Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.17 n.1 Lima abr. 2010
TRABAJOS ORIGINALES
Characterization of leaf anatomy in species of Astrocaryum and Hexopetion (Arecaceae)
Caracterización de la anatomía foliar de especies de Astrocaryum y Hexopetion (Arecaceae)
Betty Millán1 y Francis Kahn2
1 Museo de Historia Natural. Universidad Nacional Mayor de San Marcos (UNMSM). Av. Arenales 1256, Jesús María. Lima, Peru. Email Betty Millán: bmillans@unmsm.edu.pe
2 IRD, UMR188-DIAPC, DYNADIV, Casilla 18-1209, Lima, Peru. Email Francis Kahn: francis.kahn@ird.fr
Abstract
Leaf anatomy of 23 species of the genus Astrocaryum and of the two species of the genus Hexopetion is described. A total of 109 characters with at least one difference between species are reported from the lamina (83), main rib (11), petiole (9) and sheath (6). An identification key to species is given based on leaf anatomy. Distribution of the characters is discussed in order to evaluate their taxonomic relevance.
Keywords: palms, leaf, stomata, vessels, fibers.
Resumen
Se describe la anatomía foliar de 23 especies del género Astrocaryum y de las dos especies del género Hexopetion. Se identifican 109 caracteres diferenciales del limbo (83), de la nervadura principal (11) del pecíolo (9) y de la vaina (6). Se elabora una clave de identificación de las especies a partir de los caracteres anatómicos de hoja. Se discute la distribución de los caracteres en los grupos infragenéricos de Astrocaryum a partir de las especies estudiadas para evaluar su importancia taxonómica.
Palabras clave: palmeras, hoja, estomas, haces conductores, fibras.
Introduction
Astrocaryum and Hexopetion are two closely related palm genera (Burret 1934a, b). The former with 40 species is found in most ecosystems in Tropical South-America; it is well diversified in habit, either caespitose or solitary, and develops either large palms, or medium-sized to short-trunked palms with large leaves, or slender palms with medium-sized leaves, or acaulescent palms with large or with short leaves (Kahn 2008). The latter includes only two slender species with medium-sized leaves (Pintaud et al. 2008). There are few data available on leaf anatomy of these genera. Tomlinson (1961) described the leaf anatomy of Astrocaryum rostratum (=Hexopetion mexicanum), including the fibers near the hypodermis and the large vascular vessels in the mesophyll towards each surface. He also described the leaf anatomy of Astrocaryum sclerophyllum (considered a species dubia by Burret 1934a). Schlüter et al. (1993) pointed out that both leaf sides of the riparian Astrocaryum jauari are covered with a heavy wax layer that protects them from flooding. Henderson (2006) gave a first description of leaf anatomy of Astrocaryum alatum (=Hexopetion alatum). The most recent study on evolutionnary trends in lamina anatomy based on 161 palm genera does not include Astrocaryum (Horn et al. 2009). Pintaud et al. (2008) used morphological and anatomical characters to differentiate the two species of Hexopetion (H. alatum y H. mexicanum) from those of Astrocaryum.
We present the major features of leaf anatomy of 23 species of Astrocaryum and of the two species of Hexopetion. Differential characters are listed to illustrate the high diversity of leaf anatomy and to emphasize the differences among species, as well as between the two genera. A key for identification of the species based on leaf anatomy is given. Anatomical character distribution in Astrocaryum species is evaluated by using similarity methods and discussed in relation to the infrageneric classification.
Material and methods
Five individuals of each of the two species of Hexopetion and 23 species of Astrocaryum were sampled for the study. Materiel was collected from bifid leaves of seedlings or from juvenile leaves divided into a few pinnae; leaf material from an adult plant was used in Astrocaryum minus, a very rare species, only known from two living individuals and from the type specimen (Kahn & Granville 1998); fruit and seedlings of this species are still unknown. Flowering and/or fruiting individuals were collected for identifying the species. Vouchers are deposited in the herbaria USM (Lima, Peru), CAY (Cayenne, French Guiana) and UFA (Acre, Brazil). Material from Hexopetion was collected in the Fairchild Tropical Botanical Garden, Miami, USA.
Botanical references and material used — Astrocaryum: A. huaimi (F. Kahn 4464, Bolivia, USM; from juvenile); A. jauari (B. Millán 422, 423, 424, Peru, USM; from seedling); A. standleyanum (B. Millán 407, 408, Ecuador, USM; from seedling); A. vulgare (J.-J. de Granville 10004, French Guiana, CAY; from seedling); A. chambira (B. Millán 411, Ecuador, USM; B. Millán 412, 413, Peru, USM; from seedling); A. gynacanthum (J.-J. Granville 8223, French Guiana, CAY; from seedling); A. minus (J.-J. de Granville and F. Kahn 12921, French Guiana, CAY; from adult); A. paramaca (J.-J. de Granville and F. Kahn 5407, French Guiana, CAY; from juvenile); A. rodriguesii (J.-J. de Granville and F. Kahn 11199, French Guiana, CAY; from seedling); A. sciophilum (Oldeman 1088, French Guiana, CAY; from juvenile); A. carnosum (B. Millán and F. Kahn 631, 634, 638, Peru, USM; from seedling); A. ciliatum (B. Millán and F. Kahn 1315, Colombia, USM; from seedling). A. faranae (B. Millán and F. Kahn 648, Peru, USM; from seedling); A. scopatum (B. Millán and F. Kahn 531, 532, 536, Peru, USM; from seedling); A. huicungo (B. Millán and J.-C. Pintaud 448, Peru, USM; from seedling); A. javarense (B. Millán 414, 416, 417, 418, Peru, USM; from seedling); A. macrocalyx (B. Millán 428, 429, 430, Peru, USM; from seedling); A. gratum (B. Millán and J. Lingán 499, 500, Peru, USM; from seedling); A. perangustatum (B. Millán, J.-C. Pintaud, C. Vegas and R. Montúfar 831, 834, 842, Peru, USM; from seedling); A. urostachys (B. Millán 409, 410, Ecuador, USM; from seedling); A. chonta (B. Millán 420, 421, 425, Peru, USM; from seedling); A. murumuru (J.-J. de Granville and F. Kahn 11201, French Guiana, CAY; from seedling); A. ulei (J. Ribamar and E. Ferreira, Acre, Brazil, UFA; F. Kahn 4459, 4460, 4461, Peru, USM; from seedling). Hexopetion: H. alatum (J.-C. Pintaud 92350A, cultivated, Miami, FTG; from juvenile); H. mexicanum (J.-C. Pintaud 0653A, cultivated, Miami, FTG; from juvenile).
