Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.15 supl.1 Lima nov. 2008
TRABAJOS ORIGINALES
Novelties in the genus Ceroxylon (Arecaceae) from Peru, with description of a new species
Novedades en el género Ceroxylon (Arecaceae) del Perú, con la descripción de una nueva especie
Gloria Galeano1 ; María José Sanín1 ; Kember Mejía2 ; Jean-Christophe Pintaud3 and Betty Millán4
1 Instituto de Ciencias Naturales, Universidad Nacional de Colombia, sede Bogotá. Email Gloria Galeano: gagaleanog@unal.edu.co, Email María José Sanín: mjsaninp@unal.edu.co.
2 Instituto de Investigaciones de la Amazonia Peruana, Iquitos. Email Kember Mejía: kmejia@iiap.org.pe
3 IRD, UMR DIA-PC/DYNADIV, 911 Av. Agropolis, BP 64501, 34394 Montpellier cedex 5, France. Email Jean-Christophe Pintaud: pintaujc@mpl.ird.fr
4 Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima – Perú. E-mail Betty Millán: bmillans@unmsm.edu.pe
Trabajo presentado al Simposio Internacional Las palmeras en el marco de la investigación para el desarrollo en América del Sur, del 07 al 09 de Noviembre 2007, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Perú.
Abstract
A new species of Ceroxylon from Peru, C. peruvianum, is described and illustrated. The finding of disjunctive populations of C. quindiuense in Peru is discussed.
Keywords: Arecaceae, Ceroxylon, new species, phytogeography.
Resumen
Se describe e ilustra una nueva especie de Ceroxylon del Perú, C. peruvianum, y se discute el hallazgo de poblaciones disyuntas de C. quindiuense en Perú.
Palabras clave: Arecaceae, Ceroxylon, fitogeografía, nueva especie.
Introduction
The genus Ceroxylon Bonpl. is distributed all along the Andean montane forest, from Venezuela and Colombia to Ecuador, Peru and Bolivia, ranging from 800 to 3500 m in elevation. Following its description, the genus was monographed by Burret (1929), who recognized 16 species and mentioned other three names of dubious identity. In the last 30 years, five species have been described as new and many names have been reduced to synonymy, most of them corresponding to morphotypes of C. vogelianum and C. parvifrons, the two most widespread species of Ceroxylon (from Venezuela to Bolivia). The most recent attempt to clarify species identities within the genus was included in the Field Guide to the Palms of the Americas (Henderson et al., 1995), in which 11 species were recognized. This treatment was based on intensive field work in Colombia and Ecuador, but unfortunately not in the remaining Andean countries.
The Andes region in Peru is believed to harbor many unknown species; however, it is one of the most poorly collected areas in America (Gentry, 1993). This is true for many plant groups, including palms. In the case of Ceroxylon, the Peruvian Andes is an area that deserves special attention: in his monograph, Burret (1929) described seven new species, four of them from Peru, collected by the German botanist August Weberbauer between 1902 and 1915. Most of Burrets types were kept at the Berlin Herbarium and some of them were destroyed during the Second World War. For many species the only existing data are often imprecise descriptions. In consequence, the reconstruction of species identity in Ceroxylon has been an arduous task. In order to resolve doubts about taxonomic identity of these large and dioecious palms more field work needs to be done, especially in Peru.
Recent palm exploration in the Peruvian Andes has resulted in new, interesting information on the genus (Pintaud & Anthelme, 2008), and in the discovery of a new species of Ceroxylon that is here described. We also report the finding of another species, not previously recorded in Peru.
Ceroxylon peruvianum Galeano, Sanín & Mejía sp. nov.
