Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. vol.19 no.2 Lima ago. 2012
TRABAJOS ORIGINALES
Chemical composition of Apodanthera biflora, a Cucurbit of the dry forest in northwestern Peru
Composición química de Apodanthera biflora, una cucurbitácea del bosque seco del noroeste peruano
Daniel Clark1*, Maribel Tupa2, Andrea Bazán2, Lily Chang3, Wilfredo L. Gonzáles2
1 Unidad de Genómica, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia.
2 Laboratorio de Ecología Evolutiva, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia.
3 Departamento de Ciencias Exactas, Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia.
Abstract
The dry forest of northwestern Peru is the habitat of numerous native species that have received little attention beyond taxonomic purposes. Here we report the chemical composition of the roots and seeds of a set of accessions of Apondanthera biflora, a wild Cucurbit with potentialities as a food resource. The roots show features that are comparable to those from cassava roots and potato tubers: a high content of carbohydrates on a dry matter basis (77.5 - 84.9%), with starch representing around 20% of the total dry matter. The seeds fat content on a dry matter basis (22.22 - 39.37%) falls within the percentages found in oily seeds such as safflower, whereas the protein content (21.37 - 29.06%) is similar to that of flax and sunflower. The fatty acid profile of the seeds oil shows a predominance of polyunsaturated acids, in particular linoleic acid (43.81%), making it comparable to that of cotton oil. Our results confirm the nutritional value of Apodanthera biflora and set the ground for its use in domestication and food security programs.
Keywords: Yuca de monte; food security; dry forest; starch; oily seed; comestible root.
Resumen
El bosque seco del noroeste del Perú es hábitat de numerosas especies nativas que, fuera de su taxonomía, han sido poco estudiadas. En este trabajo se reporta la composición química de las raíces y semillas de un grupo de accesiones de Apodanthera biflora, una cucurbitácea silvestre con potencial nutricional. Las raíces muestran una composición comparable a la de la yuca y la papa: un alto contenido de carbohidratos en base al peso seco (77,5 - 84,9%), entre los cuales destaca el almidón con alrededor de 20% del peso seco. El contenido de grasas de la semilla en base al peso seco (22,22 - 39,37%) está dentro del rango descrito en semillas oleaginosas como las de cártamo, mientras que el contenido de proteínas (21,37 -29,06%) es similar al hallado en semillas de lino y girasol. En el perfil de ácidos grasos del aceite de la semilla se observa un predominio de los ácidos grasos poliinsaturados, en particular del ácido linoleico (43,81%), que lo hace comparable al del aceite de algodón. Los resultados confirman el valor nutricional de Apodanthera biflora y sienta las bases para su domesticación y aprovechamiento en programas de seguridad alimentaria.
Palabras clave: Yuca de monte; seguridad alimentaria; bosque seco; almidón; semilla oleaginosa; raíz comestible.
Introduction
Neotropical seasonal dry forests are ecosystems characterized by precipitations of less than 1600 mm/year, with at least 5 - 6 months of less than 100 mm rainfall (Pennington et al. 2000). In southwestern Ecuador and northwestern Peru, an area with such characteristics spans 86859 km2 of the coastal belt and shows a high degree of endemism (Aguirre et al. 2006). While taxonomic classification and geographic distribution of plant species within this area have been addressed by several investigators over the years (Svenson 1946; Ferreyra 1983; Aguirre et al. 2006), their phenology, reproductive strategies, genetic diversity, and chemical potentialities for application as food resources or in industrial processes have received little attention, in spite of the uses given to many of those species by the local population (Bussmann & Sharon 2006; Bussmann & Sharon 2009; Wilfredo Gonzáles, unpublished observations).
Apodanthera biflora Cogn. (locally known as "yuca de monte") is a perennial herbaceous vine belonging to the Cucurbitaceae family that is native to the Peruvian dry forest (Macbride 1937; Brack 1999; Jørgensen & León 1999; Mostacero et al. 2002). The aerial parts of the plant (vegetative and reproductive) grow from December through March, during the raining season.
Upon arrival of the dry season, they dry out leaving a tuberous root with latent buds buried in the soil, from which new aerial parts will grow in the following raining season (Wilfredo Gonzáles, unpublished observations).
