Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. v.19 n.1 Lima abr. 2012
TRABAJOS ORIGINALES
Decrease in spermatic parameters of mice treated with hydroalcoholic extract Tropaeolum tuberosum "mashua"
Disminución en los parámetros espermáticos de ratones tratados con el extracto hidroalcohólico de Tropaeolum tuberosum "mashua"
Jonathan H. Vásquez 1, 2,*, José M. Gonzáles 1,2 and José L. Pino 1
1 Laboratorio de Reproducción y Biología del Desarrollo
2 Laboratorio de Fisiología de la Reproducción Animal, Instituto de Ciencias Biológicas Antonio Raimoindi, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Av. Venezuela cdra. 34, Lima, Perú. Tel. +511 619 7000 extension 1529, fax: +511 4649110. PO BOX 11-0058. Lima 11.
Abstract
In this work, we provided a Tropaeolum tuberosum hydroalcoholic extract to male mice (780 mg kg-1) for 7, 14 and 21 days treatment, there was no significant difference in body weight gain, testes, epididymides and prostate weight (p> 0.05), nevertheless progressive motility decreased and immobile sperm count increased significantly after 21 days treatment (p <0.05). The sperm count in the epididymis cauda decreased in the 3 three assessments, concentration on 21 days treatment was significantly lower than those of 7 and 14 days treatments (p <0.05). Our results suggest, that T. tuberosum has a direct action on the male reproductive system decreasing spermatic parameters without exerting toxic effects on mice.
Keywords: sperm count, hydroalcoholic extract, sperm motility, sperm parameters, Tropaeolum tuberosum.
Resumen
Se proporcionó extracto hidroalcohólico de Tropaeolum tuberosum a ratones machos (780 mg kg-1) durante 7, 14 y 21 días. Los tratamientos no produjeron diferencias significativas en la ganancia de peso corporal, y en el peso de los testículos, epidídimos y la próstata. Sin embargo, la movilidad progresiva espermática disminuyó y el recuento de espermatozoides inmóviles aumentó, ambos significativamente, después de 21 días de tratamiento (p <0.05). La concentración de espermatozoides en la cola del epidídimo disminuyó en las tres evaluaciones, la concentración espermática después de 21 días de tratamiento fue significativamente menor en comparación a 7 y 14 días de tratamiento (p <0.05). Nuestros resultados sugieren que T. tuberosum tiene una acción directa sobre el sistema reproductor masculino disminuyendo los parámetros espermáticos, sin ejercer efectos tóxicos en los ratones.
Palabras clave: espermatozoides, tubérculo andino, movilidad espermática, parámetros espermáticos, Tropaeolum tuberosum.
Introduction
Plants and their active compounds have been used as an important medicinal source in several disease treatments. Therapeutic potential is based on anti carcinogenic, antidiabetic, hepatoprotective, cardioprotective, antispasmodic, analgesic properties (D'Cruz et al. 2010). However, some plants can affect negatively several physiological functions, including male reproductive function, whose adverse effects have been attributed mainly to anti-steroidogenic and antispermatogenic properties (Ashok et al. 2004, Gupta et al. 2006, Lohiya et al. 2002).
Tropaeolum tuberosum Ruiz & Pav. is a kind of tuber, belongs to the Tropaeolaceae family and grows between 2400 and 4300 m. altitude (Grau et al. 2003, Flores et al. 2003). It was domesticated in the Andes, from Venezuela to Argentina (Chirinos et al. 2008). Tropaeolum tuberosum "mashua" is in the fourth position at the nutritional tuber ranking after potato, oca and olluco (NRC 1989). On the other hand, several studies have reported medicinal uses of mashua in order to relief kidney and liver ailments (Oblitas 1969), skin eczema (Pérez Arbelaez 1947), prostate ailments (Brack 1999) and diabetes (Rea 1984), these therapeutic properties would be related to phenolic antioxidants presence and these in turn due to high anthocyanins content (Chirinos et al. 2008).
Isothiocyanates of T. tuberosum (Johns & Towers 1981, Ramallo et al. 2004) would be responsible for its properties as an antibiotic, nematicide and diuretic (Cárdenas 1958, Brack 1999). These active compounds stop metastatic cells growth (Nakamura & Miyoshi 2006, Zhang et al. 2003, 2006) inhibiting enzymes involved carcinogenics activation and activating enzymes that accelarate carcinogens inactivation (Zhang et al. 1998) or the covalent cell protein union that could be inducing apoptosis (Mi et al. 2007). Antioxidants in these crops (Campos et al. 2006, Chirinos et al. 2007) support their wide use in traditional Andean medicine (Johns et al. 1982).
