Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.15 supl.1 Lima nov. 2008
TRABAJOS ORIGINALES
Aphandra natalia (Arecaceae) – a little known source of piassaba fibers from the western Amazon
Aphandra natalia (Arecaceae) – un recurso poco conocido de piassaba en el oeste de la Amazonía
Mette Kronborg1, César A. Grández2, Evandro Ferreira3 and Henrik Balslev1
1 Department of Biology, University of Aarhus, building 1540, Ny Munkegade, 8000 Aarhus C., Denmark. Email Mette Kronborg: mettekronborg@gmail.com, Email Henrik Balslev: henrik.balslev@biology.au.dk
2 Facultad de Ciencias Biológias, Universidad Nacional de la Amazonía Peruana, Iquitos, Peru. Email: cgrandez@hotmail.com
3 Instituto Nacional de Pesquisas da Amazônia-INPA, Núcleo de Pesquisas do Acre, BR 364, km 4, Parque Zoobotânico da Universidade Federal do Acre, CEP 69.915-900, Rio Branco, Brazil. Email: evandroferreira@hotmail.com
Trabajo presentado al Simposio Internacional Las palmeras en el marco de la investigación para el desarrollo en América del Sur, del 07 al 09 de Noviembre 2007, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Perú.
Abstract
Aphandra natalia (Balslev & Henderson) Barfod is a multipurpose palm that is exploited both commercially and for subsistence purposes. Its fibers are important in Peruvian and Ecuadorean broom industries and support many people economically. In Brazil, it is found in the western part of Acre, where it is the main source for a local broom market. Data from fieldwork in Peru (2007) suggests that the variation in gross profit per kilogram of fiber is considerable among the different segments in the broom industry. Harvesters and distributors earn negligible amounts of money whereas manufacturers reap of the major part of the earnings. Fiber extraction appears to be sustainable in Ecuador and in some parts of Peru, whereas in other parts of Peru unsustainable harvest occurs, involving felling of entire palm trees for the harvest of fibers. The same destructive extraction method is used in Brazil, where the palm is becoming rare in its natural distribution area.
Keywords: agroforestry, conservation, ecological sustainability, extractivism, value chain.
Resumen
La palmera de piasaba (piassava, piassaba) Aphandra natalia (Balslev& Henderson) Barfod es una palma que se utiliza para muchos propósitos, tanto comerciales como para la subsistencia de pueblos rurales. Sus fibras son de importancia económica en industrias de escobas en Perú y Ecuador, las cuales sostienen económicamente a muchas personas. En Brasil, esta palma se encuentra en la parte oeste del estado de Acre, donde sus fibras constituyen el recurso principal para el mercado local de escobas. Información de campo originada en Perú en el año 2007, muestra que existe una importante variación en las ganancias económicas por kilo de fibra entre los diferentes sectores de la industria de escobas. Los que cosechan y distribuyen los productos obtienen ganancias muy reducidas, mientras que los productores de escobas son los que más ganan. La extracción de fibras parece ser sostenible en Ecuador y en algunas partes de Perú, mientras que en otras partes de Perú se tumban palmeras enteras para sacar la fibra, lo cual representa un método no sostenible. La misma forma destructiva de cosecha de las fibras existe en Brasil, lo cual ha traído como consecuencia que las poblaciones de la palma se encuentran muy disminuidas en su hábitat natural.
Palabras claves: agroforestería, conservación, sostenibilidad ecológica, extractivismo, cadena de valores.
Introduction
Several palms are keystone species in the Amazon and many others provide commercial and subsistence commodities of great importance to rural people in the regions rain forests. Harvesting of palms provide people with security in terms of food availability and cash income, but unfortunately palm exploitation is often destructive and sometimes leads to a decline in abundance and richness of species (Balick, 1989; Borgtoft Pedersen, 1992; Borgtoft Pedersen & Balslev>, 1992; Zambrana et al., 2007). Conservation and management of palms have therefore attracted much attention and it has been argued that sustainable management of palms is needed to fulfil future requirements of rain forest conservation and peoples economic and nutritional needs (Balick, 1989; Pinard, 1993; Borgtoft Pedersen & Skov, 2001; Vormisto, 2002a; Macía, 2004). An often discussed proposal for conserving rain forests is to use the forest through extractivism, i.e., non-destructive harvest of non-timber forest products. To many this proposal seems reasonable, but others have rejected it as inadequate, because sustainable harvest methods are lacking or insufficient (Homma, 1993; Vormisto, 2002a, 2002b). Therefore it has been suggested that other methods, for instance cultivation, should be promoted to prevent further deterioration of rain forests. Still other proposals include the development of correct valuation of ecosystem goods and services, and its subsequent influence on how to manage these natural resources (Voeks & Rahmatian, 2004).
The stiff, brown fiber called piassaba is just one example of an economically important non-timber forest product in South America. It is derived from three different and taxonomically unrelated palm species: Leopoldinia piassaba Wallace, Attalea funifera Mart. and Aphandra natalia, and used for brooms that are primarily sold on national markets (Borgtoft Pedersen & Balslev, 1992; Borgtoft Pedersen, 1992, 1996). Their management, and social and economic importance are diverse, depending on the species and the geographic area.
