Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.15 supl.1 Lima nov. 2008
TRABAJOS ORIGINALES
An overview of the taxonomy of Attalea (Arecaceae)
Una visión general de la taxonomía de Attalea (Arecaceae)
Jean-Christophe Pintaud1
1 IRD, UMR DIA-PC/DYNADIV, Laboratoire Genetrop, Centre IRD de Montpellier, 911 Av. Agropolis, BP 64501, 34394 Montpellier Cedex 5, France.
Trabajo presentado al Simposio Internacional Las palmeras en el marco de la investigación para el desarrollo en América del Sur, del 07 al 09 de Noviembre 2007, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Perú.
Abstract
The genus Attalea (Arecaceae) is distributed in continental habitats of the Neotropical region and in some Caribbean islands. Life forms of Attalea species vary from small acaulescent palms to tall and massive palms, always solitary. The ecological range of the genus encompasses most of the Neotropical ecosystems, from coastal sand dunes to sub-Andean forests up to 1600 m in elevation, lowland wet to dry forests, savannas, swamps, etc. The taxonomy of the genus has been poorly understood due to conflicting genus and species concepts that exist since the last decades. Taxonomical problems have been caused by the lack of adequate material, especially species of large size, loss of many types and difficulties in interpreting hybrids. In this article, I review the most recent taxonomic literature on Attalea. The number of species in Attalea varies from 29 to 67 depending on different authors, with a maximum estimate of 73 species when combining the revised publications. There is a consensus for the validity of 20 species among modern palm taxonomists. The most conflicting species or group of species are discussed in detail as well as the taxonomic significance of some characters such as the pattern of insertion of staminate flowers on rachillae, insertion of pinnae on rachis, and arrangement of fibrous strands in the endocarp.
Keywords: Attalea, Arecaceae, taxonomy, palms, Neotropics.
Resumen
Attalea (Arecaceae) es un género distribuido en toda la región Neotropical continental y en algunas islas Caribeñas. Las formas de vida de las especies de Attalea incluyen tanto pequeñas palmeras como plantas de gran tamaño, siempre con tallo solitario. El rango ecológico del género abarca prácticamente todos los ecosistemas neotropicales desde las dunas de arena costeras hasta el bosque sub-Andino (algunas especies llegan a 1600 m de altitud), pasando por todo tipo de bosque tropical, seco o húmedo, pantanos, sabanas, etc. La taxonomía del género ha sido poco entendida y conceptos conflictivos sobre géneros y especies existen desde hace décadas. Las dificultades taxonómicas resultan de la falta en los herbarios de material adecuado, en particular para las grandes especies, de la pérdida o destrucción de numerosos tipos y de la frecuente hibridación entre especies. En este artículo se analizan los trabajos taxonómicos más recientes sobre Attalea. El número de especies varía entre 29 y 67 según los autores, y un máximo estimado de 73 especies al combinar los diferentes trabajos; siendo 20 las especies en consenso entre autores. Las especies y grupos de especies más problemáticos se tratan detalladamente y se enfatiza el significado taxonómico de algunos caracteres como la inserción de las flores estaminadas en la raquilla, inserción de las pinas en el raquis, distribución de las fibras en el endocarpio, entre otros.
Palabras claves: Attalea, Arecaceae, taxonomía, palmeras, neotrópico.
Introduction
Attalea is one of the most conspicuous palm genera in the Neotropics. Ranging from Mexico to Bolivia, Paraguay, Southern Brazil and the Caribbean, it is found in most tropical lowland ecosystems and in the Andes up to 1200-1600 m elevation. Numerous species are massive palms forming dense stands and they are therefore remarkable elements of the landscape, but there are also smaller acaulescent species in both forests and savannas.