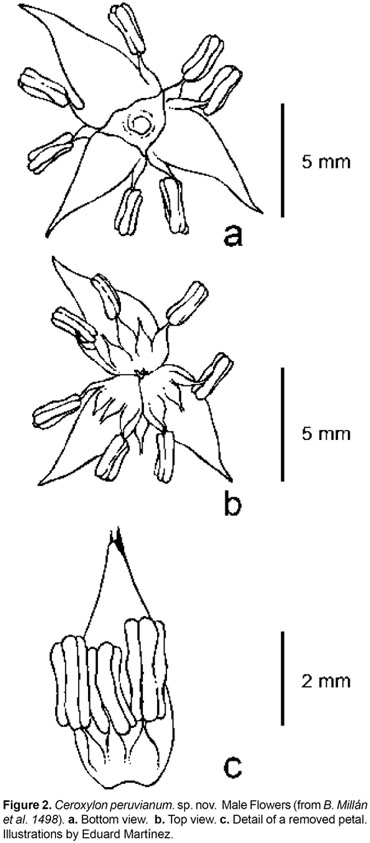
Type. PERU. Department of Amazonas. Province Bongará, District Jazán, Pedro Ruiz trail to Gocta Falls, 06°030.4 S 77°5318.2 W, 1800 m, 14 Nov 2007, B. Millán, J.C. Pintaud & L. Noblick 1488 (mat.fr.) (holotype: USM; isotypes: COL, AAU).
A Ceroxylon echinulatum differt foliis pinnis irregulariter dispositis, et floribus masculis staminibus 1215.
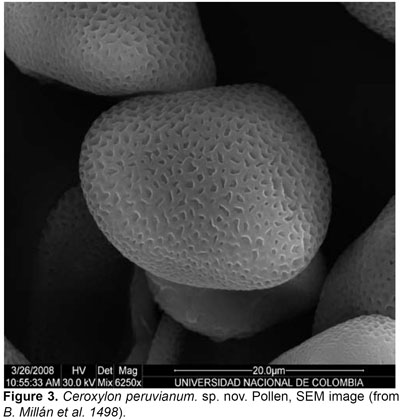
Trunk 812 m tall, 2026 cm diameter, silver to light grey with a thin layer of wax; leaf scars conspicuous, dark brown or gray. Leaves 1321, forming a hemispheric crown; sheath 130168 cm long, 57 cm wide at apex, with scarcely fibrous margins, covered with white tomentum; petiole 2560 cm long, 3,58 cm wide, flat to convex, green and with scarce indumentum above, convex and densely covered with white to light brown tomentum below; rachis 240362 cm long, the upper side flattened up to 165218 cm from base, with a well-defined hastula, and concave, covered with thick, white tomentum below; pinnae 96140 on each side, irregularly arranged in groups of 26, inserted in several planes, usually the proximal pinnae ascendant, the distal descendent, pinnae stiff until the middle of their length, then pendulous, apex slightly asymmetric, midrib prominent, adaxial surface glabrous, glossy, dark olive green, abaxial surface covered with white to yellowish scales, arranged in non-interlocking rows of persistent, elongate, thin, slightly membranous, mid-fixed, 0,51,0 mm long, 0,08 mm wide scales, consisting of a base and a short blade, and arranged in linear files, revealing the pinnae surface in between files in about the same width as the scales (with about 6 rows of scales per mm); lowermost filiform pinnae 7,041,5 x 0,21,0 cm, basal pinnae (10th pair, from base) 3751 x 0,81,3 cm, middle pinnae 6393 x 3,55 cm, apical pinnae 2146 x 0,73 cm, 29 most apical pinnae united along margins. Inflorescences 26 contemporaneous: female inflorescences 144 cm long in the fruiting stage, branched to 3rd order; peduncle 6290 cm long, 5,7 cm wide at apex; prophyll 2-keeled, 31 cm long, inserted 7 cm above the base of the peduncle; the lower peduncular bracts 118-220 cm long, inserted at ca. 27 cm above the base of the peduncle; rachis 77134 cm, with 6178 primary branches, basal branches 2179 cm long, middle branches 21-45 cm long, apical branches 2,54,3 cm long. Male inflorescences branched up to 3rd order; peduncle 4867 cm long, 4 cm wide at base; peduncular bracts 149169,5 cm long, 2327 cm wide; rachis 81102 cm long, with 7299 branches, basal branches 1836 cm, middle branches 2442,5 cm long, apical branches 36,5 cm long, not branched. Flowers pedicellate, the pedicel 0,5 mm long, subtended by a small, triangular acuminate, 2 mm long bract. Pistillate flowers not seen. Staminate flowers light yellow when fresh; sepals 3, ovate, 1 mm long, connate for 1/2 their length, lobes reaching 1/2 to the total height of the corolla tube; petals 3, ovate-acuminate, 47 mm long, including a 1 mm long acumen, connate up to 11,5 mm (1/61/4 of their length); stamens 1215, 13 opposite each sepal and 23 opposite each petal, filaments 11,5 mm long, anther 22,2 mm long, round at apex; pollen elliptical, monosulcate, tectate, 25,65 ± 1,01 µm diam, exine reticulate, exine thickness 0,52 ± 0,10 µm, with reticule aperture 0,75 ± 0,43 µm diam, reticule width 0,48 ± 0,06 µm; pistillode trimerous, minute. Fruits globose, 22,3 cm long, 22,2 cm wide, green turning red when ripe, mature exocarp densely covered with irregular and acute bulges; stigmatic residue small, lateral; seeds brown, globose, 1,5 cm diam. Fruit perianth with a persistent calyx about 1 mm long, reaching 1/2 the total height of the corolla tube; petals 45 mm long, connate for up to 2 mm (1/31/2 their length); staminodes 1213, 12 opposite each sepal, and 23 opposite each petal. Eophyll bifid, the abaxial surface covered with white tomentum.
