Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.15 no.2 Lima 2008
TRABAJOS ORIGINALES
Somatic hybrids obtained by protoplast fusion between Solanum tuberosum L. subsp. tuberosum and the wild species Solanum circaeifolium Bitter
Híbridos somáticos obtenidos por fusión de protoplastos entre Solanum tuberosum L. subsp. tuberosum y la especie silvestre Solanum circaeifolium Bitter
Rosa Espejo1, Giselle Cipriani2, Genoveva Rosel3, Alí Golmirzaie4 and William Roca3
1) Universidad Nacional Agraria. Departamento de Biología. Av. La Molina s/n La Molina. Apartado postal 12-056. Lima 12 - Perú. Email Rosa Espejo: respejo@lamolina.edu.pe
2) New York University. Department of Biology. 31 Washington Place. New York, NY 10003. Email Giselle Cipriani: pgc212@nyu.edu
3) Internacional Potato Center. Genetic Resourses Conservation and Characterization Division. Apartado 1558. Lima 12 - Perú.
4) University of Arkansas. Department of Horticulture. Fayatteville, AR 72701.
Abstract
Interspecific somatic hybrids were obtained by polyethylene glycol fusion of protoplasts from tetraploid Solanum tuberosum L. and the diploid wild species S. circaeifolium. Fusion-treated protoplasts were cultured in V-KM medium supplemented with bovine serum albumin. First cell divisions occurred within 34 days. A rapid calli proliferation was observed after colonies developed. Nineteen somatic hybrid plants were obtained and confirmed by RAPD analysis. Chromosome observations indicated that all hybrids were aneuploids. The morphology of fusion-derived regenerants was intermediate between the donor parents. This study shows that somatic hybrid potato plants can be obtained by the fusion method presented.
Keywords: Solanum, protoplast fusion, polyethylene glycol, somatic hybrid, RAPD analysis.
Resumen
Con la finalidad de obtener híbridos somáticos interespecíficos, se fusionaron protoplastos de la especie tetraploide Solanum tuberosum y de la especie silvestre diploide Solanum circaeifolium utilizando polietilenglicol. Los productos de fusión fueron cultivados en el medio V-KM suplementado con albúmina de suero bovino. Las primeras divisiones celulares ocurrieron a los 3 a 4 días de cultivo. Después de la formación de colonias se observó una rápida proliferación de callos, a partir de los cuales se regeneraron 19 plantas. El análisis molecular usando RAPD, confirmó que los regenerantes presentaban segmentos de ADN de ambos parentales, sugiriendo su posible naturaleza de híbridos somáticos. Las observaciones del número de cromosomas indicaron que todos los híbridos fueron aneuploides. En condiciones de invernadero, los regenerantes derivados de la fusión de protoplastos, mostraron características morfológicas intermedias entre las líneas parentales. Este estudio muestra la producción de híbridos somáticos de papa con el método de fusión presentado.
Palabras claves: Solanum, fusión de protoplastos, polietilenglicol, híbridos somáticos, RAPD.
Introduction
Potato, Solanum tuberosum L. (2n=4x=48, 4 EBN: endosperm balance number) is an economically important crop species. The wild species S. circaeifolium (2n=2x=24, 1 EBN) is considered to be a source of agronomic traits such as resistance to Phytophthora infestans (Mont.) de Bary (Mattheij et al., 1992; Colon, 1994). Many wild Solanum species are regarded as important sources for disease resistance and tolerance to many abiotic stresses (Hawkes, 1994) but their use in potato breeding is limited due to poor crossability and sterility of interspecific hybrids. These barriers in classical breeding can be overcome using biotechnological methods such as somatic hybridization by protoplast fusion (Carputo et al., 1995; 1998; Millam et al., 1997; Davey et al., 2005). Using chemical or electrical procedures (Jones, 1988) protoplasts from different donor plants can be fused together and somatic hybrids regenerated from the fusion products. This technique has created novel cellular genome configurations by combining sexually incongruent species.
Somatic potato hybrids have been produced by fusion of diploid wild species with tetraploid S. tuberosum (Barsby et al., 1984; Austin et al., 1993; Cardi et al., 1993;) or with dihaploid S. tuberosum lines (Austin et al., 1985; Rokka et al., 1994). Successful application of the fusion technique demands a protocol for plant regeneration from protoplasts.