Identification of Attalea species has however been difficult for several reasons. The first problem is the paucity of good herbarium collections. Most species have very large leaves, inflorescences and fruits, and also exhibit biological characteristics like very seasonal phenology and functional dioecy which make it difficult to gather complete material. Moreover, many valuable collections, including type specimens were lost or destroyed (Henderson, 1995). Consequently, several large and extremely abundant Attalea species remained undescribed until recently or are still very poorly known or of doubtful status.
The second problem is the use of different genus concepts among taxonomists. In the Field Guide to the Palms of the Americas (Henderson et al., 1995), all species of subtribe Attaleinae sensu Uhl and Dransfield (1987) are included in a broad genus Attalea, while in the Taxonomic Treatment of Palm Subtribe Attaleinae (Glassman, 1999), the group is treated as five separate genera (Attalea sensu stricto, Orbignya, Scheelea, Maximiliana and Ynesa). It is to be noted that the subtribe Attaleinae has been subsequently extended (Dransfield et al., 2005) to genera previously included in Beccariophoenicinae and Butiinae by Uhl and Dransfield (1987). Zona (2002) established the still needed corresponding names in the Attalea sensu lato concept for the species treated by Glassman in other genera, so that the two concepts are now fully compatible. However, in order to do these transfers of genera, it was needed to disentangle particularly complex nomenclatural problems. This is exemplified by the case of the babaçu, one of the best known and economically most important species (Anderson et al., 1991). The first name apparently applied to this species is Attalea speciosa Mart., described from Brazil in 1826, but although there is an indication of type locality in the original description, no specimen was cited as type for this name, so it is a nomen confusum (Glassman, 1999). Martius described again this palm in 1844, based on dOrbignys collections from Bolivia as Orbignya phalerata Mart. In the five genera concept, this should be the correct name of the babaçu, although Barbosa Rodrigues made the combination Orbignya speciosa based on the older nomen confusum. When considering a single, broad genus Attalea, it is not possible to make a new combination, Attalea phalerata, for the babaçu, because it would be homonymous with an older name for another palm, Attalea phalerata Mart. ex Spreng. described in 1825, which was latter transferred to Scheelea. A new name in Attalea is therefore needed for the babaçu if one considers that A. speciosa is not valid. Zona (2002) proposed Attalea glassmanii, but Govaerts and Dransfield (2005), following Henderson et al. (1995) and other authors, chose to conserve the old name Attalea speciosa under which this important palm has been known since a long time.
Recognizing a single genus is biologically sound since there are many fertile hybrids between Attalea sensu stricto, Orbignya, Scheelea and Maximiliana, which indicate a very close affinity of these taxa (Balick et al., 1987 a, b). At the seedling stage, Attalea sensu lato species are recognizable by the lanceolate eophylls with dentate upper margins and latter by the asymmetrical pinna apex with a lateral projection, brownish on the abaxial side (Fig. 3). These characters make Attalea s.l. instantly recognizable at any developmental stage, while any further identification generally requires flowers and fruits. Wessels Boer (1965) gave a lengthy argumentation in favor of a single genus Attalea. Henderson and Balick (1991) provided additional arguments upon examining a poorly known species, Attalea crassispatha. However, within a broad genus Attalea, there most probably exist monophyletic entities corresponding closely to taxa previously considered at the generic level. As a matter or fact, when attempting to identify a species of this group of palms, the distinction of four entities corresponding to the genera Attalea, Scheelea, Orbignya and Maximiliana, as it was adopted in Genera Palmarum (Uhl and Dransfield, 1987), remains the easiest way to proceed. These taxa differ markedly in staminate flower structure, although they are often undistinguishable vegetatively. Palms referred to as Orbignya are characterized by stamens with coiled anthers, and staminate flowers often densely packed on rachillae (Fig. 8). All the other staminate flower types have straight anthers. Maximiliana has staminate flowers with petals much shorter than the stamens. There is a single species of this type, A. maripa, which is widespread in the Amazon region. Attalea sensu stricto and Scheelea have staminate flowers with petals longer than stamens. Attalea s.s. has flattened petals which enclose the stamens (Fig. 7) while Scheelea has linear-cylindrical fleshy petals which do not enclose the stamens (Fig. 5-6). Assigning an Attalea sensu lato species to its staminate flower type narrows considerably the search for the species name at any particular location. In some places, there are species of Attalea, Scheelea and Orbignya types which are vegetatively almost identical but which can be readily recognized when staminate flowers are available.