Distribution and natural history
Known only from the Eastern slopes of the northern Peruvian Andes, this species has a rather wide ecological range, with respect to both temperature, as indicated by its altitudinal distribution (1500 to 2300 m) and humidity, as the species is found from semi-deciduous forest to wet Andean forest (after Onern, 1976), as a canopy component. Known populations develop mostly on soils derived from limestone rocks. The original vegetation of Andean forests is characterized by small to medium-sized trees (1520 m tall), with a semi-closed to closed canopy, from which the crowns of the wax palms stand out. The woody component of these forests includes species of the genera Pourouma and Cecropia (Cecropiaceae); Nectandra, Ocotea and Persea (Lauraceae); Manilkara (Sapotaceae); Calyptranthes (Myrtaceae); Schefflera (Araliaceae); Ficus and Brosimun (Moraceae); Inga (Leguminosae/Mimosoideae); Protium (Burseraceae); Licania (Chrysobalanaceae); and Clusia (Clusiaceae), as well as arborescent ferns of the genera Alsophylla and Cyathea (Cyatheaceae). These forests, specially those to the south of the Utcubamba River and on the sides of the roads between Pedro Ruiz Gallo, Pomacochas and the Abra Pardo Miguel, have been replaced by agricultural fields since the 70s, affecting palm populations. Hence, the area where C. peruvianum grows is currently heavily deforested with the palms remaining in the midst of farms. A few wild individuals were seen, and these were surrounded by forest on hardly accessible, steep slopes.
Little is known about the growth and development of this species, but the locals in the town of San Carlos reported that the palm attains chest-height in approximately 10 years.
Local names and uses
The Pona, as called by the locals, is considered a valuable ornamental plant, and it is cultivated in the town of San Carlos, being fairly common along dirt roads, the principal tracks leading to small villages and bordering the houses and chacras. Additionally, the species is often cultivated as a timber tree in coffee agroforestry systems, and the trunk is used for posts, supporting houses or huts, and to make fences; the leaves are eventually used for thatching farmyards; the fruits are fed to pigs.
The fact that this species is being cultivated is particularly interesting; the inclusion of Ceroxylon in agroforestry systems has been documented by Pintaud & Anthelme (2008) for C. echinulatum in northern Peru, and it was known also for C. sasaimae in Colombia (Galeano & Bernal, 2005). It is possible that the inclusion of C. peruvianum in agroforestry systems could constitute a real conservation opportunity despite the threat of extinction among the natural populations, a case similar to C. echinulatum (Pintaud & Anthelme, 2008).
Etymology
The species is named for Peru, where it was discovered.