Like other wild species of the Cucurbitaceae family such as Cucurbita foestidissima (DeVeaux & Shultz 1985), Cucumeropsis mannii, Cucurbita maxima, Cucurbita moschata, Lagenaria siceraria, Cucumis sativus (Achu et al. 2005), or Melothria pendula (Arazate-Fernández & Grenón-Cascales 2002), A. biflora has some features that make it a potential resource for food security and/or food industry. Its roots accumulate starch, are comestible and often used by the local population to feed livestock. Occasionally, though, they are also consumed by people who incorporate them as part of their meals. In addition, its fruit, a modified berry or pepo, contains approximately 20 seeds with significant amounts of extractable oil (Wilfredo Gonzáles, unpublished observations). Nevertheless, no studies addressing the chemical composition of the roots and seeds of A. biflora have been carried out to date.
An effort to make a full appraisal of the potentialities of A. biflora is being carried out in our laboratory, which includes studies on the phenology, reproductive biology, genetic diversity, and chemical composition of roots and seeds, taking advantage of a set of accessions from northwestern Peru. In the present article we show the relevant results on the chemical and nutritional value of A. biflora roots and seeds, emphasizing on the variability observed. The results are discussed with regard to the values reported for domesticated species of known nutritional and commercial importance.
Material and methods
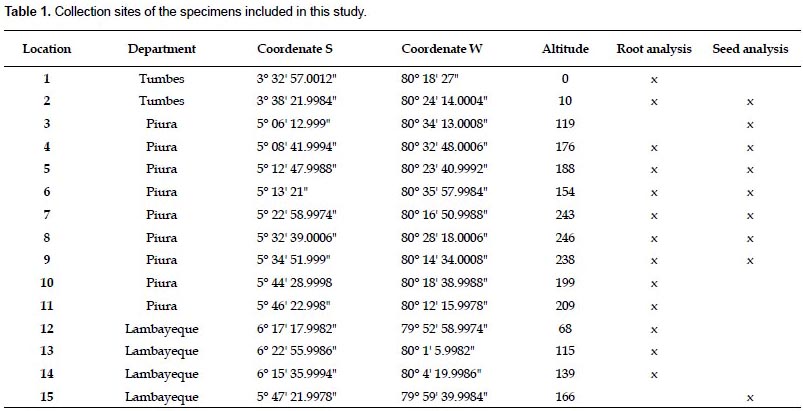
Samples.- Plant material was collected from 15 locations in the departments of Tumbes, Piura, and Lambayeque, in northwestern Peru (Table 1). The locations were at least 5 km away from each other. Approximately 10 kg/location of tuberous roots from wild adult plants were collected in Tumbes (locations 1 and 2), Piura (locations 4 - 11), and Lambayeque (locations 12 - 14) between May and June 2009, at the end of the wet season, when the local population harvest them. Seeds were obtained from fruits of wild adult plants in Tumbes (location 2), Piura (locations 3 - 9), and Lambayeque (location 15) in March 2009, when the fruits were mature. Both roots and seeds were stored in paper bags at room temperature until use.
Processing of root samples.- For each location, 2 kg of undamaged roots were washed thoroughly with running water and their cortex removed so as to leave the inner edible parts. The latter were washed with distilled water, chopped into small pieces, and ground in a food processor. The homogeneous paste was divided into portions of approximately 40 g that were accurately weighed and stored at -20 °C until analysis.
Processing of seed samples.- Clean seeds were first weighed and then desiccated in an oven at 103 °C for 17 h. After weighing the desiccated seeds, the dry matter content was calculated and the seeds ground in a mortar to determine the fat and protein content.
Determination of chemical composition of roots.-
Dry matter content: The dry matter content of the root paste was determined according to the AOAC Method 22.018 (AOAC 1980). Determinations were made in 6 replicates of 5 g each, 3 of which were utilized later to quantify the lipid content and the 3 remaining to determine the ash content. Results were expressed as percentage (w/w) of the samples fresh weight.
Ash content: The ash content was determined in triplicate using dehydrated samples (see previous section) according to AOAC Method 7.009 (AOAC 1980). Between 2 - 5 g of dehydrated material were used per replicate and the results expressed as percentage (w/w) of the samples dry weight.
Crude protein content: The crude protein content was measured in triplicate according to AOAC Method 47.021 (AOAC 1980), with 2 g of root paste per replicate. A standard conversion factor (6.25) was used to express the protein content as a percentage (w/w) of the samples dry weight.