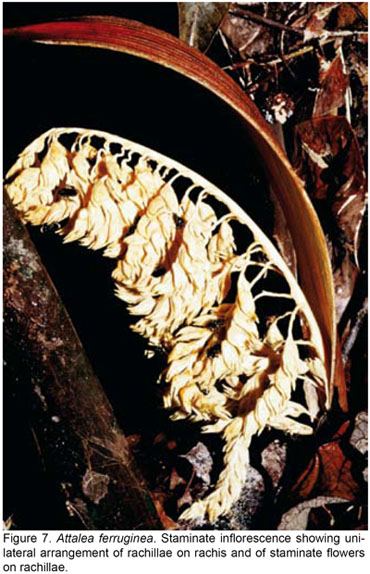

Excluding unambiguous hybrids, there are in fact only five species which do not fit well within the four genera scheme, and constitute an argument to consider a single genus Attalea (Wessels Boer, 1965; Henderson and Balick, 1991). One species from the upper Rio Negro region, A. luetzelburgii, has mixed characters of Orbignya and Scheelea and some unique characteristics which led at one time to place it in a separate genus, Parascheelea (Dugand, 1940). Another species from north-eastern Amazonia, Attalea dahlgreniana has mixed characters of Orbignya and Maximiliana and had been considered either as a distinct genus, Markleya (Bondar, 1957) or an intergeneric hybrid, ×Maximbignya (Glassman, 1999). Attalea attaleoides, from central and eastern Amazonia has characters of Scheelea and Maximiliana. Attalea colenda, a species endemic to the Pacific coast of northern South America (Fig. 1), has been placed in its own genus as well, Ynesa. Balslev and Henderson (1987) interpreted it as a typical Attalea sensu stricto, while Glassman (1999) considered it as a putative hybrid between Orbignya and Attalea or Maximiliana. The outlying Attalea crassispatha, the only species of the Greater Antilles (Haiti), has also unusual characteristics, especially in staminate flower structure and pollen morphology (Henderson and Balick, 1991). It somewhat resembles Orbignya, however, especially for the curled anthers at anthesis, and Glassman (1999) transferred it to this genus.
However, even when the staminate flower type has been identified, the conflicts between taxonomic treatments and generic concepts make it difficult to choose the appropriate name for a particular palm. For example, around Iquitos in Peru, there are three acaulescent species with pinnae regularly arranged and spreading in one plane, which key out as Attalea racemosa, Attalea microcarpa and Attalea butyracea in Hendersons Palms of the Amazon (1995), and as Attalea ferruginea, Orbignya polysticha and Scheelea plowmanii, respectively, in Glassmans (1999) monograph.
It is therefore very difficult to determine how many species of Attalea sensu lato should be recognized. Wessels Boer (1965) gave an estimate of around 100 species. The treatment of Glassman (1999), with 65 species, recognizes fine-scale variation at the species level (Zona 2002). Many of the species accepted or described as new by Glassman (1999) are based on one or very few and generally incomplete herbarium collections, preventing an adequate evaluation of the natural variability of populations. Nevertheless Glassmans treatment provides names for very distinctive species previously undescribed like A. plowmanii (Galeano and Bernal, 2002) or Attalea moorei (Fig. 5). Henderson et al. (1995) used generally a broad species concept, which in some instances, like in the complex of Attalea butyracea, has not been supported by subsequent detailed field studies (Stauffer and Fariñas, 2006). The two treatments nevertheless agree on the definition of 20 species (Table 1).