Additional specimens examined: PERU, Department Amazonas, Province Bongará, District San Carlos, cultivated in the town of San Carlos, 05°5751.1 S, 77°5650.4 W, 1830 m, 15 Nov 2007, B. Millán, G. Galeano, M.J. Sanín, J.C. Pintaud, F. Borchsenius, L. Noblick, P. Trénel & J. Roncal 1497 (mat. fr.) (USM); cultivated in the town of San Carlos 05°5757.8 S 77°5637.2 W, 1880 m, 15 Nov 2007, B. Millán, G. Galeano, M.J. Sanín, J.C. Pintaud, F. Borchsenius, L. Noblick, P. Trénel & J. Roncal 1498 (st. fl.) (AAU, COL, NY, P, USM); same locality, 15 Nov 2007, B. Millán, G. Galeano, M.J. Sanín, J.C. Pintaud, F. Borchsenius, L. Noblick, P. Trénel & J. Roncal 1499 (inm.fr.) (AAU, COL, NY, P, USM). Province Bongará, District Jazán, cultivated near way to San Pablo, Pedro Ruiz road Chachapoyas, 06º0331.8 S, 77º5538.28 W, 1569 m, 16 Mar 2006, B. Millán & J.C. Pintaud 1354 (inm. fr.)(USM); same locality, 16 Mar 2006, B. Millán & J.C. Pintaud 1356 (seedling)(USM). Province Bongará, road from Pedro Ruiz to Moyobamba, km 12, Oct 1990, F. Kahn and F. Moussa 2704 (inm.fr.) (USM). Province Bongará, Road Pedro Ruiz to Moyobamba, km 340-350, Buenos Aires, 5° 45S 77° 47W, 2300 m, 30 Aug 1983, D. Smith & S. Vásquez 4854 (mat.fr.) (MO, USM).
Comments
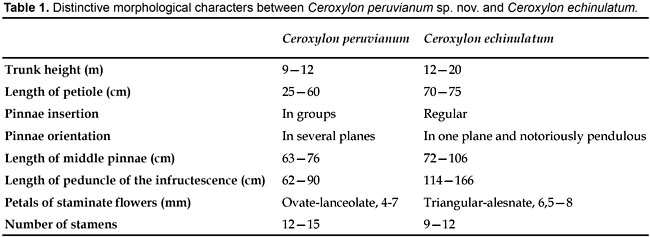
Ceroxylon peruvianum is very distinctive in its irregularly arranged pinnae, combined with staminate flowers bearing 12-15 stamens and the fruit exocarp densely covered with acute bulges. The latter character was so far only known from C. echinulatum, which has a similar altitudinal range (15002300 m) but this palm has regularly arranged pinnae set in one plane and staminate flowers with 912 stamens. Other differences between these two especies are listed in table 1. Except for the pinnae insertion character (in groups vs. regular), none of the remainder characteristics contrasted in table 2, are by themselves, strong enough for the delimitation of a Ceroxylon species; however, the combination of them makes the new species unmistakable.
Ceroxylon echinulatum has been recently found in northern Peru (Pintaud & Anthelme, 2008), at less than 100 km North from the area where C. peruvianum is known to grow. It would be very interesting to explore neighboring areas to see if the distributional ranges of these species come closer or perhaps even overlap.
Ceroxylon quindiuense (Karst.)H. Wendl.
C. quindiuense is characterized by its stout and tall stems, covered by a thick layer of white wax, hemispherical crowns of horizontal leaves with straight rachis and regularly arranged, pendulous pinnae that are covered with a thick indumentum beneath, and smooth fruits. This species was previously known only from Colombia, where it grows all along the Andes, between 2000 and 3000 m, in montane forests, usually forming characteristic, large populations of thousands of individuals (Fig. 4c). Nevertheless, recent field work in Northern Peru has revealed new records of palms that, besides growing in the same habitat type, form dense populations, and perfectly match the vegetative and reproductive macro-morphological characters of C. quindiuense (Fig. 4ab). These similarities have led us to consider that these newly found Peruvian individuals can indeed be circumscribed under C. quindiuense, despite the distributional gap.
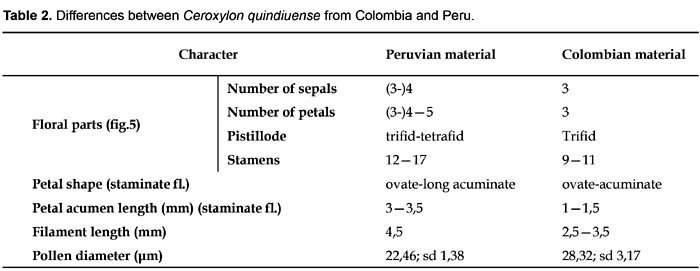
The few differences found between the Colombian and the Peruvian palms are listed in Table 2.