Leopoldinia piassaba (piassava in Brazil, chiquichiqui in Colombia and Venezuela, fibra in Colombia) is distributed in the upper Rio Negro region of Brazil, Colombia and Venezuela (Figure 1), especially on poor and sandy soils associated with black water rivers where it forms extensive single-species patches covering several hectares. The palm is solitary, and reaches a height of 10 m with its crown of pinnate leaves. Both fibers and fruits of the palm are exploited by rural people, commercially as well as a subsistence commodity. Piassaba is the remains of leaf sheaths that decay over time and subsequently turn into 11,5 m long strong and brown fibers that are collected from palm groves and sold to middlemen or directly to manufacturers of brooms and ropes (Balick, 1989; Kahn, 1991; Bernal, 1992; Lescure et al., 1992; Henderson et al., 1995). The economic importance of Leopoldinia piassaba fibers have changed over time from the 19th and early 20th centuries when they were exported internationally, to the present situation where they mostly contribute to rural peoples income through local and national sales (Bernal, 1992; Henderson et al., 1995). This change has occurred as cheaper and more accessible substitutes to piassaba fibers, such as plastic materials, became available. Exploitation of Leopoldinia piassaba is sustainable, usually involving a harvest method in which two to four younger leaves are left intact, and the palms are left to regenerate between each harvesting thereby promoting population size to stay in equilibrium (Lescure et al., 1992).
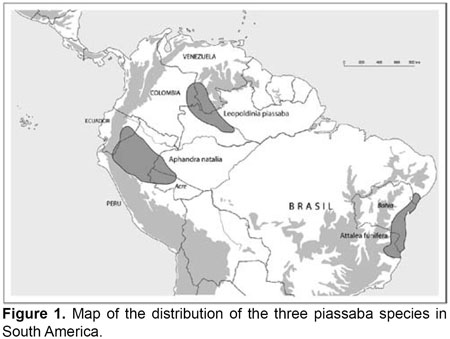
Attalea funifera (piassava or piaçava) is endemic to a belt along the Atlantic coast of Bahia in Brazil (Figure 1) where it mostly inhabits sandy and spodosolic soils on elevated terraces close to the sea. It reaches a height of 12–15 m, its leaflets are clustered, it is hermaphroditic but changes sex expressions as it grows older, and its pollination involves various insect species that depend on it for their survival (Voeks, 1988, 2002; Henderson et al., 1995). Attalea funifera fibers, derived from the sheath and petiole, are harvested mostly by rural smallholders or by specialized cutters contracted by owners of greater areas with stands of the palm. Fibers are used for brooms and thatch and are, subsequent to harvesting, sold to middlemen, broom manufacturers, or international export companies. Usually palms are exploited sustainably, and only 12 living leaves are destroyed during each harvesting although the shift to sustainable practices is new and originated in the 20th century (Voeks, 1988, 2002; Henderson et al., 1995; Voeks & Rahmatian, 2004). Management of the palm is widespread in Brazil where several initiatives were established to increase sustainability of the practices. These efforts include planting, long-fallow slash-and-burn practices or benign neglect, meaning no special care is carried out within the natural piassaba stands besides one annual harvest (Voeks, 1988, 2002; Henderson et al., 1995; Voeks & Rahmatian, 2004).
Leopoldinia piassaba and Attalea funifera are widely sold in Brazil. The annual production is estimated at more than 95,000 tons (IBGE, 2002; 2003), of which 90% are sold nationally. Fibers from Attalea funifera are of a higher use quality, possibly because they are stiffer and more robust than fibers from Leopoldinia piassaba, and they cost twice as much. Fiber production from both piassaba palms is declining; for Attalea funifera because of destruction of its natural stands and for Leopoldinia piassaba because of reduced demand for the fibers. Nevertheless, income from piassaba fibers continues to contribute substantially to Brazilian household economies both through local and national sales. In 2003 the commercial value of fibers sold in Brazil amounted to $48,6 millions (IBGE, 2003).
Whereas the piassaba palms from Bahia (Attalea funifera) and Rio Negro (Leopoldinia piassaba) were described in the scientific literature over a century and a half ago (Martius, 1824; Wallace, 1855) the western Amazon piassaba palm (Aphandra natalia) remained un-described until 20 years ago (Balslev & Henderson, 1987). Consequently the popular and scientific literature abounds with descriptions of the two former species, while the western Amazon piassaba often is not even mentioned in the piassaba literature (Balick, 1989). Nevertheless some information about Aphandra has accumulated, and we here review the literature about the biology, use and management of this economically important species, and supplement that with our own observations.
The biology of Aphandra natalia
Taxonomy
The genus Aphandra includes only one species, Aphandra natalia, which was originally described in Ammandra (Balslev & Henderson, 1987) but later transferred to the new genus Aphandra in a revision of the palm subfamily Phytelephantoideae (Barfod, 1991), which has now been merged into Ceroxyloideae.
Distribution and abundance
Aphandra natalia occurs in the western Amazon region from the foothills of the Andes in Ecuador through the northern part of Perus Amazon to the state of Acre in Brazil (Figures 1 & 2) (Borgtoft Pedersen & Balslev, 1990; Barfod, 1991; Henderson et al., 1995; Boll et al., 2005).
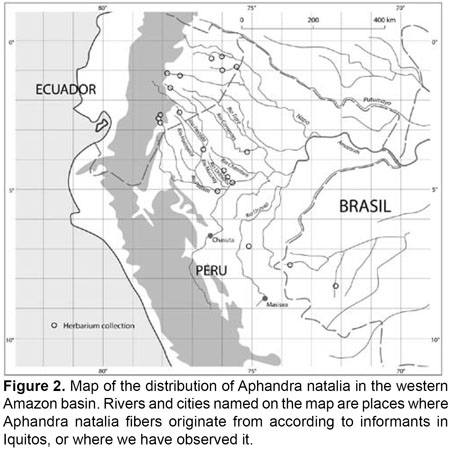
Soil moisture and elevation determine local occurrence of Aphandra natalia, and its preferred habitat is terra firme forest and low dry terraces that are inundated only after substantial rainfall. Wild populations of Aphandra natalia can be found at elevations of up to 800 m above sea level, and cultivated specimens have been found 1000 m above sea level in southern Ecuador (Borgtoft Pedersen & Balslev, 1990, 1992; Borgtoft Pedersen, 1992; Henderson et al., 1995; Boll et al., 2005; Montufar & Pintaud, 2006). The climate favoured by Aphandra natalia is the typical rain forest climate, with humidity and temperature high and constant all year round, and annual mean precipitation above 2000 mm.