Lipid content: The amount of lipids contained in the roots was determined in triplicate with previously dehydrated samples (see section 2.4.1.) according to AOAC Method 155 7.056 (AOAC 1980). Results were expressed as percentage (w/w) of the samples dry weight.
Crude fiber content: The crude fiber content was quantified in triplicate according to AOAC Method 7.061 (AOAC 1980). Two grams of defatted sample were used per replicate and the results were expressed as percentage (w/w) of the samples dry weight.
Available carbohydrate content: The percentage of available carbohydrate in roots was estimated by subtracting from 100 the sum of water, ash, protein, lipid, and crude fiber.
Ca and P contents: Samples from ashing were digested for 30 min with 6 mL of concentrated hydrochloric acid under heating. The digest volume was brought to 50 mL with double distilled water in a volumetric flask and used for Calcium and Phosphorus determinations in triplicate.
Calcium was quantified in 3 replicates by the permanganate titration method described by James (1999), with slight modifications (AOAC Method 7.096, 1980). Briefly, the diluted ash digest is brought to pH 4.5 and an excess of ammonium oxalate is added to precipitate calcium as calcium oxalate. After several washing rounds with 2% ammonium hydroxide, oxalic acid is liberated from the precipitate by adding sulfuric acid and titration of the acid is carried out afterwards with 0.01 M potassium permanganate at 70 °C to a persistent pink color. Results were expressed as milligrams of calcium per 100 g of dried sample.
Phosphorus was determined in 3 replicates with a HI 83225 photometer (Hanna Instruments) and phosphorus reagents HI 93706A-0, HI 93706B-0, and HI 93706-01 (HANNA Instruments) according to the manufacturers instructions. The kit is based on the amino acid method, adapted from Standard Method for the Examination of Water and Wastewater (1999). The reaction between phosphorus and the reagents gives a blue color in the sample. Results were expressed as milligrams of phosphorus per 100 g of dried sample.
Determination of protein, lipid content, and fatty acid composition of seeds.- Total protein and fat content in seeds were quantified in triplicate according to AOAC Methods 47.021 and 7.056, respectively (AOAC 1980) and expressed as percentage of dry matter. The fatty acid composition of the oil obtained was determined by gas chromatography in a HP 6890 apparatus (Agilent Technologies, Palo Alto, CA). Briefly, a capillary column Omegawax 100 (15 m, 0.10 mm i.d., 0.10 µm film thickness) was used for the separation of fatty acid methyl esters. The initial temperature was 140 °C for 5 min, raised to 280 °C at a rate of 40 °C/min, and kept at 280 °C for 2 min. The split ratio was 1:200 and the carrier gas hydrogen kept at a constant flow rate of 50 cm/s. The injector temperature was 250 °C and the detector temperature 280 °C. One µL of sample was injected and the running time was 60 min.
Statistical analysis.- To assess whether the chemical composition of the tuberous root is affected by the site of collection, a nested analysis of variance (ANOVA) was performed considering as factors the department (Lambayeque, Piura or Tumbes) and the location (nested in the department). Descriptive statistics were used to address the seeds chemical features of interest such as the fat and protein content, and the fatty acid composition of the oil obtained. The mean, range (minimum and maximum), and coefficient of variation (standard deviation/mean) are reported for each seed trait.
Results and discussion
Chemical composition of the root.- The results of the proximate analysis carried out with roots from 13 locations in northwestern Peru are given in Table 2. The content of calcium and phosphorus was also included. With the exception of the fiber content, all the parameters show some degree of variation at the department level (Table 3). It is worth noting the high percentage of fat and calcium content found in roots from Lambayeque as compared with those from Tumbes and Piura. The percentage of fat in Lambayeque is 2.3 - 2.5 fold higher than those in Tumbes and Piura, whereas the amount of calcium shows a 1.4 fold increase with respect to Tumbes (Table 2). Differences were also evident within departments at the location level, with fiber content as the sole exception again (Table 3). The reason for this apparent uniformity in the fiber content among accessions is unclear at present and a search in the scientific literature did not reveal a similar trend in other comestible storage roots such as cassava (Charles et al. 2005). Notable variations were observed among all the populations in the fat percentages (from 0.1 to 9.2%), as well as in the amounts of calcium (from 37.3 to 132.7%) and phosphorus (from 69 to 182.3%). All these variations were concentrated in Piura, with the exception of the fat percentage. Still, its variation in Piura was considerable (from 0.1% to 6.4%, Table 2). While part of this variability might be explained by phenotypic plasticity under different environmental conditions (precipitation, temperature, soil composition), genetic differences cannot be ruled out as an ISSR-based estimation of the genetic diversity in the sampled area resulted in a significant degree of population structure (unpublished results). The lower variability found in Tumbes and Lambayeque might have been determined by the smaller number of locations sampled.