An analysis of putative fusion products is essential to confirm hybrid status (Masuelli et al., 1995; Penner et al., 1996; Matthews et al., 1997) and expression of the desirable traits. This can be done via morphological, biochemical, cytological and molecular markers (Pinto et al., 1995), and increasingly sophisticated molecular discrimination methods have recently been reported (Provan et al., 1996; Matthews et al., 1999; Harding and Millam, 2000; Trabelsi et al., 2005; Guo et al., 2007).
In this paper we describe the fusion of leaf protoplasts of S. tuberosum L. with S. circaeifolium Bitter in order to obtain somatic hybrids. RAPD and chromosomal analysis of the hybrids are also presented.
Materials and methods
Plant material and protoplast isolation.
In vitro 46 week-old leaves of tetraploid Solanum tuberosum L. cv. Désirée plants and wild species diploid S. circaeifolium plants (accession 2.1) were used as explants sources. The latter has a natural resistance to late blight, Phytophthora infestans (Mont.) de Bary (Mattheij et al., 1992; Colon, 1994).
These plants were grown on a lacking hormones Murashige and Skoog (1962) medium, and supplemented with 25% sucrose and 3,5% phytagel, pH 5,6.Plants were maintained in a controlled environmental cabinet with 1822 ºC, 16 h photoperiod and 80% relative humidity.
For protoplast isolation, 1 g of plant material was placed in petri dishes containing a plasmolyzing solution of sorbitol 0,5 M for 12 hours. Then, leaves were cut into small pieces and incubated with 10 mL of an enzymatic solution mixture containing 0,25% macerozyme R-10, 1% cellulase R-10, 700 mg calcium chloride, 1% 2-N-morpholinoethane sulfonic acid (MES) in sorbitol 0,5 M, pH 5,6.
The enzyme treatment was carried out overnight with gentle agitation. The protoplast suspension was filtered through 50 μm nylon screens. Protoplasts were precipitated by centrifugation at 1000 rpm during 10 minutes. The pellets were mixed with 13% mannitol and protoplasts were purified by flotation over 21% sucrose solution. The protoplast band was collected and washed with 13% mannitol. Final concentration was 105-106 protoplasts per millilitre.
Protoplast fusion, culture and regeneration
Prior to fusion, protoplasts from S. tuberosum cv. Desiree and S. circaeifolium were mixed in a 1:1 ratio in a tube with 0,2% calcium chloride and 2,5% potassium chloride solution, pH 6,9. Protoplasts were precipitated by centrifugation at 800 rpm during five minutes.
Some drops of fusion solution containing 25% polyethylene glycol, (PEG, MW 1500) were placed on a plastic petri dish. Then 100 μl from a mixture of partner protoplasts were placed over each fusion solution drop. After that, protoplasts settled down. During 2030 minutes protoplasts were incubated. Fusion frequency (F.F.) was defined as Fish et al. (1988):
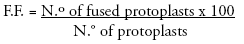
After fusion time, PEG was diluted by addition of a volume of washing solution (7,2% mannitol, pH 5,8). The mixture was incubated for thirty minutes and then protoplasts were collected by centrifugation at 800 rpm during five minutes.
The fusion-treated protoplasts were mixed with an equal volume of 2,8% sodium alginate and the suspension dropped onto a polymerization solution of 50 mM calcium chloride in sorbitol 0,4 M. The plates with alginate beads were stored during twelve hours at 4 ºC. After this time, the solution was replaced by protoplasts culture V-KM medium (Binding and Nehls, 1977) supplemented with 1% serum bovine albumin. The osmolality of this medium was fixed at 500 mOsm. Plates were incubated at 2528 ºC under dark conditions. After seven days, the culture medium was diluted 1:1 with V-KM medium of 300 mOsm. Then, at fifty days, all culture media were replaced by a 300 mOsm V-KM medium.
When colonies had developed, alginate beads were despolimeryzed using 20 mM sodium citrate solution in sorbitol 0,3 M, pH 7,4 and gently shaking. Then the suspension was centrifugated at 1000 rpm during ten minutes. After that, colonies were dispersed into plates with solid MS13K regeneration medium (Benke, 1975). The cultures were maintained in an incubation room at 2224 ºC under indirect light conditions. Regenerants from the fusion experiments were propagated in vitro using the Murashige and Skoog (1962) medium.