Populations of Aphandra natalia are often isolated from each other, clumped and with a slight dominance of male individuals (Ervik, 1993) primarily due to dispersal limitations. Although other theories have been used to explain clumped distribution of plant species (Levine & Murrell, 2003; Boll et al., 2005), dispersal limitation has been suggested as one of the most plausible reasons in the case of Aphandra natalia, mainly because its seeds are large and its dispersers (agouties and squirrels) can only carry seeds over short distances. Moreover, immature Aphandra natalia are aggregated around mature individuals, which fits the idea that dispersal limitation is the cause of its clumped distribution (Boll et al., 2005). Another explanation to the clumped distribution could be that Aphandra natalia cannot stand or germinate in inundated areas. On a larger scale the abundance of Aphandra natalia, within its distribution area, is variable and characterised by areas of different sizes inhabited by dense populations of the palm next to areas with no or very few palms (Table 1).
Morphology and reproduction
Aphandra natalia is a medium-sized, solitary palm with stem heights of 311 m, and stem diameters of 2030 cm (Balslev & Henderson, 1987; Borgtoft Pedersen & Balslev, 1990; Barfod, 1991; Henderson et al., 1995; Borchsenius et al., 1998). Its crown has 1036 leaves that are up to 8 m long, erect or spreading and with a twisting rachis. The blades are pinnate; pinnae are linear and numerous, usually 90120 per side with the basal and middle ones oriented horizontally and those near the apical end oriented vertically because of the rachis twist, still, all pinnae are distributed regularly and in the same plane. Leaf sheaths are large and disintegrate continuously over time, which creates a dense mass of long and solid brown fibers hanging 33,5 m down from the base of the crown. In one year single palms (measured in south-eastern Ecuador) produced 380442 g of fibers, depending on exposure to sunlight (Borgtoft Pedersen, 1996). Presence of fibers and the numerous black scales on the surface make it easy to distinguish Aphandra natalia from other, closely related palms such as Ammandra dasyneura (Burret) Barfod and species of Phytelephas. Aphandra natalia is dioecious with individual palms possessing either female or male inflorescences. Inflorescences are borne among the leaves while infructescences are usually borne on the stem underneath the crown where they, nevertheless, may be difficult to see because they are hidden in the mass of fibers. Staminate inflorescences can be more than 2 m long, they have a soft yellow colour and are fleshy. Staminate inflorescences also posses characters which distinguish Aphandra natalia from the other, closely related, genera Ammandra and Phytelephas; the staminate flower cluster contains additional bracteoles, which may induce a more branched staminate cluster (Barfod & Uhl, 2001). The structures constituting the staminate inflorescence are a rachis densely covered with short male flowers. Four flowers are clustered together on one single branch and each flower contains 200300 small stamens. Pistillate inflorescences are smaller and more contracted than staminate ones, and the rachises are very compact and covered by 3050 flowers. Calyx and corolla are both fleshy and similar in appearance. Styles are long and have several stigmas. Up to five infructescences can be present on a single palm; they are almost globular, brown, 3045 cm in diameter, consist of 3045 fruits, and have a hard and woody surface. The mesocarp is fleshy and light orange-yellow in colour. The endosperm has a fluid and jelly-like texture when immature and later develops into a very hard bony and homogenous substance comprised of polysaccharides of the type mannan A and B. (Timell, 1957; Borgtoft Pedersen & Balslev, 1990; Ervik, 1993; Henderson et al., 1995; Ervik et al., 1999; Barfod & Uhl, 2001)(Figure 3).
Phenology
Aphandra natalia does not appear to have a sharply delimited flowering season. It often produces multiple inflorescences and flowers continuously throughout the year, although with varying intensity and with a peak in February and March. Initial flowering occurs five years after germination (Borgtoft Pedersen & Balslev, 1990; Barfod, 1991). Several weeks prior to anthesis temperatures of staminate and pistillate inflorescences rise 1019 °C above ambient temperature. The heating is most pronounced in pistillate inflorescences immediately before anthesis and the temperature rise persists in the pistillate inflorescence for at least a week. The temperature increase is less dramatic and of shorter duration in staminate inflorescences, and lasts a shorter period of time after anthesis. The odours emitted from staminate and pistillate inflorescences resemble each other in scent chemistry, mainly consisting of a pyrazine based compound (2-methoxy-3-secbutylpyrazine), that has a strong heavy odour, unpleasant to humans, but thought to be a key factor in attracting specific pollinator species. The splitting of inflorescence bracts, which is the initial indication of anthesis, varies between sexes. Staminate inflorescences open during the day while pistillate inflorescences open during the night (Borgtoft Pedersen & Balslev, 1990; Ervik, 1993; Ervik et al., 1999; Barfod & Uhl, 2001). Pollination of Aphandra natalia is primarily by Coleoptera, although a few species of Diptera and Hymenoptera have been found to pollinate it as well. Mutualistic relationships have been found between Aphandra natalia and some beetle pollinators, i.e., species of Baradinae and Aleocharinae (Ervik, 1993). In addition, several other species have been reported as frequent or less frequent visitors to Aphandra natalia. These include Cyclocephala discolor (Dynastinae), Cyclocephala quadripunctata (Dynastinae), Philonthus sp. (Staphylininae), Xanthopygus sp. (Staphylininae), Coproporus sp. (Tachyporinae) (Table 2). The reward to these pollinators is pollen when visiting staminate inflorescences. Pollinators of Aphandra natalia use pistillate inflorescences for oviposition possibly because of increased temperature in flowers during anthesis, which may provide improved growth conditions for the larvae (Ervik, 1993; Ervik et al., 1999; Barfod & Uhl, 2001).

