pap.) 48 (11): 2521-2528.
Pacific Fisheries Environmental Laboratory, 2006. Upwelling Indices along the coast of South America. dataset, http://www.pfeg.noaa.gov/.
Parrish, R. H., A. Bakun, D. M. Husby, et al., 1983. Comparative climatology of selected environmental processes in relation to eastern boundary current pelagic fish reproduction, 731-777 pp.
Pauly, D. and I. Tsukayama, 1987. On the implementation of management-oriented fishery research: The case of Peruvian anchoveta, 1-13 pp.
Pauly, D. and V. Christensen, 1995. Primary production required to sustain global fisheries. Nature 374 (6519): 255-257.
Richardson, A. J., B. A. Mitchell-Innes, J. L. Fowler, et al., 1998. The effect of sea temperature and food availability on the spawning success of Cape anchovy Engraulis capensis in the southern Benguela. South African Journal of Marine Science/Suid-Afrikaanse Tydskrif vir Seewetenskap 19: 275-290.
Rodriguez, J., J. Tintore, J. T. Allen, et al., 2001. Mesoscale vertical motion and the size structure of phytoplankton in the ocean. Nature 410 (6826): 360-363.
Roy, C., P. Cury and S. Kifani, 1992. Pelagic fish recruitment success and reproductive strategy in upwelling areas: Environmental compromises, 135-146 pp.
Ryther, J. H., 1969. Photosynthesis and fish production in the sea. Science 166: 72-76.
Sea Around Us, 2006. A global database on marine fisheries and ecosystems. Fisheries Centre, University British Columbia, Vancouver, www.seaaroundus.org.
Serra, J. R., 1983. Changes in the abundance of pelagic resources along the Chilean coast. Proceedings of the expert consultation to examine changes in abundance and species composition of neritic fish resources, a preparatory meeting for the FAO world conference on fisheries management and development. FAO FISH. REP./FAO, INF. PESCA.: 255-284.
Serra, R. and I. Tsukayama, 1988. (A synopsis of biological and fishery data on the sardine, Sardina sagax (Jenyns 1842) in the Southeast Pacific.)
Shannon, L. J. and A. Jarre-Teichmann, 1999. A Model of trophic flows in the northern Benguela Upwelling system during the 1980s. South African Journal of Marine Science/Suid-Afrikaanse Tydskrif vir Seewetenskap 21: 349-366.
Shannon, L. J., P. M. Cury and A. Jarre, 2000. Modelling effects of fishing in the southern Benguela ecosystem. ICES Journal of Marine Science 57 (3): 720-722.
Shannon, L. J., C. L. Moloney, A. Jarre, et al., 2003. Trophic flows in the southern Benguela during the 1980s and 1990s. Journal of Marine Systems 39: 83-116.
Shannon, L. J., V. Christensen and C. J. Walters, 2004a. Modelling Stock Dynamics in the Southern Benguela Ecosystem for the Period 1978_2002. Afr. J. mar. Sci. 26 (Ecosystem Approaches to Fisheries in the Southern Benguela): 179-196.
Shannon, L. J., J. G. Field and C. L. Moloney, 2004b. Simulating anchovy-sardine regime shifts in southern Benguela ecosystem. Ecol. Model. 172: 269-281.
Sinclair, M., 1988. Marine populations. An essay on population regulation and speciation. Washington Sea Grant, Seattle, WA
Sinclair, M. and T. D. Iles, 1988. Population richness of marine fish species. Aquat. Living Resour./Ressour. Aquat. Vivantes. 1 (1): 71-83.
Sinclair, M. and T. D. Iles, 1989. Population regulation and speciation in the oceans. J. Cons. Ciem. 45 (2): 165-175.
Sun, J. and D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25 (11): 1331-1346.
Tam, J., S. Purca, L. O. Duarte, et al., 2006. Changes in the diet of hake associated with El Niño 1997_1998 in the northern Humboldt Current ecosystem. Advances in Geosciences 6: 63-67.
Ulanowicz, R. E. and C. J. Puccia, 1990. Mixed trophic impacts in ecosystems. Coenoses 5 (1): 7-16.
Van der Lingen, C. D., 1994. Effect of particle size and concentration on the feeding behaviour of adult pilchard Sardinops sagax. Marine ecology progress series 109: 1-13.
Van der Lingen, C. D., 2002. Diet of sardine Sardinops sagax in the southern Benguela upwelling ecosystem. South African Journal of Marine Science/Suid-Afrikaanse Tydskrif vir Seewetenskap 24: 301-316.
Walsh, J. J., 1981. A carbon budget for overfishing off Peru. Nature 290: 300-304.
Walters, C., D. Pauly, V. Christensen, et al., 2000. Representing Density Dependent Consequences of Life History Strategies in Aquatic Ecosystems: EcoSim II. Ecosystems 3 (1): 70-83.
Ware, D. M., 1992. Production characteristics of upwelling systems and the trophodynamic role of hake, 501-513 pp.
Watters, G. M., R. J. Olson, R. C. Francis, et al., 2003. Physical forcing and the dynamics of the pelagic ecosystem in the eastern tropical Pacific: Simulations with ENSO-scale and global-warming climate drivers. Canadian Journal of Fisheries and Aquatic Sciences 60 (9): 1161-1175.
Wolff, M., C. Wosnitza-Mendo and J. Mendo, 2003. The Humboldt Current- Trends in exploitation, protection and research.In: Hempel, G. and K. Sherman (Eds.), Large Marine Ecosystems of the World. Elsevier: 279-309.
Correspondencia
Zentrum für Marine Tropenökologie, Fahrenheitstrasse 6, 28359 Bremen, Germany.
E-mail Marc H. Taylor: marchtaylor@yahoo.com
Presentado: 27/07/2006
Aceptado: 19/12/2006