RAPD analysis
The hybridity of the fusion regenerants was confirmed by RAPD markers. For this analysis, DNA extraction was performed according to Doyle and Doyle (1990) with some modifications. Random decameric oligonucleotides from GENSET were used for PCR amplification.
PCR was carried out in a 10 μL reaction volume containing: 4,76 μL mili-Q water; 1,5 μL 10X reaction buffer; 50 mM MgCl2; 2,5 mM dNTPs; 10 ng/μL primer and 0,09 μL Taq polymerase (isolated at the International Potato Center laboratories). To this reaction mixture, 5 μL of genomic DNA and 50 μL mineral oil were added. PCR amplification was realized in a PTC-100 thermal cycler (Programmable Thermal Cycler) programmed for one cycle of 3 minutes at 94 ºC followed by 40 cycles of 1 minute at 94 ºC, 1 minute at 35 ºC, 2 minutes at 72 ºC and one cycle of 7 minutes. Amplification products were analyzed by gel electrophoresis in 1,4% agarose gels containing ethidium bromide. Gels were analyzed and photographed under UV light.
Chromosome analysis
Root tips from the regenerants and the fusion parents were pretreated with 100 ppm of Ambush pirethroid insecticide, pH 5 for 24 hours at 4 ºC. These samples were obtained from rooted plants maintained in a greenhouse. Hydrolysis was carried out with 1 N HCl at 60 ºC during 8 minutes. Root tips were stained by lacto-propionic orcein.
Results and discussion
Protoplasts fusion and plant regeneration
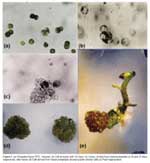
Protoplasts fusion from S. tuberosum cv. Désirée and S. circaeifolium were induced with the fusion conditions described (Fig. 1a). Fusion frequencies of 2030% were obtained. Aggregations of more than two protoplasts were also observed. Heterokaryons can not be identified because protoplasts of the parents were of the same type and shape. Due to that, fused and unfused protoplasts were cultured together.
The first cellular divisions happened within 36 days of culture in V-KM medium (Fig. 1b) further forming several colonies (Fig. 1c), indicating that the survival after fusion treatment was still high. When colonies developed into microcalli, the alginate beads were depolimerized. Microcalli were transferred onto MS-13 medium to induce shoot regeneration under indirect light conditions. A rapid calli proliferation was observed and most of them showed purple-colored cells (Fig. 1d). Shoot regeneration started twelve weeks after calli were transferred to MS-13 medium. Shoots also showed purple-colored meristematic zones. Nineteen plants were regenerated from calli (Fig. 1e). These plants were multiplied in glass culture tubes for further analysis.
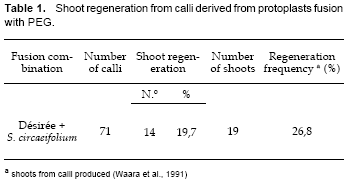
From the two fusion experiments, the regeneration frequency was 26,8% (Table 1). This value is different from that obtained by Mattheij et al. (1992), who, after protoplasts electrofusion, reported a regeneration frequency of 14%.
This difference in the regeneration frequency could be explained due to the fact that one of the parents, the tetraploid cultivar Désirée, has a very good morphogenetic capacity (Espejo et al., 1999). The other parent, the wild species S. circaeifolium, also has response for regeneration but its morphogenic capacity is lower than the Désirée cultivar (Espejo, 2000). Under these conditions, calli hybrids follow the developmental pathway of the morphogenic parents.
RAPD analysis
To select hybrids, regeneration of in vitro plants was subjected to RAPD analysis. This technique is a tool for somatic hybrid characterization (Baird et al., 1992; Yong-Sheng, 1993; Takemori, 1994; Rasmussen and Rasmussen 1995; Penner et al., 1996; Henn et al., 1998; Rokka et al., 1998).
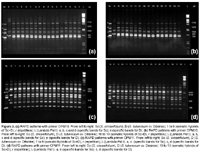
The RAPD analysis was carried out for all regenerated plants derived from calli of the combination S. tuberosum cv. Désirée + S. circaeifolium. The RAPD markers showed that all of the regenerants were somatic hybrids between S. tuberosum and S. circaeifolium. The primer OPM10 (5-TCTGGCGCAC-3) amplified a S. tuberosum specific band of 805 bp which appeared in the fusion-derived regenerants. The same primer amplified four specific bands of 740, 1000, 1270 and 1750 bp in S. circaeifolium. These bands also appeared in the fusion regenerants (Figs. 2a, b), indicating that parts of the genomes from both parents were combined in the hybrids.