Optical microscopy. Fresh portions of 0.5-1 cm taken from apical, middle and basal portions of the bifid leaf of seedling or of pinnae of juvenile, and portions of 1 cm from petiole and sheath were conserved in FAA solution (formalin-acetic-ethanol). For Astrocaryum minus, a fresh portion of 1 cm was taken from the midpart of medial pinnae of adult leaves. Ten histological slides were prepared from each sample. Transverse sectioning was done by hand and processed using a modification of the standard procedure (Johansen 1940). Microscope preparations were made of either unstained sections or sections bleached for 15-20 min in 50% commercial bleach (sodium hypochlorite), washed in water, and stained with safranin and cresil violet (1%); temporary preparations were mounted in gelatin-glycerin. Microphotographs were taken with Canon PS 5.5 mp equipment at 4x, 10x y 40x using a Leica binocular microscope. The images were adjusted for contrast and color level in Adobe Photoshop CS. Optical microscopy was used for describing transerve sections of the lamina, main rib, petiole and sheath. The petiole was not described in Hexopetion alatum and H. mexicanum, nor was the sheath in Astrocaryum minus, A. paramaca, A. sciophilum, H. alatum and H. mexicanum. Some quantitative characters were not taken into account because they increase with age; these include: adaxial and abaxial prominency of the main rib, number of layers of parenchyma cells, of fibrous strands under the adaxial and above the abaxial hypodermis, of layers of sclereids, of chlorenchyma and spongious parenchyma.
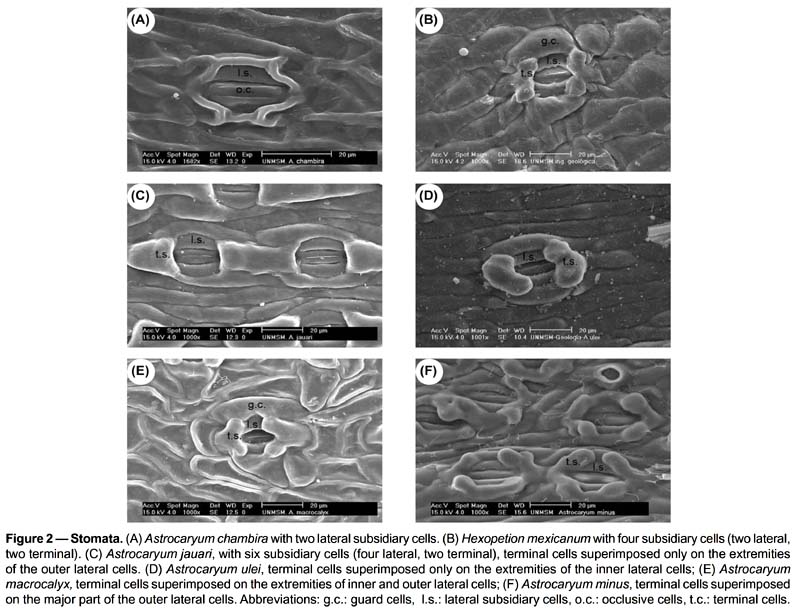
Scanning electron microscope. Portions of 1 cm of bifid seedling leaf or pinnae conserved in FAA solution were used to extract the adaxial and abaxial epidermis. Wax of the abaxial side was first eliminated with petroleum ether. Material was fixed in Carnovski, post fixation in osmium, dehydratation in alcohol, dessicating in a critical point dryer with carbon dioxide, and coated with gold-palladium. Observations at 100x, 250x, 500x y 1000x were made with SEM Philips XL 30 at Geological School of San Marcos University. SEM was used to describe the shape of epidermal cells as well as stomata (shape of guard cells, number, location and form of subsidiary cells, and stomata arrangement in the epidermis).
Analysis of phenotypic data. For cluster analysis, the data including 109 differencial characters (Tab. 1) were converted into a binary matrix, where the digit 1 represents the presence of a phenotypic character, and the digit 0 its absence. The similarity matrix was generated by Euclidean distances, which were used to build a tree with the unweighted pair group mean averages (UPGMA) algorithm. Analysis of phenotypic data was performed using the software PAST (Hammer et al. 2001). One thousand resamplings were used to evaluate robustness of the inferred trees.
Results
Characters common to all the species. Several anatomical characters are common to all the species studied:
Lamina. Leaf surface with epidermal cells and guard cells of stomata polygonal in shape, stomata only present on abaxial side; in tranverse section, adaxial epidermis cells more or less rectangular in shape with inner wall thin, hypodermis with only one layer of cells; non-vascular fibers with estegmata, silica bodies cap-shaped; presence of chlorenchyma and spongious parenchyma in mesophyll; major vascular bundle with small vessels of protoxylem towards the adaxial side; vascular bundles of the sheath with only one layer of parenchymatic cells.
Main rib. Cells of adaxial and abaxial hypodermis similar to that of the lamina; colorless parenchyma located in the inner part with the collateral vascular bundles, these including xylem, vessels of protoxylem and metaxylem, xylematic parenchyma and fibers; phloem is composed of sieve cells and phloematic fibers.
Petiole and sheath. Inner wall of epidermic cells are thin; parenchyma composed of polyhedral cells; collateral bundles with a set of sclerenchyma fibers are located at the periphery, each collateral bundle includes two lateral strands of phloem, a central bundle composed of small strands of phloem surrounded with phloematic fibers, a large vessel of metaxylem, several small vessels of protoxylem and xylematic parenchyma.