Combinations such as 3 sepals-5 petals-trifid pistillode, or 4 sepals-4 petals-tetrafid pistillode, were common in the single Peruvian staminate individual observed (Fig. 5). While variation in the number of floral parts has been experimentally induced in diverse angiosperm taxa of ornamental plants, this phenomenon has also been reported in wild population. Such variations can occur, for example, between populations, which is the case of Sanguinaria canadensis (Spencer, 1994), or along different parts of the inflorescence, which is the case for Drimys winteri (Winteraceae)(Doust, 2001). The latter type of variation could also occur in Ceroxylon, but it has not been observed in detail, especially as the Ceroxylon inflorescence can reach considerable size and weight, reason for which just small portions are collected in the field and kept in herbarium specimens. As for other palm genera, this particularity has not been discussed in detail. However, in Wettinia, flowers of some species, can have 3(-4) sepals and/or 3(-4) sepals (Uhl & Dransfield,1987).
Another aspect of floral morphology that needs more attention is the variation in the number of stamens and staminodes, which has been usually thought of as determinant for some species in the genus (ex. C. alpinum, C. parvum, C. vogelianum). Nevertheless, it is known, for example, that in Allagoptera, the number of stamens varies among the species, but, interestingly, A. leucocalix has 9-15 stamens, with 14-15 stamens in the proximal flowers of the inflorescence, and 9-10 in the distal ones (Moraes, 1996).
The difference in number of stamens found in the Peruvian material could be attributed to the high proportion of tetramerous and pentamerous staminate flowers found, but at least one individual with 3 petals and 15 stamens was observed, indicating that more stamens can occur in spite of an otherwise normal floral morphology.
On the other hand, the long acumens of the petals and the greater length of the filaments in the staminate flowers of the Peruvian individual studied constitute a difference from the known specimens of C. quindiuense from Colombia. In the polyandrous Iriarteinae with stamen numbers that range from 17 to 145, Henderson (1990) observed that, in some species, the proximal flowers on a rachilla are larger and have more stamens than the apical flowers on the same rachilla. This is an example of how quantitative characters are only trustworthy when sampling has been exhaustive and discreteness in an otherwise continuous numerical scale can be affirmed. The range for many quantitative characters has been proven to be quite large, and one individual displaying an outlying score cannot be considered to accurately cover the variability within a population, and thus doom it to exclusion from a species taxonomic domain.
Likewise, the differences in pollen measurements can not be evaluated as weighty evidence, since, for example, pollen grain diameter in C. amazonicum Galeano ranges from 19,6 to 27,6 µm in different individuals.
Consequently, it is here remarked that without substantial sampling and knowledge of more Peruvian individuals, it is hard to decide whether these differences constitute character states or traits. We emphasize that studies of all kinds are however still needed, including morphological, and molecular ones, in order to prove whether this material deserves the status of species.
On the other hand, when comparing material from both countries, we found an additional shared character, interestingly found nowhere else within the genus, which is the pollen form and aperture. The pollen of Ceroxylon has been said to be monosulcate and elliptical. The pollen sample of C. quindiuense from Colombia can have both monosulcate or trichotomosulcate aperture and globose to elliptical or triangular ambit (Fig. 6). The sample from Peru has only trichotomosulcate, triangular pollen (Fig. 6a), but still, these are two morphologies nowhere else seen in the genus. Trichotomosulcate apertures have been considered as typically derived from the monosulcate condition (Walker & Doyle, 1975), the latter of which is considered ancestral among the monocots (Penet et al., 2004), and pollen shape or ambit can be considered to be closely related with the type of aperture. Some authors (Penet et al., 2004; Harley, 2004) consider that trichotomosulcate pollen is related to regular tetrahedral tetrads in the developmental pathway, and that developmental options eventually select against one of the morphologies. Additionally, trichotomosulcate pollen is only known in the subfamily Ceroxyloideae from Pseudophoenix sargentii subsp. saonae, where it has been observed as a mixed condition, involving pollen with both one and three apertures (Machado, 2003). The same scenario could be considered for C. quindiuense: a pollen dimorphism in which both the derived and ancestral states are simultaneously present in one species, probably due to active differentiation processes.