With the primer OPM11 (5-GTCCACTGTG-3), these two parental lines exhibited clear differential banding patterns which also confirmed the hybrid nature of regenerants (Figs. 2c, d). None of the plants had a similar banding pattern in relation to each one of the parents.
Identification of somatic hybrids is a requisite for the effective exploitation of the protoplasts fusion in potato improvement. In this study, confirmation of fusion-derived regenerants by the use of RAPD markers represented an effective system for the detection of somatic hybrids from S. tuberosum cv. Désirée and S. circaeifolium.
Chromosome analysis
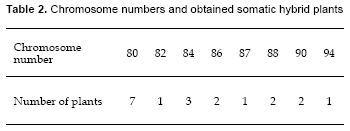
The numbers of chromosome of S. tuberosum was 2n=2X=48 and S. circaeifolium was 2X=2X=24. For the obtained somatic hybrid plants, the chromosome number varied from 80-94 (Table 2). These results showed that all hybrids were aneuploids (Fig. 3). None of the regenerants had 72 chromosomes, which results from the protoplasts fusion of a tetraploid with a diploid genome.
The production of somatic hybrid plants with a chromosome number deviating from the expected is well documented in many fusion combinations where potato protoplasts have been used as one or both fusion parents (Austin et al., 1985; Fish and Karp, 1986; Fish et al., 1988; Perl et al., 1990; Waara et al., 1991; Masuelli et al., 1995; Pinto et al., 1995).
After protoplasts fusion and during plant regeneration it frequently happens that genetic changes such as variations in chromosome number occur. The origin of this chromosome instability has been studied elsewhere in potato (Kumar, 1994).
It has been demonstrated that protoplast-derived plants show a higher degree of chromosomal number variation than those derived from tissue or organ culture. It has been suggested that protoplasts are more prone to chromosome instability because they undergo a long period of greater stress during initial stages of cell division and dedifferentiation (Pijnaker and Sree Ramulu 1990; Carrasco et al., 1998).
The ploidy of the genotype used in plant regeneration has been shown to profoundly affect the type of variation observed in morphological characters and chromosome numbers among regenerant plants. Plant regeneration from mono- and dihaploid potato genotypes often results in ploidy changes, but little aneuploidy; on the other hand, regenerated plants from tetraploid genotypes produce a wide range of aneuploidy (Kumar, 1994). For these reasons and considering the obtained results, it can be suggested that there is a high probability of fusion-derived regenerants which are aneuploids with an increase or loss of some chromosomes when one or both fusion partners have a high ploidy level.
Somatic hybrids were confirmed morphologically under greenhouse conditions, when intermediate characteristics between parent lines, such as shoot coloration, leaf shape and pubescence were observed.
Potato is an example of a crop in which advantages can be obtained by the use of alternative techniques which exchange and introgress new genetic information, conferring beneficial traits from wild species into conventional cultivars (Millam et al., 1997). Tissue culture procedures to produce somatic hybrids and the application of molecular biological tools for their analysis are well established.
In conclusion, somatic fusions between the cultivated potato Solanum tuberosum and the wild species S. circaeifolium were produced in order to incorporate desirable traits into the potato gene pool. Nineteen somatic hybrid plants were obtained from fusion experiments using PEG in an effort to combine elite traits from both parents. Cytological and RAPD marker analysis confirm their hybrid nature. Further work is needed to evaluate their resistance level to Phytophthora infestans (Mont.) de Bary.

Acknowledgments
The authors would like to thank the International Potato Center for financial support and to Martha Williams de Castro who revised the English version of this article.
Literature cited
Austin S., M. A. Baer & J. P. Helgeson. 1985. Transfer of resistance to potato leaf roll virus from Solanum brevidens into Solanum tuberosum by somatic fusion. Plant Sci. 39: 75-82.
Austin S., J. D. Pohlman, C. R. Brown, H. Moitahedi, G. S. Santo, D. S. Douches & J. P. Helgeson. 1993. Interspecific somatic hybridization between Solanum tuberosum L. and S. bulbocastanum Dum. as a means of transferring nematode resistance. Am. Potato J. 70: 485-496.