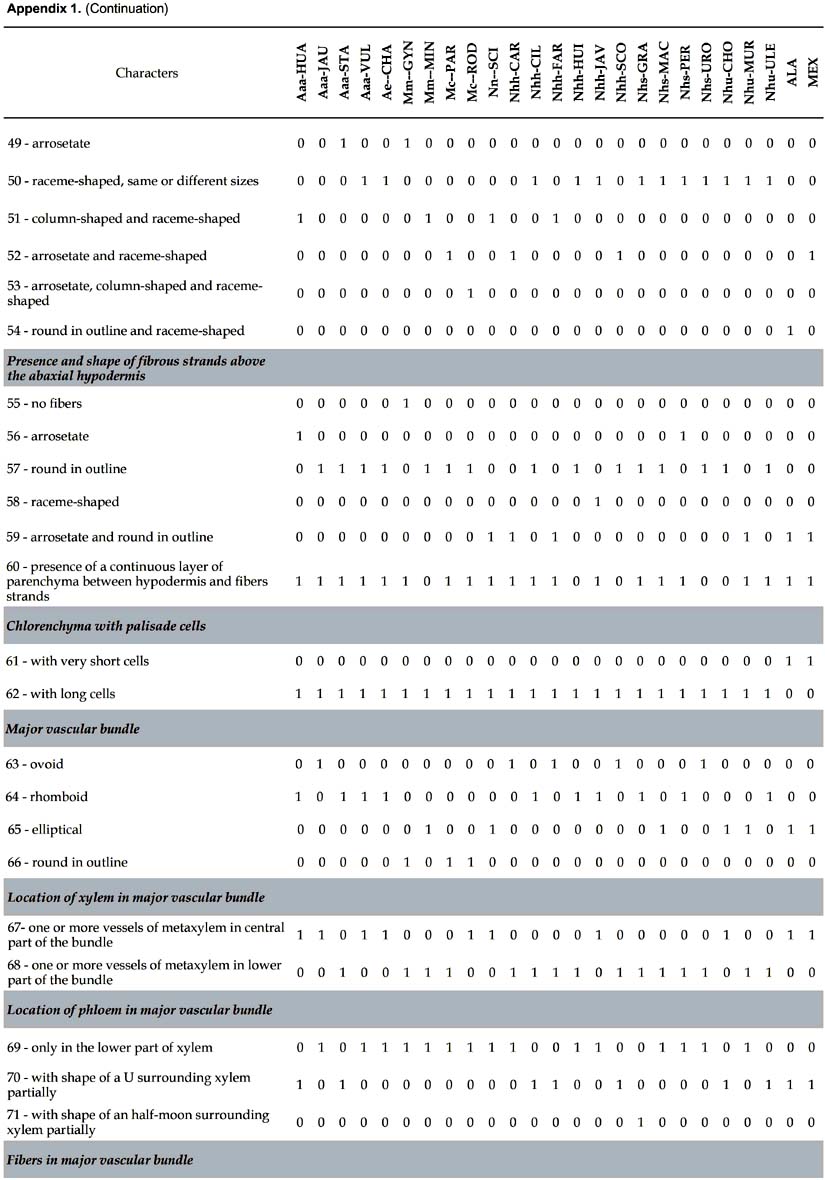
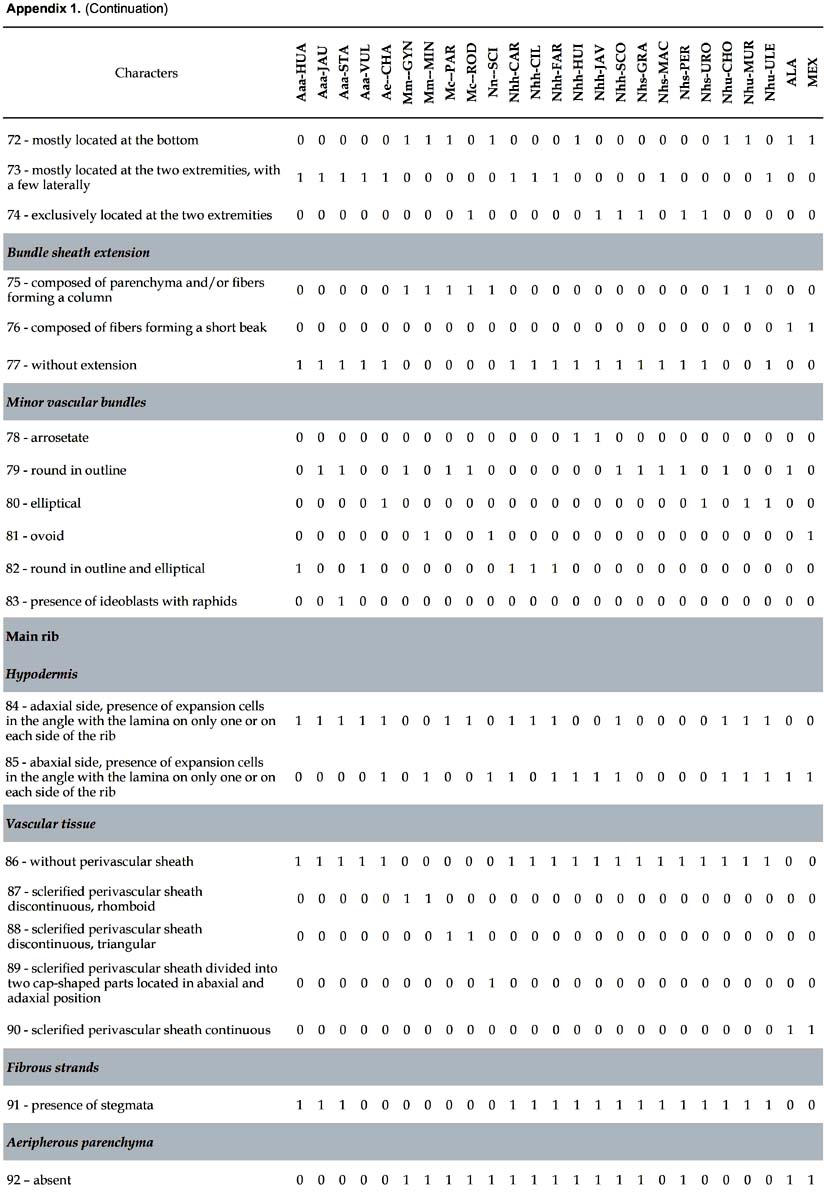
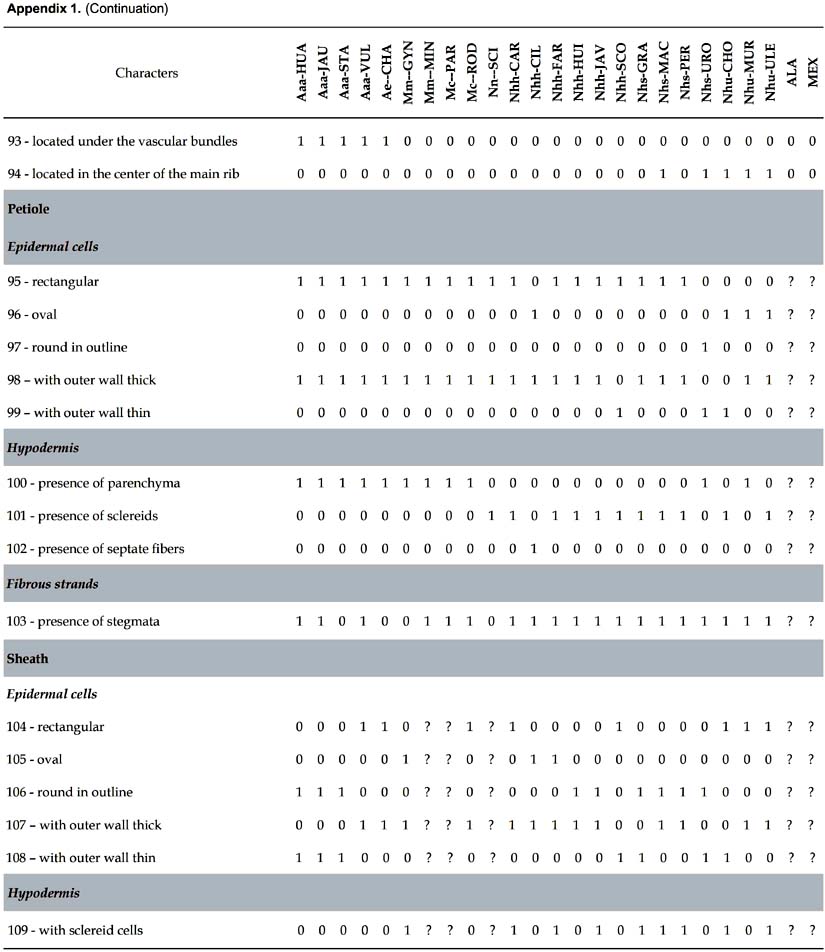
Differential characters. 109 characters with at least one difference among species are described. The number given in parenthesis in the text below refers to the numeration of the characters in Appendix 1.