Ethnobotany of Aphandra natalia
Local names
Aphandra natalia is known under the vernacular names Chilli, Chilli-punschu, Chirisi, Fibra, Kinchuk, Kintiuk Sili, Tindiuqui, Tintiuk, Wamowe (Ecuador) and Piassaba, Tintuki (Peru) and Piassaba, Piassava (Brazil) (Barfod, 1991; Borgtoft Pedersen & Balslev, 1990, 1992; Macía, 2004).
Uses
Aphandra natalia is a multipurpose palm and its different parts together provide numerous products. In addition to the commercial and subsistence commodities Aphandra natalia provides indirect and non-commercial benefits such as shade for cattle and reduction of erosion in fields. Subsistence uses of Aphandra natalia depend on most of the palms parts (Borgtoft Pedersen & Balslev, 1990, 1992; Borgtoft Pedersen, 1992; Macía, 2004; Boll et al., 2005).
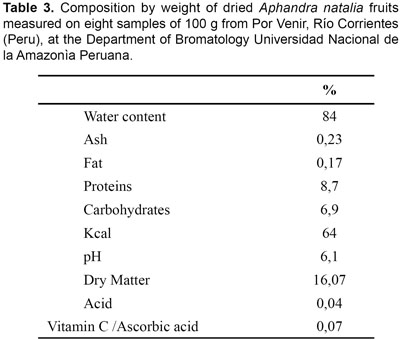
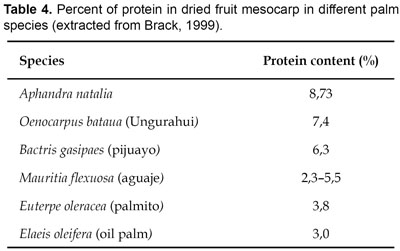
The mesocarp and immature endosperm are of nutritional value to humans (Table 3), having a high protein content compared to other palm fruits (Table 4). The oily orange mesocarp is eaten after boiling and the fluid endosperm is consumed as a beverage. In Ecuador, fruits are sold on markets whereas in Peru we have only observed their use for subsistence purposes. Furthermore it has been reported that wild animals eat the fruits, and stands of Aphandra natalia are therefore recognised as excellent hunting grounds (Borgtoft Pedersen & Balslev, 1990).
Palm heart harvested from new leaves or from the core of the stem, is of nutritional value to humans. Harvest of the heart is unsustainable because the whole palm has to be felled.
Larvae of the beetle Rhynchophorus palmarum inhabiting the trunk is a food-item connected to Aphandra natalia, which is recognised as a delicacy in many South American cultures. Although not a part of the palm it is mentioned here because of the obvious and direct association with Aphandra natalia. Its presence is a problem to fiber harvesters, because larvae often destroy palms and make them useless for other purposes.
Male inflorescences are used for cattle fodder, either eaten directly from the palm or collected and fed to the cattle. The potential of inflorescences as fodder has to be further investigated, because other plant species that, like Aphandra natalia, contain calcium oxalate crystals have been shown to cause internal bleedings in humans and cattle (Borgtoft Pedersen & Balslev, 1990).
Leaves are used for thatch, although the quality is not as good as, e.g., Lepidocaryum tenue. They are also used for blowgun darts, made of the leaf rachis, and for woven baskets, mats and nets.
Economy and management of Aphandra natalia
Commercial exploitation of the palm generates essential and necessary income to many rural people. Especially harvesting of fibers and the subsequent sale to middlemen or broom manufacturers, constitute a substantial source of income to these people (Borgtoft Pedersen & Balslev, 1992). Fibers are harvested intensively in Peru and Ecuador and supplied to the broom industry, whereas in Brazil commercialisation of fibers is less common. Both Ecuadorean and Peruvian markets support extensive parts of western Amazon households and shops with brooms (Boll et al., 2005). The Ecuadorean broom market has in recent years been investigated, but we know much less about the Peruvian broom market. In Ecuador the industry has flourished and sale of fibers and brooms has had important impacts on rural peoples economy. Before they were used commercially for brooms in Ecuador they were woven into ropes used to tie cattle in the fields. Because of this, together with its multipurpose nature Aphandra natalia survived periods where shifts in land-use were common from primary rain forest to pastures or agricultural fields (Borgtoft Pedersen & Balslev, 1990, 1992). In Peru the importance of the broom industry has been less pronounced, still, many people are today engaged in the industry. The potential of the industry in Peru is however at present not fully exploited, when compared to the evident abundance of Aphandra natalia in the Amazon forests of Peru.
Production and the value chain
Value chains within broom industries in Peru and Ecuador are similar, and are most often comprised of four segments; harvesters, middlemen, broom manufacturers and local or national distributors (Figure 4). In Brazil the value chain only comprises three segments; harvester/home manufacturers, middlemen and local distributors.