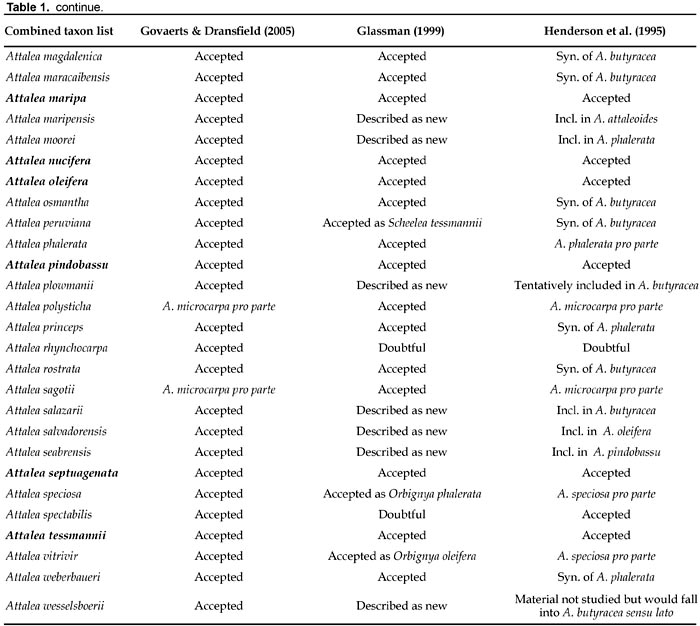
The World Checklist of Palms (Govaerts and Dransfield, 2005), mostly based on Glassmans treatment, includes 67 accepted species in the genus Attalea. These authors tentatively accept Attalea blepharopus from Bolivia, A. hoehnei from Brazil and A. rhynchocarpa from Colombia, all considered as uncertain names by Glassman (1999) and Henderson et al. (1995). Henderson (1995) listed A. blepharopus as a synonym of A. phalerata while Moraes (2004) included it as a synonym of A. butyracea. Govaerts and Dransfield (2005) and Henderson et al. (1995) also accept Attalea dahlgreniana, A. spectabilis and A. attaleoides while Glassman (1999) considers the first a hybrid and the latter two doubtful names. Henderson (1995) discussed various hypotheses about the uncertain nature of A. spectabilis. Four species, Attalea burretiana, A. liebmannii, A. lundellii and A. sagotii are accepted by Glassman (1999) but not by Govaerts and Dransfield (2005) nor by Henderson et al. (1995). Both Glassman (1999) and Govaerts and Dransfield (2005), listing 65 and 67 species, respectively, are far from the 29 species estimate of Henderson et al. (1995). Correspondences of species names (when possible) are given in Table 1. The first column of the table includes all taxa accepted in at least one of four most recent partial or complete treatments of the genus (Glassman 1999; Govaerts and Dransfield, 2005; Henderson et al., 1995; Noblick, 2007). This results in the highest possible estimate of 73 species in Attalea, among which only 20 species have been uncontroversial among modern palm taxonomists.
Uncontroversial species of Attalea
These 20 species include Attalea maripa, which has leaves arranged in five vertical ranks or orthostichies, corresponding to a 2/5 phyllotactic fraction, while most other species of Attalea sensu lato and Cocoseae in general have a 3/8 fraction. This species has a unique staminate flower structure and has been placed in its own genus, Maximiliana. Other very distinctive species include A. luetzelburgii, also formerly placed in a separate genus, Parascheelea, A. colenda, formerly placed in Ynesa (Fig. 1), and the morphologically and geographically isolated A. crassispatha. Within Attalea sensu stricto, A. tessmannii is highly distinctive vegetatively with its rigid leaflets dark glaucous-green above and whitish beneath (Fig. 2 and 4); within the Orbignya group, A. cuatrecasana is remarkable for its very large fruits to 15 cm long and 10 cm in diameter, only surpassed in size by the coconut within Arecoideae. The most problematic group remains that of Scheelea, in which a single species, A. insignis, has been uncontroversial among authors.