1. Astrocaryum
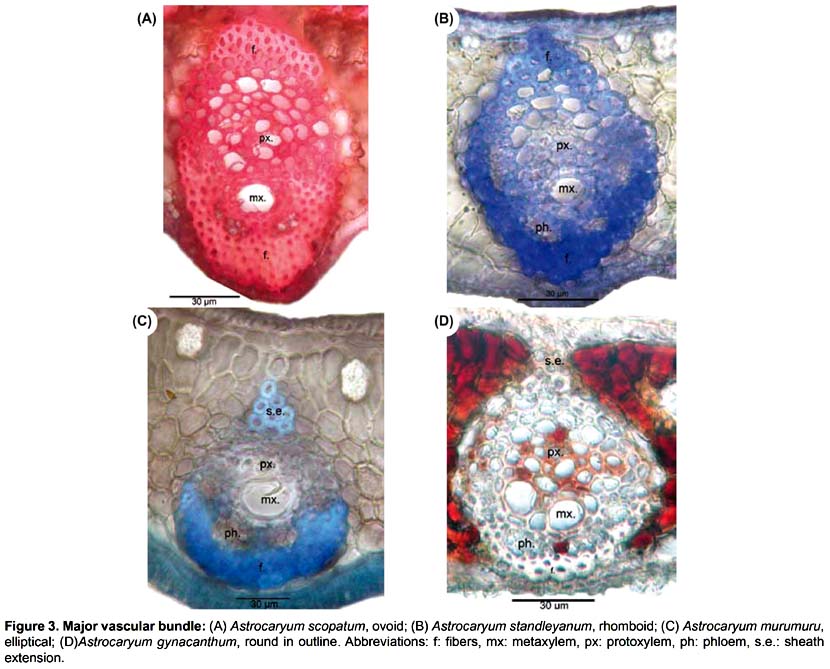
Lamina (Figs. 1-3). 83 binary-coded characters from epidermis, hypodermis, mesophyll, and vascular tissues: Epidermis — Differences are found in the shape and thickness of outer and inner wall of the cells; epidermal cells on adaxial side with outer wall thin (1) or thick (2); abaxial epidermis with cells round in outline (3), rectangular (4), or oval (5), with outer wall thin (6) or thick (7); epidermal indumentum is absent (8) or present, either papillate (9) or composed of cone-shaped hairs with pluricellular base (10). Stomata present six structural patterns. The first is made of only 2 lateral subsidiary cells around the two guard cells, no terminal cells at the ends of guard cells (11). The other five include 6 subsidiary cells, 4 of them laterally disposed (2 on each side: lateral inner and lateral outer) and two terminal cells (13): (i) the two terminal cells are superimposed only on the extremities of the outer lateral subsidiary cells and rise above them (25, 30); they are shortly triangular (14), round in outline (15), long kidney-shaped (17); (ii) the two terminal cells are superimposed on the major part of the outer lateral subsidiary cells (28); they are long V-shaped (19) and their extremities do not rise above them, or rise above them (33); they are short kidney-shaped (18) and their extremities do not rise above them, or rise above them (33); (iii) the two terminal cells are superimposed on the major part of the inner lateral subsidiary cells (29) and rise above them (34), they are lobed (21); (iv) the two terminal cells are superimposed on the extremities of both outer and inner lateral subsidiary cells (27) and their extremities do not rise above them; they are short V-shaped (20); or their extremities rise above them (32), they are then either long Y-shaped (23), or short Y-shaped (22), or short V-shaped (20), or round in outline (15), or oval (16); (v) the two terminal cells are superimposed on the extremities of the inner lateral subsidiary cells (26) and their extremities rise above them (31); they are short V-shaped (20), or long V-shaped (19). The stomata are laid out regularly or irregularly in the epidermis (35, 36). Hipodermis with cells variable in size (37-39) and in thickness of inner and outer walls (40-47). Fibrous strands located under the adaxial hypodermis have only one shape (48 columned-shaped, 49 arrosetate, 50 raceme-shaped variable in size), or several shapes (51 column-shaped with raceme-shaped, 52 arrosetate with raceme-shaped, 53 arrosetate with column-shaped and raceme-shaped, 54 round in outline with raceme-shaped). Fibrous strands located above the abaxial hypodermis are absent (55) or present forming one or two rows with one or several shapes (56 arrosetate, 57 round in outline variable in size, 58 raceme-shaped, 59 arrosetate mixed with round in outline); chlorenchyma is continuous above abaxial hypodermis (60) or discontinuous, then outer fibrous strands are directly above the hypodermis. Vascular tissue — Four shapes of major vascular bundle are observed: ovoid (63), rhomboid (64), elliptical (65), round in outline (66); one or more vessels of metaxylem are located either in the central part or in the lower part of the major vascular bundle (67, 68); location of phloem, only in the lower part of xylem (69), or surrounding it partially, either U-shaped (70) or half-moon shaped (71); fibers in major vascular bundle are either mostly located at the bottom (72), or located at the two extremities with a few laterally (73), or exclusively located at the extremities (74). Bundle sheath extension is absent (77) or present and composed of parenchyma cells and/or with fibers forming columns (75). Five shapes of minor vascular bundles are found: arrosetate (78), round in outline (79), elliptical (80), ovoid (81), or mixed round in outline and elliptical (82); idioblasts with rachids are present or absent (83).