Johns et al. (1982) suggests that T. tuberosum has estrogenic activity, is thought to suppress sexual appetite, decreases reproductive potential and erectile men function (León 1967, Oblitas 1969, Brack 1999). According to the folklore, Incas used to fed his troops with mashua in order to forget their wives while they were at war (Patiño 1964) and rural women in Cusco (Peru) prevented husband's infidelities provided him of mashua (Hermann 1992). Oblitas (1969) indicates its use like a "menstruation inducer"
There are only 2 reports that attempt to clarify these beliefs and show experimentally that may not be unfounded. Johns et al. (1982), fed rats with T. tuberosum, then, they observed a 45% decrease in testosterone / dihydrotestosterone levels but rats retained their impregnate ability. Nevertheless, there was not a proper monitoring of the animals ingested dose which make difficult the results interpretation. Cárdenas-Valencia et al. (2008) report that aqueous extract of mashua produced a testicular function decrease by reducing spermatids and sperm densities, as well as sperm daily production and spermatozoa transit time through the epididymis. However, no changes in testosterone levels were observed during treatments.
The present study aims to evaluate the effects of T. tuberosum hydroalcoholic extract ingestion on mice sperm functional parameters.
Material and methods
Animals.- 60 males mice (Mus musculus) 6–7 weeks old, Swiss Rockefeller strain, from The Animal Care House of Facultad de Ciencias Biologicas, Universidad Nacional Mayor de San Marcos (Lima, Perú), were kept under physiological conditions: temperature (25 – 27 ºC), balanced food (Purina-Peru), water ad libitum and a photoperiod of roughly 14/10 hours (light / dark).
Plant and preparation of Tropaeolum tuberosum paste.- Tubers were obtained from Ayacucho city (Ayacucho, Peru, at 2700 m of altitude) and were identified in the Departameto de Botanica of Universidad Nacional Mayor de San Marcos. Tubers paste was prepared by macerating 500 g of T. tuberosum with 700 mL of 96° ethanol for a period of 12 days under constantly agitation. Supernatant was filtered twice through a 40 and 20 microns Whatman filter paper, and then the extract was placed in an oven at 40 °C for 2 weeks until pasty consistency was achieved. We determined paste dry weight in order to calculate mashua concentration; thus, T. tuberosum paste was diluted with distilled water up to a 780 mg raw material per kilogram of body weight concentration (this dose showed significant effects in our laboratory preliminary tests). This solution was stored in vials at 4 °C until use.
Experimental design.- We had 6 treatment groups: T7, T14 and T21, representing treated animals that were administered with aqueous extract of T. tuberosum at 780 mg kg-1 for 7, 14 and 21 days respectively with a single daily dose (Piña-Guzmán et al. 2005 ) and their respective controls C7, C14, C21 which were administered distilled water by the same route. Both solutions were administered using an intubation needle N° 18 (Fisher Scientific, Pittsburgh, PA, USA). These treatments times with T. tuberosum derive from established times for secondary spermatocytes, testis spermatids and epididymis sperm assessment (Oakberg 1956).
After treatment body weight was recorded and animals were euthanized. Reproductive organs were weighed: testes, epididymides and prostate. Sperm were obtained from the epididymis tail and sperm motility count was registered.
Testes and sperm retrieval.- Each epididymis obtained was washed with PBS (7.4) at 37 °C, several incisions were made at the epididymis tail and spermatozoa were released by pressing the incision region manually (Martins et al. 2007). The sperm content was fully recovered in 1.5 mL polypropylene tubes (Axygen Scientific) and 0.5 mL Flushing medium (MediCult®, Copenhagen, Denmark).
Sperm motility assessment.- A drop of sperm sample was placed on a slide tempered at 37 °C in a CO2 incubator and directly observed at 400X in a phase contrast microscope (AJ Seitz, San Francisco, USA). This procedure was repeated twice, and results were showed as an average of both assessments. At least two hundred sperm were evaluated. Sperm cells were designated as having progressive motility (PM) when displacement was observed; non progressive motility (NPM), when in-situ movement without displacement was observed, and immotility (IM), when no form of movement was perceived (WHO 2010).