Nomenclatural and taxonomic problems in Attalea
The Attalea attaleoides complex
Attalea attaleoides, A. camopiensis, A. degranvillei, A. guianensis, A. maripensis
Attalea attaleoides is based on Maximiliana attaleoides Barb. Rodr. (not to be confused with Scheelea attaleoides H. Karst. which is a synonym of A. insignis). Glassman (1999) considered Maximiliana attaleoides as a species confusa belonging in fact to the Scheelea group. Henderson (1995) considered that A. attaleoides is part of the Maximiliana group, in accordance with its original assignment. Glassman (1999) described four new species from French Guyana in the genus Scheelea, latter transferred to Attalea by Zona (2002), on the basis of material previously assigned (at least tentatively) to Attalea attaleoides by Henderson (1995). However Glassman (1999) did not provide any statement on the material from Suriname and Brazil also included in A. attaleoides by Henderson (1995). Govaerts and Dransfield (2005), in accepting all four French Guyanan species (A. camopiensis, A. degranvillei, A. guianensis, A. maripensis) and A. attaleoides, implicitly consider that some material from outside French Guiana do correspond to the latter species. In fact, the material of A. guianensis, A. maripensis from French Guyana and A. attaleoides from Suriname and Brazil is very similar and it is likely that all these taxa represent the same species, which would be A. attaleoides if this name is accepted. On the other hand Henderson (1995) noted that the two specimens now known as A. camopiensis and A. degranvillei did depart from the typical morphology of A. attaleoides.
Attalea dahlgreniana
This species, known from Suriname and eastern Brazil is accepted by Henderson (1995) and Govaerts and Dransfield (2005). They followed Wessels Boer (1965) who argued about the distinctiveness of this species and the fact that it was probably not of hybrid origin since it is fully fertile, very abundant and morphologically homogeneous from Suriname to Brazil. It was originally described as a new genus, Markleya by Bondar (1957), who regarded it as a possible hybrid between A. maripa and A. speciosa. These two species grow sympatrically with A. dahlgreniana near the mouth of the Amazon river but allopatric populations of the latter exist in Suriname. Glassman noted that the staminate flowers of A. dahlgreniana do not match in any way those of the putative parent species A. maripa and A. speciosa, but nevertheless accepted A. dahlgreniana as an intergeneric hybrid called ×Maximbignya.
The Orbignya group
The Attalea speciosa complex
Attalea brejinhoensis, A. speciosa, A. spectabilis, A. vitrivir
This widespread peri-Amazonian group, the babaçu has been intensively studied due to the usefulness and extension of its populations. Nevertheless, its taxonomy remains controversial. Nomenclatural problems associated with the name A. speciosa have been described in the introduction of this paper. Henderson (1995) considered A. speciosa (= O. phalerata) to include A. vitrivir (= O. oleifera). This latter name corresponds to populations restricted to Minas Gerais and Bahia. Balick et al. (1987), Anderson and Balick (1988) and Glassman (1999) nevertheless provide convincing elements to maintain this geographically and morphologically distinct population as a separate species. Glassman (1999) described a third species in the complex, A. brejinhoensis, from Bahia. Henderson (1995) considered also the acaulescent A. spectabilis as part of the complex, being either an acaulescent form of A. speciosa or a hybrid with A. microcarpa. Glassman (1999) considered both A. spectabilis and A. microcarpa as doubtful species, since description of staminate flowers is ambiguous or lacking, and no material, type or otherwise mentioned in the literature, if it ever existed, is presently available for study.