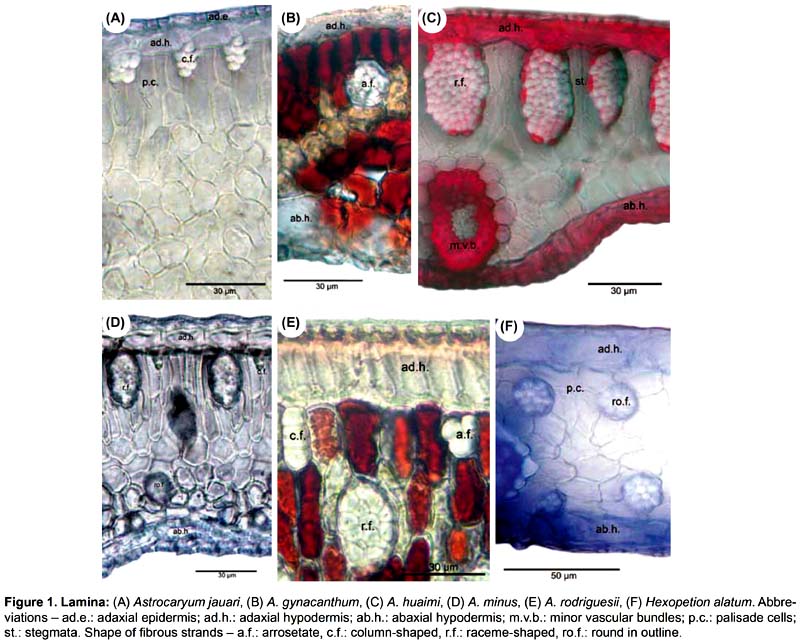
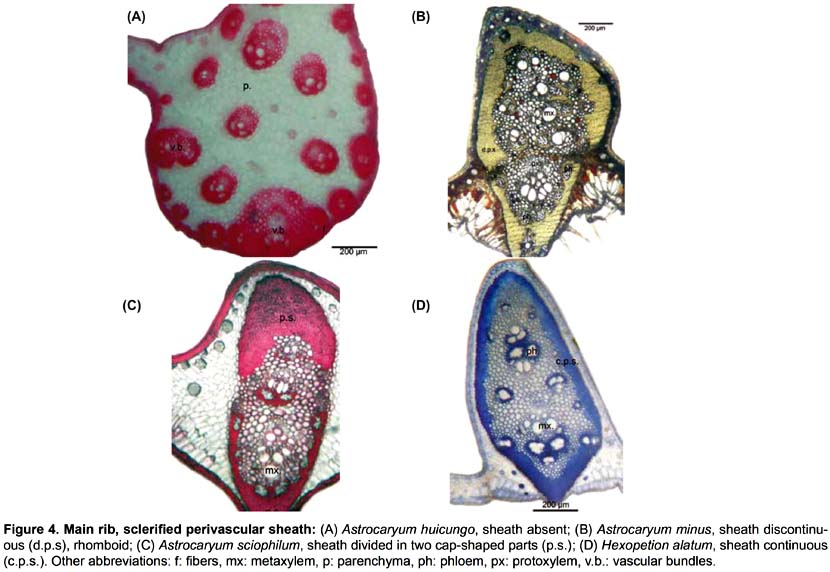
Main rib (Fig. 4). Eleven differential characters are identified. These include: hypodermis with expansion cells in only one or in the two angles with the lamina, on abaxial and adaxial sides (84, 85); vascular tissue without (86) or with discontinuous perivascular sheath of sclerified fibers, either rhomboid (87) or triangular (88), or divided in two cap-shaped parts located in adaxial and abaxial position, respectively (89); fibrous strands with stegmata (91); aeripherous parenchyma absent (92) or present and located under the vascular bundles (93) or in the central part of the main rib (94).
Petiole. Nine differential characters: epidermal cells rectangular (95), oval (96), round in outline (97), with outer wall thick or thin (98, 99); hypodermis with parenchyma (100), sclereids (101) or septate fibers (102); fibrous strands with stegmata (103).
Sheath. Six differential characters: epidermal cells rectangular (104), oval (105) or round in outline (106) with outer wall thick (107) or thin (108); hypodermis with sclereid cells (109).
2. Hexopetion
The two species are distinguished using several characters of stomata, lamina and vascular vessels: (i) stomata with terminal cells like dumbbells (24, Hexopetion mexicanum), or like short V (20, Hexopetion alatum); (ii) adaxial epidermis cells with thin outer wall (1, Hexopetion mexicanum), with thick outer wall (2, Hexopetion alatum); (iii) under the adaxial hypodermis, fibrous strands are arrosetate and raceme-shaped (52, Hexopetion mexicanum), or round in outline and raceme-shaped (54, Hexopetion alatum); (iv) minor vascular bundles ovoid (81, Hexopetion mexicanum) or round in outline (79, Hexopetion alatum).
3. Astrocaryum versus Hexopetion
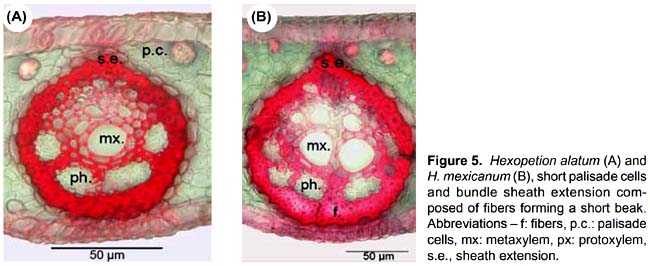
Four anatomical characters separate the 23 species of Astrocaryum from the two species of Hexopetion: (i) stomata (Fig. 2) with 2 or 6 subsidiary cells around guard cells in Astrocaryum, 4 in Hexopetion (11-13); (ii) chlorenchyma with palisade cells composed of short cells in Hexopetion and of long cells in Astrocaryum (61-62); (iii) fibers of bundle sheath extension (Fig. 5) forming a short beak in Hexopetion (76), when present in Astrocaryum extension forms a column composed of parenchyma cells and/or fibers (75); (iv) Hexopetion develops a continuous sclerified perivascular sheath in the main rib (90, Fig. 4), while this sheath is either discontinuous, or divided in two parts, or absent in Astrocaryum (86-89, Fig. 4).