Baird E., S. Cooper-Bland, R. Waugh, M. De Maine & W. Powell. 1992. Molecular characterization of inter- and intra-specific somatic hybrids of potato using randomly amplified polymorphic DNA (RAPD) markers. Mol. Gen. Genet. 233: 469-475.
Barsby L., J. F. Shepard, R. J. Kemble & R. Wong. 1984. Somatic hybridization in the genus Solanum: S. tuberosum and S. brevidens. Plant Cell Rep 3: 165-167.
Behnke M. 1975. Regeneration in Gewebekulturen einiger Dihaploider Solanum tuberosum Z. Pflanzenzucht. 75: 262-265.
Binding H. and R. Nehls 1977. Regeneration of isolated protoplasts to plants in Solanum dulcamara. L. Z. Pflanzenphysiol. 85: 279-280.
Cardi T., F. D. Ambrosio, D. Consoli, K. J. Puite & K. S. Ramulu. 1993. Production of somatic hybrids between frost - tolerant Solanum commersonii and Solanum tuberosum: characterization of hybrids plants. Theor. Appl. Genet. 87: 193-200.
Carputo D., T. Cardi, T. Chiari, G. Ferraiolo & L. Frusciante. 1995. Tissue culture response in various wild and cultivated Solanum germplasm accessions for exploitation in potato breeding. Plant Cell Tissue Organ Cult 41: 151-158.
Carputo D., P. Garreffa, M. Mazzei, L. Monti & T. Cardi. 1998. Fertility of somatic hybrids Solanum commersonii (2x, 1 EBN) (+) S. tuberosum haploid (2x, 2 EBN) in intra-and inter-EBN crosses. Genome 41: 776-781.
Colon L. T. 1994. Resistance to Phytophthora infestans in Solanum tuberosum and wild Solanum species. Thesis. Wageningen Agricultural University. Netherlands.
Carrasco A., J. Ruiz de Galarreta y E. Ritter. 1998. Caracterización morfológica, cariotípica y molecular de tres somatoclones de Solanum tuberosum L. obtenidos mediante cultivo de protoplastos. Invest. Agr. Prod. Prot. Veg. Vol. 9: 347-357.
Davey M. R., P. Anthony, J. B. Power & K. C. Lowe. 2005. Plant protoplasts: status and biotechnological perspectives. Biotechnol. Adv. 23: 131-171.
Doyle J. and J. L. Doyle. 1990. Isolation of plant DNA from fresh tissue. Focus 12.
Espejo R., G. Cipriani y A. Golmirzaie. 1999. Regeneración de plantas a partir de protoplastos en papa (Solanum tuberosum L.) y en la especie silvestre Solanum papita. Anales Científicos UNALM, Volumen XXXVIII: 263 – 268.
Espejo R. 2000. Fusión de protoplastos entre especies cultivadas y silvestres del género Solanum. Tesis, Magíster en Mejoramiento Genético de Plantas. Escuela de Post-Grado. Universidad Nacional Agraria La Molina.78 pp.
Fish N. & A. Karp. 1986. Improvements in regeneration from protoplasts of potato and studies on chromosome stability. 1. The effect of initial culture media. Theor. Appl. Genet. 72: 405-412.
Fish N., A. Karp & M. Jones. 1988. Production of somatic hybrids by electrofusion in Solanum. Theor. Appl. Genet., 76: 260-266.
Guo W. W., R. C. Wu, Y. J. Cheng & X. X. Deng. 2007. Production and molecular characterization of Citrus intergeneric somatic hybrids between red tangerine and citrange. Plant Breed. 126: 72-76.
Harding K. & S. Millam 2000. Analysis of chromatin, nuclear DNA and organelle composition in somatic hybrids between Solanum tuberosum and Solanum sanctae-rosae. Theor. Appl. Genet. 101: 939-947.
Hawkes J. G. 1994. Origins of cultivated potatoes and species relationships. In: Potato genetics. Edited by J. E. Bradshan and G. R. Mackay (Eds.). Wallingford (UK). Center for Agriculture and Brosciences International (CABI), 3-42.
Henn H., R. Wingender & H. Schnabl. 1998. Regeneration of fertile plants from Helianthus nuttalli and Helianthus giganteus L. mesophyll protoplast. Plant Cell Rep 18: 288-291.
Jones M. G. K. 1988. Fusing plant protoplasts. Trends Biotechnology 6: 153-158.