The Attalea microcarpa complex
Attalea polysticha, A. sagotii
This species is accepted by Henderson (1995) and also by Govaerts and Dransfield (2005). As mentioned above, Glassman (1999) considered A. microcarpa doubtful and instead recognized two species, A. polysticha and A. sagotii. These species differ in the arrangement of staminate flowers on the rachillae. In A. polysticha, staminate flowers are inserted all around the rachillae (fig. 8) while in A. sagotii, they are clearly unilateral. This distinction is known to be significant in many other groups of Attalea (Fig. 5-7) and has been widely used by Glassman (1999) to distinguish species and groups of species. The recent discovery of A. polysticha and A. sagotii growing almost sympatrically in French Guyana (de Granville, pers. com. and specimens at CAY) can be interpreted in two ways: 1) the two species are clearly distinct, and maintain their differences in sympatry due to reproductive isolation, or 2) it is the same species which locally presents a polymorphism in the insertion of the staminate flowers. It is to be noted that while the A. polysticha morph is widespread, the A. sagotii morph is restricted to northeastern Amazonia.
The Attalea cohune complex
Attalea cohune, A. guacuyule
The question about this group is to determine whether A. guacuyule from Mexico is distinct or not from A. cohune from Guatemala, Belize, Honduras and Nicaragua. Galeano and Bernal (2002) confirmed the presence of typical A. cohune in the Magdalena valley of Colombia, separated by about 1300 km from the previously known Central American populations.
The Scheelea group
Scheelea is the most complex and controversial entity within Attalea s.l. Excluding the Attalea attaleoides complex, previously treated, which has been assigned either to the Maximiliana or the Scheelea group, the other species could tentatively be organized as follows.
Attalea phalerata complex
Attalea amylacea, A. anisitsiana, A. moorei, A. phalerata, A. weberbaueri
This group is defined by the unilateral staminate flowers on staminate rachillae and conspicuous fiber clusters organized in one circle in the thick endocarp (Fig. 5). Within this group, A. moorei is distinguished by its leaves with regularly arranged pinnae, A. weberbaueri and A. amylacea by wide pinnae (> 5 cm), A. anisitsiana by a comparatively thin peduncular bract, all other species of this group having a very thick peduncular bract. A. weberbaueri has been described as acaulescent, but recent observations of this species in Junín and Pasco (Peru) showed that although it actually begins to flower at an acaulescent stage, it eventually develops a short aerial trunk, obscured by persistent leaf bases. This group is well characterized and composed of evidently related species. Although Henderson et al. (1995) put all of them is synonymy of A. phalerata, there are evidences to maintain them separated (Noblick, 2007 and pers. obs.). The newly described A. moorei is particularly noteworthy for the very regular arrangement of pinnae, spreading in one plane, a feature not shared with the other members of the group. This species grows sympatrically with A. phalerata in Madre de Dios, Peru, without evidence of hybridization, and allopatrically all along the Huallaga valley.
Attalea butyracea complex
Attalea butyracea, A. kewensis, A. macrocarpa, A. magdalenica
This group is defined by epetiolate leaves with very regularly arranged pinnae, these with a prominent spine-shaped auricle basally (Fig. 4), peduncular bract thin, staminate flowers spirally arranged on staminate rachillae and dispersed fibers within a thin endocarp (Fig. 6). All species of this group are tall-stemmed. Typical A. butyracea has small (4-5 cm long) fruits, turning yellow at maturity, with inconspicuous endocarp fibers, and with two seeds (Fig. 6), leaf segments are stiff, glossy green adaxially and waxy-glaucous abaxially (Fig. 4). A. macrocarpa is distinguished by larger fruits (8-9 cm long) and A. kewensis has very short staminate petals. Characters of A. butyracea and A. magdalenica merge when a large number of specimens are examined, so that the latter is better considered as a synonym. Attalea kewensis has been described in cultivation but some specimens from Peru match relatively well the type (Glassman, 1999).