Sperm concentration measurement.- Sperm concentration was measured using a 1:20 dilution: 10 µL of sample was diluted with 190 µL of fixative (WHO 2010). Sperm suspension was placed on both sides of Neubauer's hemocytometer and allowed to settle for 5 minutes. The number of spermatozoa in the squares of the hemocytometer was counted under the microscope at 400X magnification. Sperm concentration was expressed in millions per milliliters.
Statistical analysis.- Data were analyzed using SPSS 17.0 for Windows. Bartletts test was performed to determine the homogeneity of variances. When variances were homogeneous, differences between groups were assessed by analysis of variance (ANOVA). ANOVA test was used followed by Tukey post hoc test for physiological data analysis. Results were expressed as mean ± SE (standard error) and p<0.05 was considered statistically significant.
Results
Body weight and organs.- Body weights, testes, epididymides and prostate weights difference are shown in Table 1. No significant differences were observed in body weight gain, testes, epididymides and prostate weight (p> 0.05) between T. tuberosum group and control group (vehicle).
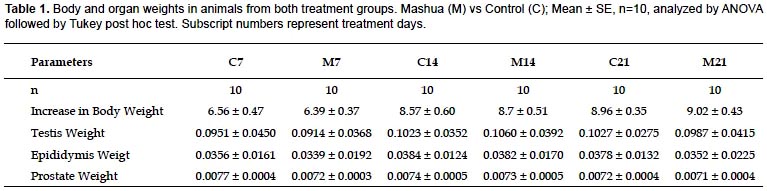
Motility and sperm concentration.- Significant differences were observed after 21 days of treatment among control group (C21) and T. tuberosum group (M21) in the values of sperm with PR, NP and IM. It showed significant decreases in PR (C21: 52.56 ± 0.78 vs. M21: 45.83 ± 0.80, p <0.05), treatments for 7 and 14 days did not differed respect to their controls (C7: 52.89 ± 0.73 vs. M7: 50.96 ± 0.72 and C14: 53.21 ± 0.70 vs. M14: 51.11 ± 0.56 respectively, p> 0.05). Regarding NP (C21: 8.47 ± 0.42 vs. M21: 5.17 ± 0.60 p <0.05), treatments for 7 and 14 days did not differed respect to their controls (C7: 9.20 ± 0.40 vs. M7: 9.57 ± 0.59 and C14: 9.42 ± 0.52 vs. M14: 7.66 ± 0.59 respectively, p> 0.05). Regarding IM (C21: 38.97 ± 0.66 vs. M21: 49.00 ± 1.22 p <0.05), treatment for 7 and 14 days did not differed respect to their controls (C7: 37.91 ± 0.83 vs. M7: 39.47 ± 0.81 and C14: 37.37 ± 0.85 vs. M14: 41.23 ± 0.81 respectively, p> 0.05). Sperm motility results are summarized in (Fig. 1).
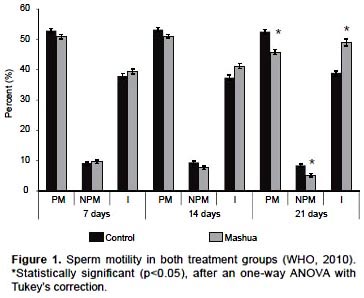
Epididymis tail sperm concentration decreased with T. tuberosum treatments during the three evaluation times (p <0.05): 7 days treatment (C7: 24.96 ± 0.61 vs M7: 19.11 ± 0.67), 14 days treatment (C14: 25.21 ± 0.64 vs M14: 18.55 ± 0.48) and 21 days treatment (C21: 25.06 ± 0.72 vs M21: 13.45 ± 0.83) (Fig. 2).
Discussion
Our research is about T. tuberosum effect on mice´s sperm parameters. It has been postulated the use of some plants as male contraceptives (Ashok et al. 2004, Gupta et al. 2006). In order to be useful, these plants should exert localized effects on the reproductive system, without systemic alterations that may alter many others physiological functions (Gupta et al. 2000, Sharma and Jocob 2001, Venma et al. 2002). In this paper we suggest a direct action of T. tuberosum on reproductive system because there is no change in body weight (Table 1, p> 0.05) but there is evidence of decrease in motility and sperm concentration values (Figs. 1 and 2, p <0.05) after T. tuberosum treatment, and this observation supports our previous results in which different mashua ecotypes did not exert genotoxic action on hematopoietic lineage (data not published). Testes, epididymides and prostate are androgen-dependent organs (Trentacose et al. 2001), we did not observe weights changes so T. tuberosum would not be exerting an anti-androgen on male reproductive system (Manivannan et al. 2009). Cardenas-Valencia et al. (2008) found no differences in serum testosterone levels in animals that ingested T. tuberosum and their respective controls.