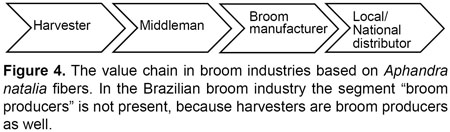
In Peru and Ecuador harvesters are primarily small-scale farmers in remote rural areas of the rain forest. They either own a small piece of land (usually not larger than one hectare) populated by Aphandra natalia or live relatively close to wild stands of Aphandra natalia. After harvest, fibers are transported by boat or sometimes on balsa floats in Peru, and in Ecuador on trucks or by plane (Borgtoft Pedersen & Balslev, 1990) to the main sites of broom production. The initial transportation is conducted by harvesters if transport opportunities are accessible but more often the fibers are purchased and transported by middlemen. The main broom production sites are Cuenca, Quito, Sto. Domingo and Guayaquil in Ecuador (Borgtoft Pedersen & Balslev, 1990) and Iquitos in Peru. Apparently, Iquitos is the main site of broom production in Peru, currently with 48 small factories, each with a handful of employees and several smaller family-based cottage industries in which production is carried out in peoples private homes and not in dedicated buildings. In the Brazilian broom industry fiber harvesters are also broom producers owning a piece of land up to 300 hectares with stanads of Aphandra natalia. Middlemen are in this case those who transport the manufactured brooms to the distributors.
Income generated by Ecuadorean harvesters who live in places that are well situated relative to transportation facilities, appears to be higher than the income generated by farmers concentrating on other crops or cattle (Borgtoft Pedersen, 1992). New data substantiating this is however missing, and further research about financial gains within the individual segments in the Ecuadorean broom industry is needed. In Brazil the segment that generates the largest profit is the distributors (Ferreira, personal observation, 2005).
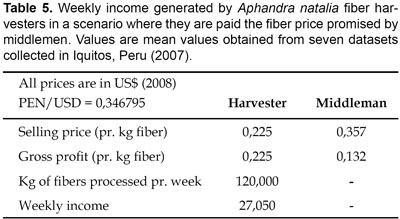
During fieldwork in Peru (2007) we collected data on the economics of the broom industry. Interviews with harvesters suggested that middlemen often deceive harvesters, since they over time tend to create an unfavourable financial situation to harvesters either by luring them into accepting untenable loans (processed food, personal hygiene items, medicaments, etc.) that they most likely will never be able to repay, or the middlemen pay the harvesters significantly below the promised priced (Table 5). Hence, a harvester will on a weekly basis earn ($12,5) which is $15 less than the income promised to him ($27; Table 6). We observed that alternative incomes, e.g., from performing agricultural activities, selling artisan products to tourists, working in the forest for a woodworking industry, etc., would amount to approximately $21 on a weekly basis (6 days working week with a daily income of $3,5). When comparing the possible income from alternative activities ($21) with the possible income from fiber harvesting ($12,5) it is not surprising that fiber harvesting is seen as not very attractive. If harvesters were paid the promised price they might stay in the broom industry and the middlemen would still have a reasonable profit of $0,132 pr. kg fiber sold to manufacturers (Table 5). The middlemens weekly income will of course depend on the amount of fibers transported to the main sites of production, but data showing this is missing and further research is therefore needed in order to comprehend the magnitude of middlemens income through sale of unprocessed fibers.
Economic tendencies within the Peruvian broom industry, including gross profits within the different segments of the value chain are presented in Table 6. Our data are – so far – very limited, and our conclusions should be taken as only preliminary.
Middlemen sell their fibers to broom manufacturers, who then process the fibers into brooms. Prices paid to middlemen by broom manufacturers is not high either, but still middlemens profit are, as mentioned above, well above harvesters profit although transportation costs from the rain forest to manufacturing sites are not included. Manufacturers are the segment in the value chain that receives the largest profit from processing one kilogram of fiber. An assumption made is that the time spent producing a small and a large broom is more or less the same, and, as a consequence of this, the gross profit of producing large brooms will in real life probably be somewhat lower and more similar to the profit of producing small brooms. Still, the greatest gross profit in the value chain seems to be in producing the brooms, although expenses to transportation, buildings, machines and electricity are not subtracted from the gross profit depicted in Table 6. Distributors generate – like harvesters – no pronounced income by selling brooms. Their gross profit seems to be lower than what they could earn from an alternative income when comparing $16,60 to $21,00. It therefore appears to be reasonable that manufacturers, who are those profiting the most, have to lower their selling price in order to improve distributors economic situations, - while demanding additional charge for the brooms from the consumers seems untenable. Currently there is no international export of brooms from Peru, Ecuador or Brazil, however many manufacturers would indeed appreciate opportunities for international export but several obstacles prevent this; production expenses are too high, transportation possibilities from remote areas like Iquitos are limited and connections to foreign buyers have not been established. It is obvious that transportation difficulty is one of the major bottlenecks within the pathway from producer to consumer, better transportation would both increase harvesters financial situation and the chances of creating a sustainable export market.
Harvesting and processing
Harvesting of fibers is, as mentioned above, conducted by small-scale farmers in Ecuador and Peru or larger extrativists in Brazil. During our fieldwork, in Peru in 2003 and 2007, we observed harvesters cutting fibers; the following is based on these observations when nothing else is noticed (Fig. 5).
Harvest is conducted by cutting the entire leaf and thereby enabling the collection of fibers attached to the base of the leaf sheath. Harvesting is usually carried out from the ground. This is possible because of the limited height of the palm, but if palms are too high a ladder is used (Ecuador, Borgtoft Pedersen, 1992), or the whole tree is cut down (Peru and Brazil). The only tool needed for harvesting is a machete, which does not constitute any notable expense to the harvester. Each palm is on average harvested every 1,55 years (Borgtoft Pedersen, 1996) and 45 leaves are left intact during harvesting, ensuring future growth and reproduction. Because Aphandra natalia occurs in stands, time spent walking from one palm to the next is limited, and the most time consuming element is the transport from the stand to a harvesters home. When a suitable palm is located the initial step is to clean it for vines and other organic material attached to the trunk or leaves. The next step is to cut, with a machete, on each side of the petiole to free the fibers from the leaf. Next the petiole is cut horizontally just below the base of the fibers, in order to separate it from the palm. Then the leaf is tilted backwards and the entire bundle of fibers is collected. Subsequently the bundle of fibers (≈ 1,5 m long) is cleaned by shaking it intensively back and forth and the basal part of the bundle is cleaned for remaining sheath residues to ensure easier processing of fibers at the broom factories. Finally the bundles of fibers (sometimes from more than one palm tree) are tied into bigger bundles by wrapping liana around, which makes it easier to transport them.