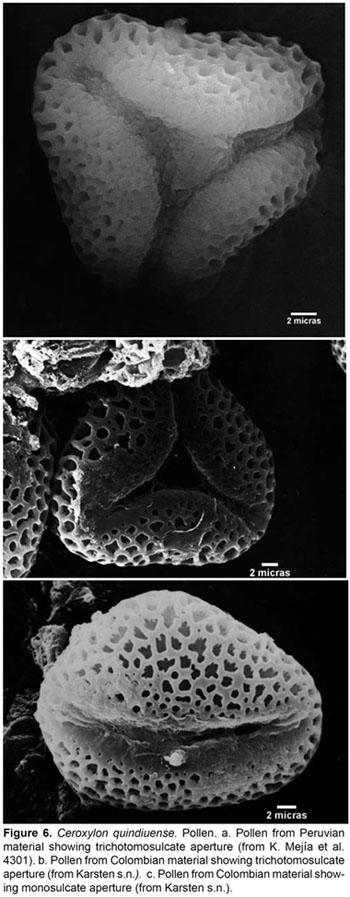
The relevance of this character for the delimitation of species in Ceroxylon is a topic that deserves more attention, as it could be a valuable source of morphological information. Thus, the data available, as well as the micro-morphological character provided, constitute the reasons for which, until more morphological and molecular observations and studies can be gathered, we consider it is most reasonable to include these Peruvian records under C. quindiuense.
We also note with interest that the distribution range of C. ventricosum, morphologically the most similar species to C. quindiuense, matches closely the distributional gap of about 800 km in Ecuador and southern Colombia, where C. quindiuense has not been recorded yet. Moreover, Trénel (2007) proposed, in his phylogeny of Ceroxylon based on molecular data, that C. ventricosum and C. quindiuense form a species complex, given that C. ventricosum was recovered as a paraphyletic assemblage, with the two analyzed accessions of C. quindiuense nested inside C. ventricosum.
These new insights into the distribution range of C. quindiuense impinge on other significant topics. C. quindiuense is known to have been a very abundant species in Colombia until the beginning of the last century, and even if some numerous populations persist in the Central Cordillera, it has been classified as endangered according to the IUCN categories (Galeano & Bernal, 2005). Most of the forests where this species grows have been turned into pastures devoted to massive livestock production, and while the adult palms are sometimes left standing, the young seedlings do not grow. Additionally, it was only until a few years ago that the leaves of this species were used for Palm Sunday celebrations during Holy Week, causing in some cases, the death of many individuals, and in other cases, a delay in normal growth and development rates. What is worse, in the last decade a new and unknown disease has been causing the death of many adult palms. In this panorama, the new records for C. quindiuense are very encouraging. However, the conservation situation in Peru does not differ substantially from the Colombian one. In the province of Chachapoyas, few individuals were seen surrounded by forest and were standing on very steep slopes, suggesting that all those which had grown on more even landscapes had been cut down. This palm has been seen in numerous and dense populations in Ocol, the District of Molinopampa, but, as locals report, it is being widely harvested as wood for housing and posts, and the trunk ripped for covering indoor walls.
Ecological research of all scopes is seriously needed, including phenological, pollination, and demographic studies. As these new records also raise many issues in taxonomic, distributional, geographical, and evolutionary domains, genetic population structuring of these accessions is strongly advocated.
Specimens examined: Peru, Department Amazonas, Province Chachapoyas, District Leimebamba, dirt road to the Archeological Museum, 06°4514.7 S 77°483.7 W, 2523 m, 13 Nov 2007, B. Millán, G. Galeano, M.J. Sanín, J.C. Pintaud, F. Borchsenius, L. Noblick, P. Trénel & J. Roncal 1487 (inm.fr.) (AAU, COL, P, USM). Department Amazonas, Province Chachapoyas,District Molinopampa,locality Ocol, 06°1548 S 77° 3441 W, 2373 m, 20 Sep, 2007, K. Mejía et al. 4301 (st. fl.)(USM). Department Amazonas, Province Chachapoyas, District Molinopampa, locality Puma Armana near Ocol, 06º1527.72 S 77º3424.54 W, 2360 m, 16 Mar 2006, B. Millán & J.C. Pintaud 1352 (inm.fr.)(USM).