4. Result of phenetic analysis
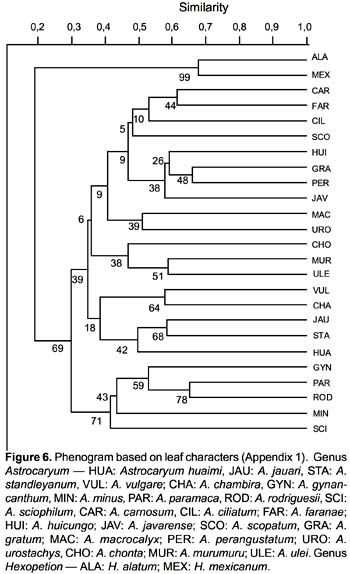
The phenogram is shown in Fig. 6. The species are distributed in two clusters, corresponding to Astrocaryum and Hexopetion, respectively. Astrocaryum is divided into two main clusters: one groups A. gynacanthym, A. paramaca, A. rodriguesii, A. minus and A. sciophilum, the other is divided into two groups, one with A. vulgare, A. chambira, A. jauari, A. standleyanum, and A. huaimi; the other group includes two clusters, one with A. chonta, A. murumuru and A. ulei, the other with three subgroups: (i) A. urostachys and A. macrocalyx, (ii) A. javarense, A. perangustatum, A. gratum and A. huicungo, (iii) A. scopatum, A. ciliatum, A. faranae and A. carnosum.
5. Key to Astrocaryum and Hexopetion species based on anatomical characters of the leaf
1a. Stomata with 4 subsidiary cells; sclerified perivascular sheath continuous — 2
1b. Stomata with 2 or 6 subsidiary cells; sclerified perivascular sheath discontinuous — 3
2a. Adaxial epidermal cells with outer wall thin; under the adaxial hypodermis, fibrous strands arrosetate and raceme-shaped; minor vascular bundles ovoid; stomata with subsidiary cells like dumbbells — Hexopetion mexicanum
2b. Adaxial epidermal cells with outer wall thick; under the adaxial hypodermis, fibrous strands round in outline and raceme-shaped; minor vascular bundles round in outline; stomata with subsidiary terminal cells short V-shaped — Hexopetion alatum
3a. Stomata with 2 lateral subsidiary cells, no terminal cells — Astrocaryum chambira
3b. Stomata with 6 subsidiary cells, two of them terminal — 4
4a. Terminal cells superimposed on both outer and inner lateral subsidiary cells — 5
4b. Terminal cells superimposed on outer or on inner lateral subsidiary cells — 15
5a. Extremities of the terminal cells do not rise above the lateral cells — 6
5b. Extremities of the terminal cells rise above the outer and inner lateral cells — 8
6a. Major vascular bundle ovoid — Astrocaryum faranae
6b. Major vascular bundle romboid — 7
7a. Fibrous strands round in outline, no layer of chlorenchyma above the abaxial hypodermis — Astrocaryum huicungo
7b. Fibrous strands raceme-shaped, with a layer of chlorenchyma above the abaxial hypodermis — Astrocaryum javarense
8a. Under the adaxial hypodermis, fibrous strands raceme-shaped only, or raceme-shaped and column-shaped — 9
8b. Under the adaxial hypodermis, fibrous strands arrosetate and raceme-shaped — 10
9a. Terminal cells not oval (short V-shaped, short or long Y-shaped, round in line); under the adaxial hypodermis, fibrous strands raceme-shaped only — 11
9b. Terminal cells oval; under the adaxial hypodermis, fibrous strands raceme-shaped and column-shaped — Astrocaryum huaimi
10a. Above the abaxial hypodermis, fibrous strands round in outline — Astrocaryum scopatum
10b. Above the abaxial hypodermis, fibrous strands arrosetate and round in outline — Astrocaryum carnosum
11a. Terminal cells short V-shaped — 12
11b. Terminal cells not V-shaped — 13
12a. Above the abaxial hypodermis, fibrous strands round in outline — Astrocaryum ciliatum
12b. Above the abaxial hypodermis, fibrous strands arrosetate — Astrocaryum perangustatum
13a. Without a discontinuous layer of chlorenchyma above the abaxial hypodermis; terminal cells long Y-shaped; major vascular bundle ovoid — Astrocaryum urostachys
13b. With a continuous layer of chlorenchyma above the abaxial hypodermis; terminal cells short Y-shaped or round in outline; major vascular bundle not ovoid —14
14a. Terminal cells short Y-shaped; major vascular bundle elliptical — Astrocaryum macrocalyx
14b. Terminal cells round in outline; major vascular bundle rhomboid — Astrocaryum gratum
15a. Terminal cells superimposed on the outer lateral cells — 16
15b. Terminal cells superimposed on the inner lateral cells — 22
16a. Terminal cells superimposed on the major part of the outer lateral cells — 17
16b. Terminal cells superimposed only on the extremities of the outer lateral cells — 20
17a. Terminal cells long V-shaped — 18
17b. Terminal cells shortly kidney-shaped — 19
18a. Terminal cell extremities rise above the lateral cells; under the adaxial hypodermis, fibrous strands raceme-shaped and column-shaped; above the abaxial hypodermis, fibrous strands round in outline; major vascular bundle elliptical — Astrocaryum minus
18b. Terminal cell extremities do not rise above the lateral cells; under the adaxial hypodermis, fibrous strands arrosetate; above the abaxial hypodermis, fibrous strands absent; major vascular bundle round in outline — Astrocaryum gynacanthum
19a. Terminal cell extremities rise above the lateral cells; under the adaxial hypodermis, fibrous strands arrosetate, column-shaped and raceme-shaped — Astrocaryum rodriguesii
19b. Terminal cell extremities do not rise above the lateral cells; under the adaxial hypodermis, fibrous strands arrosetate and raceme-shaped — Astrocaryum paramaca
20a. Major vascular bundle romboid — 21
20b. Major vascular bundle ovoid — Astrocaryum jauari
21a. With fibrous strands raceme-shaped under the adaxial hypodermis — Astrocaryum vulgare
21b. With fibrous strands arrosetate under the adaxial hypodermis — Astrocaryum standleyanum
22a. Terminal cells lobed, superimposed on the major part of the inner lateral cells — Astrocaryum sciophilum
22b. Terminal cells short or long V-shaped, superimposed only on the extremities of the inner lateral cells — 23
23a. Terminal cells short V-shaped; above abaxial hypodermis, fibrous strands round in outline — 24
23b. Terminal cells long V-shaped; above the abaxial hypodermis, fibrous strands arrosetate and round in outline — Astrocaryum murumuru
24a. Without a layer of chlorenchyma above the abaxial hypodermis — Astrocaryum chonta
24b. With a layer of chlorenchyma above the abaxial hypodermis — Astrocaryum ulei
Discussion
A good diagnostic character is one shared exclusively by all the elements of a taxon. The character closely related to environmental factors cannot be a good diagnostic character because it appears in different groups under the same contraints. The leaves are parts of the plant immediately in contact with the environment. They constitute the interchange surfaces where light is absorbed and water is lost. The leaf can be reasonably thought to be more reactive to a changing environment, and its structure more variable as a result, than organs that appear over a short period, such as the perianth of the fruit or the calyx and corolla of the flowers. Horn et al. (2009) conclude that evolution of the lamina anatomy of palms is highly homoplasious, but not at random as indicated by the recurrent evolution of different cohorts of correlated character states. That fact also suggests that leaf anatomy should be able to provide some characters useful for identifying species and for supporting a typological classification.