The overall process of extracting fibers is time consuming and physically demanding. The total amount of time spent harvesting one palm is 1266 minutes, and depends on number of leaves and the height of the palm (Borgtoft Pedersen, 1996). Average amount of fibers harvested from one palm is 3,4 kg and the daily maximum of fibers harvested per harvester is 20 kg (Borgtoft Pedersen, 1996; personal observation, 2007).
Several kilograms of fibers are transported to manufacturing sites where the final processing of fibers into brooms is carried out. A minimum of 1774 kg of fibers were in February 2008, observed being transported from the rain forest, in the Iquitos Region, to Iquitos harbour (César Augusto Grández Rios, personal observation). In the factory, preparation of fibers includes cleaning on a primitive comb – a piece of wood with protruding nails – and cutting the fibers into a suitable length. Fibers are then ready to be inserted into brooms heads by the means of big solid staples. The broom handles and heads are made of Cedrela >sp. (Meliaceae) or Virola sp. (Myristicaceae). Broom handles and heads are bought at local factories specialised in producing these parts, but on occasion, they can also be produced in situ at the broom factory. When the brooms are finished, either an employee from the factory, depending on factory size, or a distributor carries out the onward resale to shops or private households.
Management and sustainability of fiber extraction
Sustainability of fiber extraction has in recent years been an important discussion issue. The tendency is that harvesting in Ecuador is thought to be sustainable while harvesting in Peru and Brazil is seen as unsustainable (Borgtoft Pedersen & Balslev, 1990; Borgtoft Pedersen 1996; Boll et al., 2005). In Brazil, the overall process of extracting fibers from the palms is similar to the one described for Ecuador, including the tools used in the process. A marked difference is the fact that harvesting in Brazil is, in most cases, predatory and thousands of Aphandra individuals are slashed annually. As a result, large populations are becoming rare close to populated areas. Harvesters now prefer to slash the older palms, which, in some cases, can produce fibers enough to make up to 10 brooms (around 4 kg). The fiber extraction and transportation from the forest to the home is done by men. Fiber processing and broom manufacturing is often conducted by the older woman or the young boys in the homes. Each broom is an artefact, made individually and entirely without machines. The brooms are made by the harvesters and sold by the distributors without the handle. In Brazil sustainable extraction was observed only in one locality along Rio Tejo (a tributary of Rio Juruá near the Peruvian border). In all other locations harvest techniques observed were destructive (Ferreira, personal observation). During fieldwork in Peru 2007, we observed a different harvesting pattern in Peru which seemed sustainable, because the whole palm was not felled and some leaves were left intact. These observations are limited, and more data is needed to confirm the sustainable fiber harvest in Peru. In context of sustainability in Aphandra natalia stands, correct and optimised management is of the outmost importance in order to keep the populations in equilibrium and secure the economic situation of future harvesters.
Information on management of Aphandra natalia in Peru is lacking, although from our personal observation it appears that most harvesting is conducted in wild stands of the species. Data on this matter is therefore very much needed, in order to assess and improve the current management activities. In Brazil management of Aphandra natalia is limited or rarely present, and harvesting is mostly conducted from wild stands. The following section on management is therefore based on knowledge from Ecuador.
Several initiatives for management of Aphandra natalia have been started, although most fiber is still harvested from unmanaged stands. Aphandra natalia is cultivated in agroforestry land-use systems, but the extent of management is limited to spreading of seeds by hand in nearby areas followed by no particular nursing (Borgtoft Pedersen, 1992). Besides cultivation, three types of management have been observed in context of wild populations of Aphandra natalia: managed forest, managed regeneration and managed in pastures (Borgtoft Pedersen, 1992).
Managed forests are widespread and an effective way of promoting this species. Part of the surrounding vegetation is cut back giving increased sunlight, which subsequently leads to enhanced production of fibers and improved conditions for juvenile individuals. Managed regeneration is when all surrounding vegetation is cleared and no grazing is permitted. Weeding and cleaning of the area is undertaken continuously but no actual planting of seeds occurs. This type of management leads to dense stands of Aphandra natalia with all age stages represented in the stand, providing a continuous production of fibers ready to be harvested. When the stands get very dense, thinning is necessary to secure light for younger and smaller individuals. When managed in pastures Aphandra natalia is left when areas are cleared for cattle grazing. These silvopastoral land-use systems combine palms with grass for cattle. Palms create shadow for cattle, but they are also an opportunity for extra income to farmers through fiber harvesting. Yet, seedlings and juvenile individuals are often destroyed by the cattle or by exposure to too much sunlight. Palms also compete strongly for the water, especially in periods of limited precipitation, and thereby reduce pasture and the cattles growth.
Some future recommendations for the management of Aphandra natalia
The overall situation for Aphandra natalia in Ecuador and Peru does not appear alarming from a conservation point of view and, based on our data and other published data, the use of the palms in these two countries appears to be sustainable. In contrast, the situation in Brazil is less favorable, all harvesting taking place being unsustainable. Still, many scientists emphasize the ongoing importance of sustainable harvesting techniques together with continuous awareness of the specific needs of Aphandra natalia, especially if the importance of extractivism increasing in the future. From a management perspective some issues have to be considered to create a sustainable future for the palm. These can be separated into two categories; economic and natural resource sustainability. We therefore suggest further research within the following areas.