Spermatogenesis is a highly synchronized and dynamic process that takes place in seminiferous tubules. In mice this process lasts about 35 days, the period known as spermiogenesis is divided into 16 steps (Oakberg 1956). The evaluation times in this study, cover different stages of spermatogenic cycle: spermatogenesis, spermiation and epididymis transport. After 21 days of T. tuberosum treatment, effects on secondary spermatocytes and round spermatids was assessed, elongated spermatids at 14 days treatment, spermiation and sperm transit through epididymis at 7 days treatment (Piña-Guzmán et al. 2004). Decrease in PR, and increase in IM, were observed only in 21 days treatment (Fig. 1, p <0.05); this suggests that T. tuberosum would be exercising some kind of effect in secondary spermatocytes and/or round spermatids, nevertheless this effect was not observed after 14 or 7 days treatment, suggesting there would not be an effect on elongated spermatids, spermiation and sperm maturation in epididymis in relation to sperm motility.
An important event during round spermatid stage is sperm nuclear proteins mRNA transcription, nuclear transition proteins (TP1 and TP2) and protamines (P1 and P2). In spermatogenesis, transcription is strongly activated during step 7 round spermatids, decreases sharply in 8, 9 step and at 10 step is undetectable (Zheng et al. 2008). In sperm nuclear murine mRNA species, proteins are initially detected in step 7 round spermatids (Mali et al. 1989), a stage corresponding to the T. tuberosum 21-days exposed group. Because of a decrease in sperm motility observed in this group as well as a greater decrease in sperm concentration compared to groups treated for 7 and 14 days (Fig. 2, p <0.05), T. tuberosum may be altering these genes transcription. It has been reported that protamines expression levels alterations are related to both sperm motility and concentration decreased (Carrell & Liu 2001, Aoki et al. 2005), and it is known that protamines condense strongly paternal genome inside sperm nucleus (Aoki & Carrell 2003), then sperm acquire a hydrodynamic shape that allows enhanced motility (Oliva & Dixon 1991).
Isothiocyanates are the most abundant T. tuberosum compounds (Ramallo et al. 2004), and can bind covalently to proteins, inactivating enzyme activities (Nakamura et al. 2006, Zhang et al. 1998, 2003, 2006). Mi et al. (2007) observed in vitro that phenethyl isothiocyanate (PEITC) binds covalently to cellular proteins and induces apoptosis in cancer cell lines. Isothiocynates present in T. tuberosum could be acting on transcription regulatory, remodeling factors, histone deacetylases, heterochromatin binding proteins and/or topoisomerase in order to prevent gene transcription (Zheng et al. 2008).
Johns et al. (1982) suggested that T. tuberosum has an estrogenic effect, however, sperm transport within and through epididymis is facilitated by the movements of efferent ducts epithelial cells cilia (Ilio & Hess 1994). These microvilli are estrogen receptor α-dependent (ERα) (Hess et al. 2001), and mice with ER haploinsufficiency had low epididymis tail sperm counts, cilia number and microvilli height alterations (Hess et al. 2000). Valencia Cardenas et al. (2008) reported that aqueous extract of T. tuberosum decreases spermatozoa transit time through epididymis. In this paper, sperm concentration decreased in all 3 treatment groups (Fig. 2, p<0.05), which suggests T. tuberosum could be interfering with ERα function.
Several contraceptives obtained from plants have proceeded on epididymis tail motility decrease and sperm concentration (Verma et al. 2002, Sharma et al. 2003), important features in order to ensure fertilization success (Bedford 1983). Valencia-Cardenas et al. (2008) showed that aqueous extract of T. tuberosum had contraceptives effects; we observed similar effects using hydroalcoholic extract. In conclusion, ingestion of T. tuberosum hydroalcoholic extract reduces sperm motility and concentration values during spermatogenesis in mice. Further research is needed to determine action mechanisms on male germ cells accurately. In order to achieve it, genes expression quantification would be an interesting alternative.
Acknowledgement
This research was supported by a Grant from Vicerrectorado de Investigación (VRI), Universidad Nacional Mayor de San Marcos, Project CON-CON RR. N° 00251-R-11.
Literature cited
Aoki V.W. & D.T. Carrell. 2003. Human protamines and the developing spermatid: their structure, function, expression and relationship with male infertility. Asian J. Androl. 5:315-324.