The anatomical description of vegetative parts of palms was formerly proposed by von Mohl (1824), Solereder and Meyer (1928), Zawanda (1890) and Pfister (1892). More recently, Cheadle and Uhl (1948) highlighted the presence of two characteristic types of vascular vessels in Roystonea regia and Acoelorrhaphe wrightii. Tomlinson (1959) described sclereids in several palm genera. By examining 250 species of 137 genera, he concluded that the great sub-groups of palms could be distinguished by a combination of anatomical characters (Tomlinson 1961). Stebbins and Khush (1961) analyzed the variation of stomata in species of Calamus and Caryota. Read (1968) pointed out similarities between Pseudophoenix and Ceroxylon. Barfod (1988, 1991) studied leaf anatomy of the genera Ammandra, Palandra, Phytelephas and identified two groups: the first includes Ammandra decasperma and Aphandra natalia, with small guard cells, thick cuticle and sclerenchyma sheath around vacular bundles; the second joins together Palandra (=Phytelephas) aequatorialis and Phytelephas karstenii, P. microcarpa, P. macrocarpa, P. schottii, P. seemanni y P. tumacana, with large guard cells, thin cuticle and thin sheath around vascular bundles. Anatomical characters were also used in cladistics of the Euterpeinae (Henderson 1999). Dransfield et al. (2008) used leaf anatomy to describe Tahina, a new palm genus from Madagascar, known from one species, T. spectabilis; Tahina differs from the other genera of Chuniophoeniceae (Coryphoideae) by having non-vascular fibers, frequently solitary, always free in the mesophyll and located alongside the abaxial side of the lamina. Hexopetion shares the continuous sclerified perivascular sheath with the genera Acrocomia, Aiphanes, Bactris and Desmoncus, while Astrocaryum is an exception in Bactridinae with a discontinuous perivascular sclerified sheath or without sclerified sheath (Pintaud et al. 2008). The present study shows that the 23 Astrocaryum species differ from Hexopetion species by three other differential characters.
Glassman (1972) dealing with 51 species of Syagrus provided the most extensive species-level analysis of leaf anatomy in palms. He separated the species by combining forms and organization of several tissus; these included: non-vascular fibrous strands, vascular bundles, expansion tissue, abaxial and adaxial hypodermis and epidermis. Read (1975) in Thrinax found that anatomical characters of the lamina can be used to distinguish the species, such as the presence of palisade tissue and the shape of guard cells. Uhl (1978) pointed out differential characters in terminal subsidiary cells, intermediate veins, fibrous strands and hypodermis, as well as combinations of characters to separate Hyophorbe verschaffeltii, H. indica, H. lagenicaulis, H. amaricaulis, H. vaughanii. The two species of Hexopetion differ in outer wall thickness of adaxial epidermal cells, in the shape of fibrous strands under the adaxial hypodermis, of minor vascular bundles and of subsidiary terminal cells.
Some trends can be drawn from the distribution of anatomical characters of the leaf in relation to infrageneric classification in Astrocaryum (Kahn 2008). The sample of 23 species includes (Appendix 1): (i) 5 of 16 species in subgenus Astrocaryum — 4 of 15 species from section Astrocaryum, 1 species of the monotypic section Euchambira; (ii) all of the 4 species of subgenus Munbaca — the 2 species of section Munbaca, and the 2 species of section Mumbacusu; (iii) 14 of 20 species of subgenus Monogynanthus — 1 of 3 from section Monogynanthus, 13 of 15 from section Huicungo (with 6 of 7 from subsection Huicungo, 4 of 5 from subsection Sachacungo, and the 3 species of subsection Murumuru); no species were studied from the monotypic sections Ayri and Guatinajo. Only a few characters, essentially those of stomata, are distributed according to the infrageneric classification. The four species of subgenus Munbaca present the same pattern (28, subsidiary terminal cells superimposed on the major part of the outer lateral cells). In subgenus Monogynanthus, the three species of subsection Murumuru develop the same pattern (26, subsidiary terminal cells superimposed only on the extremities of the inner lateral cells), while another pattern is shared by the species of the two subsections Huicungo and Sachacungo (27, subsidiary terminal cells superimposed only on the extremities of the inner and outer lateral cells). This pattern is also found in one species of subgenus Astrocaryum (A. huaimi); the other species of subgenus Astrocaryum develop two different patterns: (i) 25, subsidiary terminal cells superimposed only on the extremities of the outer lateral cells, found in three species of section Astrocaryum, and (ii) 11, only two lateral subsidiary cells, no terminal cells, found in the monotypic section Euchambira. The other anatomical characters of lamina, main rib, petiole and sheath cannot be related to infrageneric groups. However, combinations of several characters make it possible to separate the species as shown in the key. Besides the characters of the stomata, the combinations include: fibrous strands disposition and shape under adaxial and above abaxial hypodermis, presence of chlorenchyma above abaxial hypodermis, and shape of the major vascular bundle.