First, the potential of Aphandra natalia in agroforestry systems should be investigated, for instance by combining Aphandra natalia with various types of crops. Several features of Aphandra natalia, some of which we list here, favour the use of the palm in agroforestry systems:
- Aphandra natalia does not require specific light intensities to grow and reproduce and occupy various niches. Competition for light between crops and Aphandra natalia is limited and the leaves can be cut continuously for harvest.
- The presence of Aphandra natalia is not likely to induce soil-nutrient depletion because only limited amounts of nutrients are removed through fiber harvest, and nutrient leaching from the system is limited because of the palms extensive root systems.
- Harvesting requires only very simple equipment and investments required for this kind of management are limited.
- Aphandra natalia can be harvested throughout the year and growing it together with other crops seem favourable to harvesters because harvesting of fibers can easily be adjusted to other agricultural activities. Adding to this, fibers can be stored for long periods of time without deteriorating.
A problem often encountered is the clearing of vegetation, with no commercial value, to promote species such as Aphandra natalia. This may have detrimental consequences for the composition of the surrounding rain forest. In this respect agroforestry is clearly more sustainable than other systems such as pastures or monocultures. But agroforestry is only beneficial to the rain forest if it replaces more modern agricultural practices – not if it eradicates old primary or secondary rain forest.
Second, cultivation of Aphandra natalia was seen to gradually increase in southern Ecuador during the 1990s (Borgtoft Pedersen & Balslev, 1990; Sirén, 2007) and nurseries growing Aphandra natalia and Attalea funifera are already established in Itacaré in the state of Bahia, Brazil (Carlos Alex Guimarães, personal communication, 2008). The potential and sustainability of cultivation should be investigated further. From a traditional perspective, cultivation is usually not viewed as sustainable natural resource management, but if the nurseries are located in areas already damaged by previous clearing, nurseries/plantation can improve situations for the remaining wild stands of Aphandra natalia, while exploitation of these can be diminished. In addition, the fiber load differs between individuals (Borgtoft Pedersen & Balslev, 1990). Therefore, fiber load per palm can be increased by selecting individuals with the highest yield and subsequently promoting their reproduction and growth.
Third, misunderstandings between harvesters and manufacturers is, in context of fiber availability, obvious from the available data. This problem has to be solved in order to fulfil manufacturers demands for fiber. Harvesters resist harvesting because financial benefits are small, and at the same time manufacturers believe that there is not enough fiber available to be harvested. A solution to this could be to set up business-agreements between individual harvesters and middlemen to ensure fair and reasonable prices for fiber above the alternative income threshold of $21. Data on transportation costs is still missing and must be gathered before the full economic situation of the middlemen can be understood.
Finally, thes natural fibers should be promoted on Eurpean and US markets, none of which currently import the product. We assume such initiatives could be lucrative in times where focus in the trading business is very much on terms like fair trade and ecological sustainability. Establishment of a fair trade organisation within the broom industry seems plausible when looking at other industries where the concept of fair trade has been incorporated such as the banana and coffee industries (Raynolds, 2002; Shreck, 2002). Still, it has to be kept in mind that, it is not only the potential Eurpean and US maakets that must show interest for the products. Harvesters, manufacturers, etc., also must be willing to change their practices to those of fair trade.
Extraction of fiber from Aphandra natalia is of great economic value to many rural people and the potential of this industry is not yet fully explored. In order to comprehend the broom industry and all its segments, we stress the need for further research in the field. Meanwhile, proper management recommendations should be set up to promote the survival and sustainable future for this new and little known rain forest non-timber product.
Acknowledgements
We thank Guillermo Criollo, Thea Kristiansen, Dennis Pedersen and Sandie Lykke Hansen for companionship and assistance during our field work in Peru (2007), Mikkel Boel Sørensen for photographs of Aphandra natalia and Stine Wendelboe Bjorholm and Flemming Nørgaard for making the maps. We are grateful for funding from the Danida Research Council to Henrik Balslev (104.Dan.8-764) for studies of Aphandra natalia and The Danish Natural Science Research Council to Henrik Balslev (272-06-0476) for supporting our palm studies. Mette Kronborgs participation in the fieldwork in Peru (2007) was made possible by a WWF/Novozymes Research Grant to Henrik Balslev. Work of Evandra Ferreira in Acre, Brazil, was supported by funding from the Conselho Nacional de Pesquisa-CNPq (Auxílio 479637/2003-2).
Literature cited
Balick M.J. 1989. Native Neotropical palms: a resource of global interest. In: Wickens, G. E., Haq, N., & Day, P. (Eds.), New crops for food and industry. Chapman and Hall, London, United Kingdom.
Balslev H. & A. Henderson. 1987. A new Ammandra (Palmae) from Ecuador. Systematic Botany 12: 501–504.
Barfod A.S., & N.W. Uhl. 2001. Floral development in Aphandra (Aracaceae). American Journal of Botany 88: 185–195.
Barfod A.S. 1991. A monographic study of the subfamily Phytelephantoideae (Arecaceae). Opera Botanica 105: 1–73.
Bernal R.G. 1992. Colombian palm products. Pp. 158–172 In: Plotkin, M. & Famolare, L. (Eds). Sustainable harvest and marketing of rain forest products. Island Press, Washington D. C., USA.