Aoki V.W, L. Liu, D.T. Carrell. 2005. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum. Reprod. 20:1298–1306.
Ashok P. & B. Meenakshi. 2004. Contraceptive effect of Curcuma longa (L.) in male albino rat. Asian J. Androl. 6:71–74.
Bedford J.M. 1983. Significance of the need for sperm capacitation before fertilization in eutherian mammals. Biol. Reprod. 28:108–120.
Brack A. 1999. Diccionario enciclopédico de plantas útiles del Perú. Centro de Estudios Regionales Andinos Bartolomé de las Casas, Lima, Perú.
Campos D., G. Noratto, R. Chirinos, et al. 2006. Antioxidant capacity and secondary metabolites in four species of Andean tuber crops: native potato (Solanum sp.), mashua (T. tuberosum Ruiz & Pavón), Oca (Oxalis tuberosa Molina) and ulluco (Ullucus tuberosus Caldas). J. of Sci. Food Agri. 86:1481–1488.
Cárdenas M. 1958. Estudios sobre tubérculos alimenticios de los Andes. II Informe sobre los trabajos hechos en Bolivia sobre oca, ulluco y mashua. Comunicación de Turrialba 63:5–21.
Cárdenas-Valencia I., J. Nieto, M. Gasco, et al. 2008. T. tuberosum (Mashua) reduces testicular function: effect of different treatment times. Andrologia 40:352-357.
Carrell D.T. & L. Liu. 2001. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflec to ther abnormalities of spermiogenesis. J. Androl. 22:604–610.
Chirinos R., D. Campos, C. Arbizu, et al. 2007. Effect of genotype, maturity stage and post-harvest storage on phenolic compounds, carotenoid content and antioxidant capacity of Andean mashua tubers (Tropaeolum tuberosum Ruiz & Pavón). J. of Sci. Food Agri. 87:437-446.
Chirinos R., D. Campos, N. Costa, et al. 2008. Phenolic profiles of andean mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers: Identification by HPLC - DAD and evaluation of their antioxidant activity. Food Chemistry 106:1285-1298.
D'Cruz S.C., S. Vaithinathan, R. Jubendradass, et al. 2010. Effects of plants and plant products on the testis. Asian J. Androl. 12:468-479.
Flores H., T. Walker, R. Guimaraes, et al. 2003. Andean Root and Tuber Crops: Underground Rainbows. Hortscience 38:161-167.
Grau A., R. Ortega, C. Nieto, et al. 2003. Mashua (Tropaeolum tuberosum Ruiz and Pavon). In: Engels J., ed. Promoting the conservation and use of underutilized and neglected crops. The International Plant Genetic Resources Institute (IPGRI), Rome. Pp. 1–27.
Gupta R.S., P. Kumar, V.P. Dixit, et al. 2000. Antifertility studies of the root extract of the Barleria prionitis Linn in male albino rats with special reference to testicular cell population dynamics. J. Ethnopharmacol. 70:111-117.
Gupta R.S. & R. Sharma. 2006. A review on medicinal plants exhibiting antifertility activity in males. Nat. Prod. Radiance 5:389–410
Hermann M. 1992. Andean roots and tubers: research priorities for a neglected food resource. International Potato Center, Lima, Perú.
Hess R.A. 2000. Oestrogen in fluid transport and reabsorption in efferent ducts of the male reproductive tract. Rev. Reprod. 5:84–92.
Hess R.A., Q. Zhou, R. Nie, et al. 2001. Estrogens and epididymal function. Reprod. Fertil. Dev. 13:273-283.
Ilio K.Y. & R.A Hess. 1994. Structure and function of the ductuli efferentes: a review. Microsc. Res. Tech. 29:432–467.
Johns T. & G.H.N. Towers. 1981. Isothiocyanates and thioreas in enzyme hydrolysates of Tropaoelum tuberosum. Phytochemistry 20:2687-2689.
Johns T., W.D. Kitts, F. Newsome, et al. 1982. Anti-reproductive and other medicinal effects of Tropaeolum tuberosum. J. Ethnopharmacol. 5:149-161.
Leon J. 1967. Andean tuber and root crops. Origin and variability. In: Tai E.A., Charles W.B., Iton E.F., Haynes P.H., Leslie K.A, eds. Proceedings of the International Symposium on Tropical Root Crops. University of West Indies, Trinidad. Pp, 118-123.