Attalea macrolepis complex
Attalea bassleriana, A. cephalotes, A. fairchildensis, A. huebneri, A. lauromuelleriana, A. leandroana, A. macrolepis, A. maracaibensis, A. peruviana, A. princeps, A. wesselsboerii
These species share many characters with the A. phalerata complex but have spirally arranged staminate flowers on staminate rachillae similar to those of the A. butyracea complex. They all have grouped pinnae (A. peruviana = Scheelea tessmannii only known from the type lacking leaves is tentatively included here). Henderson et al. (1995) put A. princeps, A. lauromuelleriana and A. leandroana in synonymy of A. phalerata but included the other ones in A. butyracea. Stauffer and Fariñas (2006) showed that A. macrolepis is a distinct species with a unique combination of characters. Since several species included here are very poorly known, it is not possible to determine to which extent this group is natural.
Attalea rostrata complex
Attalea osmantha, A. rostrata
This Caribbean-Central American group seems distinct from the western South-American A. butyracea complex, especially in having petiolate leaves. The two species differ in petiole length, fruit size and pattern of endocarp fibers.
Attalea liebmannii complex
Attalea liebmannii, A. lundellii
This group is the northern-most one (Mexico and Guatemala). These two species were included in synonymy of A. rostrata by Govaerts and Dransfield (2005), but this seems unjustified since A. rostrata has regularly pinnate leaves while the other two have clustered pinnae. In other instances (e.g. A. moorei vs A. phalerata, A. butyracea vs. A. macrolepis), leaf arrangement proved to be very consistent within species. Little differentiation was found between A. liebmannii and A. lundellii in a chemotaxonomic survey of flavonoids (Williams et al., 1983).
Attalea plowmanii and A. salazarii
These are two species recently described from the Peruvian Amazon (Glassman, 1999). Attalea plowmanii is very distinctive by its acaulescent habit, and has been later found in Colombia (Galeano and Bernal, 2002). Henderson (1995) tentatively included it in A. butyracea, but suggested that it may be a new species. A. salazarii is known from very few collections around Iquitos and is still in need of further studies to confirm its identity and relationships.
The Attalea sensu stricto group
This group is mostly diversified in southeastern Brazil (especially Bahia), and to a lesser extent in Central Brazil, with only three, not problematic species in the Amazon forest (A. ferruginea = A. racemosa, A. septuagenata and A. tessmannii). Contrary to the Scheelea and Orbignya groups which reach southern Mexico and the Caribbean, the Attalea sensu stricto group is almost exclusively South American, just reaching Panama (de Nevers, 1987) with two species, A. allenii and A. iguadummat. Two more species, A. amygdalina and A. nucifera grow in inter-Andean valleys of Colombia and A. colenda grows on the Pacific coast of southern Colombia and Ecuador.
Taxonomic problems in Attalea sensu stricto are restricted to southeast and central Brazil.
The Attalea apoda complex
Attalea apoda, A. brasiliensis
Attalea apoda is clearly a species of the Attalea sensu stricto group, but a confusion arose because Henderson et al. (1995) put it in synonymy of A. speciosa, which is a totally unrelated species of the Orbignya group. Glassman (1999) described a new species allied to A. apoda: A. brasiliensis.
The Attalea oleifera complex
A. burretiana, A. compta, A. oleifera, A. pindobassu, A. salvadorensis, A. seabrensis
Henderson et al. (1995) had a broad concept of this complex, recognizing two species: A. oleifera (including A. burretiana and A. compta in synonymy) and A. pindobassu. Glassman (1999) considered these two species but also A. burretiana and A. compta as distinct species, and described two new species, A. salvadorensis and A. seabrensis. He considered A. pindobassu, A. burretiana, and A. seabrensis most closely related to each other. The status of A. salvadorensis remains doubtful, it may be a mere synonym of A. burretiana (Noblick, pers. com.), or it may be a hybrid (Glassman, 1999).