Acknowledgements
We thank the IRD and the Museum of Natural History at the Universidad Nacional Mayor de San Marcos for organizing the Simposio Internacional de Palmeras (Lima, November 2007) and the IRD for supporting field work after the Simposium, when these palms could be collected. We also thank: F. Borchsenius, P. Trénel, L. Noblick, J. Roncal, A. Delgado, K. Martínez, S. Vaca, P. Talavera, M. Pérez Ojeda, L. Santa Cruz and M. Sosa for the valuable help provided during the field work; Joaquina Albán, chief curator at USM; Filomeno Encarnación, for some of the photographs; Laboratorio de Microscopía Electrónica (Departamento de Geociencias, Universidad Nacional de Colombia) and the Laboratory at L.H. Bailey Hortorium (Cornell University) for SEM images; Eduard Martínez for the flower illustrations, Lauren Raz for helping us with our writing in english and two anonymous reviewers for critical comments on the manuscript.
Literature cited
Burret M. 1929. Die Gattung Ceroxylon Humb. et Bonpl. Notizbl. Bot. Gart. Berlin-Dahlem 10: 824-844.
Doust A.N. 2001. The Developmental Basis of Floral Variation in Drimys winteri (Winteraceae). Int. J. Plant Sci. 162(4):697–717.
Galeano G. & R. Bernal. 2005. Palmas. In: Calderón, E., G. Galeano y N. García (eds.). Libro Rojo de las Plantas de Colombia. Volumen 2: Palmas, Frailejones y Zamias. Serie de Libros Rojos de Especies Amenazadas de Colombia. Bogotá, Colombia. Instituto Alexander von Humboldt –Instituto de Ciencias Naturales de la Universidad Nacional de Colombia – Ministerio de Medio Ambiente, Vivienda y Desarrollo Territorial. Pp. 59-224.
Gentry A. 1993. Overview of the Peruvian Flora. Pp. xxix-xl. In: Brako, L. & J. Zarucchi (eds.). Catálogo de las Angiospermas del Perú. Monographs in Systematic Botany from the Missouri Botanical Garden vol. 45.
Harley M. 2004. Triaperturate pollen in the monocotyledons: configurations and conjectures. Plant Systematics and Evolution 247 (1-2):75-122.
Henderson A. 1990. Arecaceae. Part I. Introduction and the Iriarteinae. Flora Neotropica, Monograph 53. The New York Botanical Garden. New York.
Henderson A., G. Galeano & R. Bernal. 1995. Field guide to the palms of the Americas. Princeton University Press, Princeton.
Machado S. 2003. Variaciones en la morfología polínica de Arecaceae en Cuba: abertura tricotomosulcada y estratificación de la exina. Revista del Jardín Botánico Nacional 24(1-2): 71-79.
Moraes M. 1996. Allagoptera (Palmae). Flora Neotropica, Monograph 73. The New York Botanical Garden. New York.
ONERN. 1976. Mapa Ecológico del Perú. Guía Explicativa y Mapa. Oficina Nacional de Evaluación de Recursos Naturales Lima. Perú.
Penet L., S. Nadot, A. Ressayre, A. Forchioni, L. Dreyer & P.H. Gouyon. 2004. Multiple developmental pathways leading to a single morph: Monosulcate pollen (examples from the Asparagales). Annals of Botany 95 (2): 331-343.
Pintaud J.C. & F. Anthelme. 2008. Ceroxylon echinulatum in an agroforestry system of northern Peru. Palms 52 (2):96-102.
Spencer W. 1944. Variation in petal number in the bloodroot, Sanguinaria canadensis. The American Naturalist 78(774): 85-89.
Trénel P. 2007. Evolutionary studies in the wax palm subfamily (Ceroxyloideae, Arecaceae). Ph.D. Thesis. Department of Systematic Botany, Institute of Biological Science, University of Aarhus, Denmark.
Uhl N. & J. Dransfield. 1987. Genera Palmarum -A classification of Palms Based on the Work of Harold E. Moore Jr. International Palm Society & L.H. Bailey Hortorium, Cornell University.
Walker J.W. & J.A. Doyle. 1975. The Bases of Angiosperm Phylogeny: Palynology. Annals of the Missouri Botanical Garden 62(3): 664-723.

