Lohiya N.K., B. Manivannan, P.K. Mishra, et al. 2002. Chloroform extract of Carica papaya seeds induces long-term reversible azoospermia in langur monkey. Asian J. Androl. 4:17-26.
Mali P., A. Kaipia, M. Kangasniemi, et al. 1989. Stage-specific expression of nucleoprotein mRNAs during rat and mouse spermiogenesis. Reprod. Fertil. Dev. 1:369–382.
Manivannan B., R. Mittal, S. Goyal. 2009. Sperm characteristics and ultrastructure of testes of rats after long-term treatment with the methanol subfraction of Carica papaya seeds. Asian J. Androl. 11:583-99.
Martins C.F., R. Rumpf, D.C. Pereira, et al. 2007. Cryopreservation of epididymal bovine spermatozoa from dead animals and its uses in vitro embryo production. Anim. Reprod. Sci. 101:326-331.
Mi L., X. Wang, S. Govindn, et al. 2007. The Role of Protein Binding in Induction of Apoptosis by Phenethyl Isothiocyanate and Sulforaphane in Human Non–Small Lung Cancer Cells. Cancer Res. 67:6409–6416.
Nakamura Y. & N. Miyoshi. 2006. Cell death induction by isothiocyanates and their underlying molecular mechanisms. Biofactors 26:123-134.
National Research Council (NRC). 1989. Lost Crops of the Incas: Little Known Plants of the Andes with Promise for Worldwide Cultivation. National Academy Press, Washington D. C.
Oakberg E.F. 1956. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Amer. J. Anat. 99:507–516.
Oblitas E. 1969. Plantas medicinales de Bolivia. Editorial los Amigos del Libro, Cochabamba, Bolivia.
Oliva R. & G.H. Dixon. 1991. Cohn W.E, Moldave K., eds. In: Progress in Nucleic Acid Research and Molecular Biology. Academic Press, San Diego. Pp, 26-96.
Patiño V. 1964. In: Plantas cultivadas y animales domesticados de América equinoccial. UK. Imprenta Departamental, Cali, Colombia, 2:40-41
Pérez-Arbelaez E. 1947. Plantas útiles de Colombia. Ensayo de botánica colombiana aplicada. Imprenta Nacional, Bogotá, Colombia.
Piña-Guzmán B., M.J. Solís-Heredia, B. Quintanilla – Vega. 2005. Diazinon alters sperm chromatin structure in mice by phosphorylating nuclear protamines. Toxicol. Appl. Pharmacol. 202:189-198.
Ramallo R., J.P. Wathelet, É. Le Boulengé, et al. 2004. Glucosinolates in isano (Tropaeolum tuberosum) tubers: qualitative and quantitative content and changes after maturity. J. of Sci. Food. Agri. 84:701-706
Rea J. 1984. Germoplasma boliviano y calidad bromatológica de Tropaeolum tuberosum. In Memorias: 4 Congreso internacional de cultivos andinos, Instituto Colombiano Agropecuario (ICA); Centro Internacional de Investigación para el Desarrollo (CIID). Pasto, Colombia. Pp, 381–386.
Sharma N. & D. Jocob. 2001. Antifertility investigation and toxicological screening of the petroleum ether extract of the leaves of Mentha arvensis L. in male albino mice. J. Ethnopharmacol. 75:5-12.
Sharma A., P.K. Verma, V.P. Dixit. 2003. Effect of Semecarpus anacardium fruits on reproductive function of male albino rats. Asian J. Androl. 5:121-124.
Venma P.K., A. Sharma, A. Mathur, et al. 2002. Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J. Androl. 4:43-47.
World Health Organization . 2010. WHO laboratory manual for the Examination and processing of human semen, fifth ed. Switzerland.
Zhang Y. & P. Talalay. 1998. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 58:4632–4639.
Zhang Y., L. Tang, V. Gonzalez. 2003. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol. Cancer Ther. 2:1045–1052.
Zhang R., S. Loganathan, I. Humphreys, et al. 2006. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J. Nutr. 136:2728–2734.
Zheng J., X. Xia, H. Ding, et al. 2008. Erasure of the paternal transcription program during spermiogenesis: the first step in the reprogramming of sperm chromatin for zygotic development. Dev. Dyn. 237:1463-1476.
* Corresponding author,
Email: Jonathan Vásquez:
jvcbioreprod@gmail.com
Presentado: 03/01/2012
Aceptado: 16/07/2012
Publicado online: 01/10/2012