Conclusion
Despite the publication of several recent accounts on the genus, the taxonomy of Attalea is still confusing. However, most problems are restricted to particular complexes of species, which have suffered excessive lumping (Attalea butyracea group), or possibly excessive splitting (Attalea attaleoides group) obscuring species delimitation. The identity and variability of many species still need to be checked in the field. New and ongoing studies, including DNA analyses, should disentangle most of the remaining problems.
Acknowledgements
I am grateful to Betty Millán for help with field and herbarium work in Peru, to Gloria Galeano and Rommel Montúfar for providing photographs, and to Larry Noblick for fruitful discussions and information on some species.
Literature cited
Anderson A.B. & M.J. Balick. 1988. Taxonomy of the Babassu complex (Orbignya spp.: Palmae). Systematic Botany 13: 32-50.
Anderson A.B., P. May & M.J. Balick. 1991. The subsidy from Nature. Columbia University Press, New York.
Balick M.J., A.B. Anderson & J.T. Medeiros-Costa. 1987. Hybridization in the Babassu palm complex II. Attalea compta × Orbignya oleifera (Palmae). Brittonia 39: 26-36.
Balick M.J., C.U.B. Pinheiro & A.B. Anderson. 1987. Hybridization in the Babassu palm complex: I. Orbignya phalerata × O. eichleri. American Journal of Botany 74: 1013-1032.
Balslev H. & A.Henderson. 1987. The identity of Ynesa Colenda (Palmae). Brittonia 39: 1-6.
Bondar G. 1957. Novo genero e nova especie de palmeiras da tribo Attaleinae. Arq. Jard. Bot. Rio de Janeiro 15: 49-55.
Dransfield J., N.W. Uhl, C.B. Asmussen, W.J. Baker, M.M. Harley & C.E. Lewis 2005. A new phylogenetic classification of the palm family, Arecaceae. Kew Bulletin 60: 559-569.
Dugand A. 1940. Palmas de Colombia. Clave diagnostica de los géneros y nomina de las especies conocidas. Caldasia 1: 20-83.
Galeano G. & R. Bernal. 2002. New species and new records of Colombian palms. Caldasia 24: 277-292.
Glassman S.F. 1999. A taxonomic treatment of the palm subtribe Attaleinae (Tribe Cocoeae). Illinois Biological Monographs 59.
Govaerts R. & J.Dransfield. 2005. World checklist of palms. Royal Botanic Gardens, Kew, UK.
Henderson A. 1995. The palms of the Amazon. Oxford University Press, 362 p.
Henderson A. & M. Balick 1991. Attalea crassispatha, a rare and endemic Haitian palm. Brittonia 43: 189-194.
Henderson A., G. Galeano & R. Bernal. 1995. Palms of the Americas. Princeton University Press, Princeton, New Jersey, USA. 352 p.
Nevers de G.C. 1987. The genus Attalea (Palmae) in Panama. Annals of the Missouri Botanical Garden 74: 505-510.
Moraes M. 2004. Flora de palmeras de Bolivia. Herbario Nacional de Bolivia, La Paz.
Noblick L. 2007. Palms of Paraguay, Uruguay and Argentina. Libro de resumenes, p. 30. Simposio Internacional: las palmeras en el marco de la investigación para el desarrollo en America del Sur. Lima, 7-9 Noviembre 2007. 72 p.
Stauffer F. W. & J. G. Fariñas. 2006. The identity of Attalea macrolepis (Burret) Wess. Boer (Arecaceae). Candollea 61: 83-88.
Uhl N.W. & J. Dransfield. 1987. Genera Palmarum. Allen Press, Lawrence, Kansas, USA.
Wessels Boer J.G. 1965. The indigenous palms of Suriname. E. J. Brill, Leiden.
Williams C., J.B. Harborne & S.F. Glassman. 1983. Flavonoids as taxonomic markers in some cocosoid palms. Plant Systematic and Evolution 142: 157-169.
Zona S. 2002. Name changes in Attalea. Palms 46: 132-133.