Boll T., J.-C.Svenning, J. Vormisto, S. Normand, C.Grández, & H. Balslev. 2005. Spatial distribution and environmental preferences of the piassaba palm Aphandra natalia (Arecaceae) along the Pastaza and Urituyacu rivers in Peru. Forest Ecology and Environmeny 18: 175–183
Borchsenius F., H. Borgtoft Pedersen, & H. Balslev. 1998. Manual to the palms of Ecuador. AAU Reports 37.
Borgtoft Pedersen H. 1992. Uses and management of Aphandra natalia (Palmae) in Ecuador. Bull. Inst. fr. études andines 21: 741–753.
Borgtoft Pedersen H. 1996. Production and harvest of fibers from Aphandra natalia (Palmae) in Ecuador. Forest Ecology and Management 80: 155–161.
Borgtoft Pedersen H. & H. Balslev. 1990. Ecuadorean palms for agroforestry. AAU Reports 23
Borgtoft Pedersen H. & H. Balslev. 1992. The Economic botany of Ecuadorean palms. Pp. 173–191 In: Plotkin, M. & Famolare, L., (Eds.), Sustainable harvest and marketing of rain forest products. Island Press, Washington, D.C., USA.
Borgtoft Petersen H. & F. Skov. 2001. Mapping palm extractivism in Ecuador using pair-wise comparisons and bioclimatic modeling. Economic Botany 55: 63–71.
Brack A. 1999. Diccionario enciclopédico de plantas útiles del Perú. Centro Bartolomé de Las Casas, Cusco, Peru.
Ervik F. 1993. Notes on the phenology and pollination of the dioecious palms Mauritia flexuosa (Calamoideae) and Aphandra natalia (Phytelephantoideae) in Ecuador. Pp. 7–12 In: Barthlott, W., Naumann, C.M., Schmidr-Loske, K. & Schuchmann, K.-L. (Eds.), Animal-plant interactions in tropical environments. Zoologisches Forschungs-institut und Museum Alexander Koenig, Bonn, Germany.
Ervik F., L. Tollsten & J.T. Knudsen, 1999. Floral scent chemistry and pollination ecology in phytelephantoid palms (Arecaceae). Plant Systematics and Evolution 217: 279–297.
Henderson, A., Galeano, G. & Bernal, R. 1995. Field Guide to the Palms of the Americas. Princeton University Press, New Jersey, USA.
Homma A.K.O. 1993. Extrativismo vegetal na Amazônia – Limites e oportunidades. Brasília: EMBRAPA-SPI.
IBGE. 2002. Produção da Extração Vegetal e da Silvicultura, Rio de Janeiro 17: 1–36.
IBGE. 2003. Produção da Extração Vegetal e da Silvicultura, Rio de Janeiro 18: 1–43.
Kahn F. 1991. Palms as key swamp forest resources in Amazonia. Forest Ecology and Management 38: 133–142.
Lescure J.-P., L. Emperaire & C. Franciscon. 1992. Leopoldinia piassaba Wallace (Arecaceae): a few biological and economic data from the Rio Negro region (Brazil). Forest Ecology and Management 55: 83–86.
Levine J.M. & D.J. Murrell. 2003. The community-level consequences of seed dispersal patterns. Annual Review of Ecology, Evolution and Systematics 34: 549–574.
Macía M.J. 2004. Multiplicity in palm uses by the Huaorani of Amazonian Ecuador. Botanical Journal of the Linnean Society 144: 149–159.
Martius C. 1823–1837. Historia Naturalis Palmarum. Vol 2: Genera et Species. Weigel, Leipzig.
Montufar R. & J.-C. Pintaud. 2006. Variation in species composition, abundance and microhabitat preferences among western Amazonian terra firme palm communities. Botanical Journal of the Linnean Society 151: 127–140.
Paniagua N.Y., J.-C.Svenning, M.Moraes, C. Grández, & H. Balslev. 2007. Diversity of palm uses in the western Amazon. Biodiversity and Conservation 16: 2771–2787.
Pinard M. 1993. Impacts of stem harvesting on populations of Iriartea deltoidea (Palmae) in an extractive reserve in Acre, Brazil. Biotropica 25: 2–14.
Raynolds L.T. 2002. Poverty alleviation through participation in fair trade coffee networks: Existing research and critical issues. Background paper prepared for project funded by the Community and Resource Development Program, The Ford Foundation, New York, USA.
Shreck A. 2002. Just bananas? Fair trade banana production in the Dominican Republic. International Journal of Sociology of Agriculture and Food 10: 13–23.
Sirén A.H. 2007. Population growth and land use intensification in a subsistence-based indigenous community in the Amazon. Journal of Human Ecology 35: 669–680.
Timell T.E. 1957. Vegetable ivory as a source of a mannan polysaccharide. Canadian Journal of Chemistry 35: 333–338.
Voeks R.A. 1988. The Brazilian fiber belt: harvest and management of the piassava fiber palm (Attalea funifera Mart.). Advances in Economic Botany 6: 254–267.
Voeks R.A. 2002. Reproductive ecology of the piassava palm (Attalea funifera) of Bahia, Brazil. Journal of Tropical Ecology 18: 121–136.
Voeks R.A. & M. Rahmatian. 2004. The providence of nature: Valuing ecosystem services. International Journal of Environmental Science & Technology 1: 151–163.
Vormisto J. 2002a. Palms as rainforest resources: how evenly are they distributed in Peruvian Amazonia? Biodiversity and Conservation 11: 1025–1045.
Vormisto J. 2002b. Making and marketing chambira hammocks and bags in the village of Brillo Nuevo, northeastern Peru. Economic Botany 56: 27–40.
Wallace A. 1853. Palm Trees of the Amazon and their Uses. Van Hoorst, London.


