Kumar A. 1994. Somaclonal variation in potato. In: J. E. Bradshaw and G. R. Mackay (Eds.). Potato Genetics. CAB International, pp: 197-212.
Masuelli R. W., E. Y. Tanimoto, C. R. Brown & L. Comai. 1995. Irregular meiosis in a somatic hybrid between S. bulbocastanum and S. tuberosum detected by species-specific PCR markers and cytological analysis. Theor. Appl. Genet. 91: 401-408.
Mattheij W. M., R. Eijlander, J. R. A. De Koning & K. W. Louwes. 1992. Interspecific hybridization between the cultivated potato Solanum tuberosum subspecies tuberosum L. and the wild species S. circaeifolium subsp. circaeifolium Bitter exhibiting resistance to Phytophthora infestans (Mont) de Bary and Globodera pallida (Stone) Behrens. Theor. Appl. Genet. 83: 459-466.
Matthews D., K. Harding, M. Wilkinson & S. Millam. 1997. A slot-blot hybridization method for screening somatic hybrids. Plant Mol. Biol. Rep. 15: 62-70.
Matthews D., J. Mc Nicoll, K. Harding & S. Millam. 1999. 5-Anchored simple sequence repeat primers are useful for analyzing potato somatic hybrids. Plant Cell Rep 19: 210-212.
Millam S., P. Davie, K. Harding & M. Dale. 1997. Non-transgenic applications of plant tissue culture in potato. Ann. Rep. Scot. Crop Res. Inst 1996, pp 50-52.
Murashige T. & F. Skoog. 1962. A revised medium for rapid growth bioassays with tobacco tissue culture. Physiol. Plant. 15: 473 – 479.
Penner G., S. Lee, L. Bezte & E. Ugali. 1996. Rapid RAPD screening of plant DNA using dot blot hybridization. Mol. Breed. 2: 7-10.
Perl A., D. Aviv & E. Galun. 1990. Protoplast-fusion derived Solanum cybrids: application limitations; phylogenetic limitations. Theor. Appl. Genet. 79: 632-640.
Pijnacker V. & K. Sree Ramulu. 1990. Somatoclonal variation in potato: a kariotipic evaluation. Acta Botánica Neerlandica. 39: 163-169.
Pinto F., Y. Chupeau & V. Cabrera. 1995. Molecular genetic characterization of plant somatic hybrids. In Vitro Cell Dev. Biol. 31: 96-100.
Provan J., A. Kumar, L. Shepard, W. Powell & R. Waugh. 1996. Analysis of intra-specific somatic hybrids of potato (Solanum tuberosum) using simple sequence repeats. Plant Cell Rep. 16: 196-199.
Rasmussen J. & O. Rasmussen. 1995. Characterization of somatic hybrids of potato by use of RAPD markers and isozyme analysis. Physiol. Plant. 93: 357-364.
Rokka V. M., Y. S. Xu, J. Kankila, A. Kuusela, S. Pulli & E. Pehu. 1994. Identification of somatic hybrids of dihaploid S. tuberosum lines and S. brevidens by species specific RAPD patterns and assessment of disease resistance of the hybrids. Euphytica 80: 207-217.
Rokka V., A. Tauriainen, L. Pietilä & E. Pehu. 1998. Interspecific somatic hybrids between wild potato Solanum acaule Bitt. and anther-derived dihaploid potato Solanum tuberosum L.). Plant Cell Reports. 18: 82-88.
Takemori N., K. Shinoda & N. Kadotani. 1994. RAPD markers for confirmation of somatic hybrids in the dihaploid breeding of potato (Solanum tuberosum L.). Plant Cell Rep. 13: 367-371.
Trabelsi S., R. Gargouri-Bouzid, F. Vedel, A. Nato, L. Lakhoua & N. Drira. 2005. Somatic hybrids between potato Solanum tuberosum and wild species Solanum vernei exhibit a recombination in the plastome. Plant Cell Tissue Organ Cult. 83: 1-11.
Waara S., A. Wallin & T. Ericksson. 1991. Production and analysis of intraspecific somatic hybrids of potato (Solanum tuberosum L.). Plant Science 75: 107-115.
Yong-Sheng X., M. Clerk & E. Pehu. 1993. Use of RAPD markers to screen somatic hybrids between Solanum tuberosum and S. brevidens. Plant Cell Rep. 12: 107-109.
____________________
Presentado: 16/11/2007
Aceptado: 20/05/2008