The phenetic analysis based on leaf anatomy only partially recognizes the infrageneric classification, which is essentially based on flower and fruit characters. The species of Astrocaryum are distributed in two main clusters : (i) One is divided in two groups that join together the five species of subgenus Astrocaryum and the 13 species of section Huicungo (subgenus Monogynanthus). In this section, the three species of subsection Murumuru form a group apart, while the species of the two other subsections, Huicungo and Sachacungo, are distibuted in three subgroups, one with two closely related species of subsection Sachacungo (Astrocaryum macrocalyx and A. urostachys, Pintaud & Kahn 2002, Vargas & Pintaud 2008), one with four species of subsection Huicungo, and the third group with four species, two of each subsection. (ii) The other main cluster is composed of the four species of subgenus Munbaca associated with Astrocaryum sciophilum (subgenus Monogynanthus, section Monogynanthus). This similarity in leaf anatomy between subgenus Munbaca and A. sciophilum can be related to the similarity in leaf morphology. Pinnae are plicate in subgenus Munbaca (except for A. paramaca), as well as in the three species of section Monogynanthus (of which only A. sciophilum is studied here), while they are not plicate in the species of section Huicungo, nor in the species of subgenus Astrocaryum.
Acknowledgments
This work was supported by the agreement between San Marcos National University, Peru and IRD-Research Institute for Development (UMR DIAPC), France. Data were formerly presented by B. Millán in a doctoral dissertation at San Marcos National University, Lima, Peru. We thank J.-C. Pintaud and Kenneth Young for their helpful comments on the manuscript, and Nancy Rojas for the preparation of specimens for SEM.
Literature cited
Barfod A. 1988. Leaf anatomy and its significance in phytelephantoid palms (Arecaceae). Nord. J. Bot. 8 (4): 341-348.
Barfod A. 1991. A monographic study of the subfamily Phytelephantoideae (Arecaceae). Opera Bot. 105: 1-73.
Burret M. 1934a. Die Palmengattung Astrocaryum G.F.W. Meyer. Repert. Spec. Nov. Regni Veg., 35: 114-158.
Burret M. 1934b. Palmae neogeae. Notizblatt Bot. Gart. Mus. Berlin-Dahlem, 12: 151-159.
Cheadle V.I. & N.W. Uhl. 1948. Types of vascular bundles in the Monocotyledoneae and their relation to the late metaxylem conducting elements. Am. J. Bot., 35 (8): 486-496.
Dransfield J., M. Rakotoarinivo, W.J. Baker, R.P. Bayton, J.B. Fisher, J.W. Horn, B. Leroy & X. Metz. 2008. A new Coryphoid palm genus from Madagascar. Bot. J. Linn. Soc., 156: 79 -91.
Glassman S.F. 1972a. Systematics studies in the leaf anatomy of palm genus Syagrus. Am. J. Bot., 59 (8): 775-788.
Hammer Ø., D.A.T. Harper & P.D. Ryan. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeont. Electr. 4:1-9.
Henderson A. 1999. A phylogenetic analysis of the Euterpeinae (Palmae; Arecoideae; Areceae) based on morphology and anatomy. Brittonia, 51 (1): 106-113.
Henderson F.M. 2006. Morphology and anatomy of palm seedlings. Bot. Rev. 72 (4): 273-329.
Horn J.W., J.B. Fischer, P.B. Tomlinson, C.E. Lewis & K. Laubengayer. 2009. Evolution of lamina anatomy in the palm family (Arecaceae). Am. J. Bot. 96 (8): 1462-1486.
Johansen D.A. 1949. Plant microtechnique. Mac Graw - Hill Book co. Inc. New York.
Kahn F. 2008. The genus Astrocaryum (Arecaceae). Rev. peru. biol. 15 (supl. 1): 31-48.
Kahn, F. & J.-J. de Granville. 1998. Astrocaryum minus Trail (Palmae), rediscovered in French Guiana. Principes, 42: 171-178.
Mohl, H. von. 1824. De structura Palmarum in Von Martius. Historia Naturalis Palmarum.
Pfister, R. 1892. Beitrag zür vergleichender Anatomie der Sabaleen-Blatter. Diss. Zürich.
Pintaud J.-C. & F. Kahn. 2002. Endémisme et spéciation radiative chez les palmiers de forêt dense humide : les Iguanurinae de Nouvelle-Calédonie et le genre Astrocaryum en Amazonie. Biosystema, 20 : 81-88.
Pintaud J.-C., B. Millán & F. Kahn. 2008. The genus Hexopetion. Rev. peru, Biol. 15, supl. 1: 49-54.
Read R.W. 1968. A study of Pseudophoenix (Palmae). Gentes Herb. 10: 169-213.
Read R.W. 1975. The genus Thrinax (Palmae: Coryphoideae). Smithsonian Contr. Bot. 19: 1-96.
Schlüter U.B., B. Furch & C.A. Joly. 1993. Physiological and anatomical adaptations by young Astrocaryum jauari Mart. (Arecaceae) in periodically inundated biotopes of Central Amazonia. Biotropica 25(4): 384-396.
Solereder H. & F.J. Meyer. 1928. Palmae in Heft 3. Systematische Anatomie der Monokotiledonen: 3-85.
Stebbins G.L. & G.S. Khush. 1961. Variation in the organization of the stomatal complex in the leaf epidermis of monocotyledons and its bearing on their phylogeny. Am. J. Bot., Vol. 48 (1): 51-59.
Tomlinson P.B. 1959. Structure and distribution of sclereids in the leaves of palms. New Phytologist, Vol. 58 (3): 253-266.
Tomlinson, P.B. 1961. Anatomy of Monocotyledons. II Palmae. Oxford University Press.
Uhl N.W. 1978. Leaf anatomy in the species of Hyophorbe (Palmae). Gentes Herb., 11 (4): 268-283.
Vargas V. & J.-C. Pintaud. 2008. Caracterización de una zona de contacto parapátrico entre Astrocaryum macrocalyx y Astrocaryum urostachys en el límite entre la planicie Marañon-Pastaza y el Arco de Iquitos. Rev. peru, Biol. 15, supl. 1: 79-83.
Zawanda K. 1890. Das anatomische Verhalten der Palmenblätter dem System dieser Familie. Diss. Erlangen.
Appendix
Trabajo presentado a la XVIII Reunión Científica del Instituto de Investigaciones en Ciencias Biológicas Antonio Raimondi, 200 años del nacimiento de Charles Darwin y el 150 aniversario de la publicación de On the Origin of Species by Means of Natural Selection. Del 19 al 21 de agosto de 2009.
Publicado impreso: 20/10/2010
Publicado online: 29/09/2010