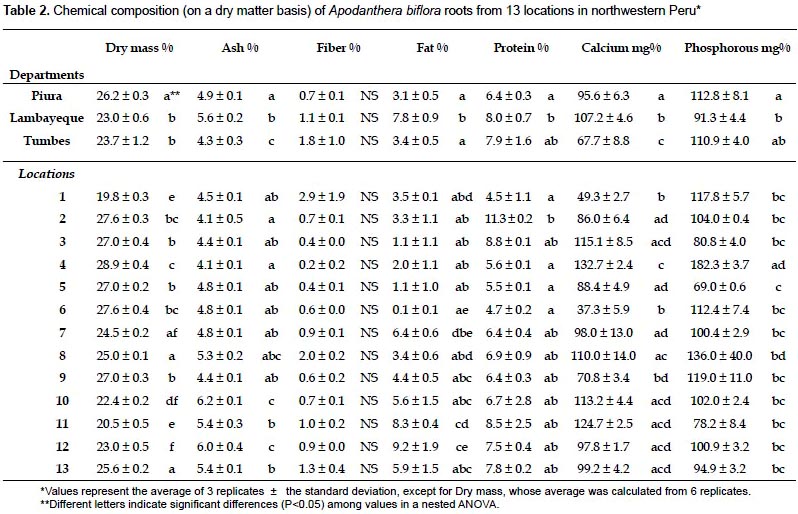
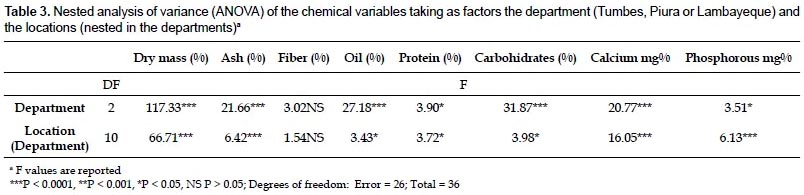
Interestingly, the carbohydrate content of A. biflora roots is high (77.5 - 84.9%) and comparable to those reported for cassava roots (94%; USDA, National Nutrient Database for Standard Reference) or potato tubers (86.94%; Jiménez 2005). This attribute is considered an important nutritional feature of comestible storage roots/tubers, and our results confirm the nutritional value of the A. biflora roots as a source of carbohydrates. Preliminary results with a few accessions indicated that starch represents approximately 20% of the dry matter, a percentage that is lower to that obtained with cassava (around 30%; Luna et al. 2006), but higher than the percentage reported for potato (13 - 15%; Lisinska & Leszczynski 1989). We are currently confirming these results by assessing further accessions, as well as characterizing the starch by physicochemical methods.
A somewhat unexpected finding was that, although low (3.1 -7.8%), the fat content is 4 - 11 fold higher than of cassava (USDA, National Nutrient Database for Standard Reference) and 2 - 5 fold that of potato (Jiménez 2005). As for the rest of attributes, the dry weight (23 - 26.2%) and the protein content (6.4 - 8%) are comparable to those found in potato (26.8% and 7.46%, respectively; Jiménez 2005), but clearly different from those in cassava (40.3% and 3.4%; USDA, National Nutrient Database for Standard Reference); ashes content were comparable to those of cassava or potato (Charles et al. 2005; Jiménez 2005); fiber (0.7 − 1.8%) was lower than values reported for cassava and potato (4.5% and 2.6%, respectively; USDA, National Nutrient Database for Standard Reference; Jiménez 2005); phosphorous levels (91.3 − 112.8 mg%) were between those found in cassava (66.9 mg%; USDA, National Nutrient Database for Standard Reference) and potato (194 mg%; Jiménez 2005), while calcium levels (67.7 - 107.2 mg%) were higher than those of cassava and potato (39.7 mg% and 22.4 mg%, respectively; USDA, National Nutrient Database for Standard Reference; Jiménez 2005).
Taken together, our results are consistent with the use of A. biflora root as a source of carbohydrates in the regular diet and its inclusion as part of food security programs, especially in northwestern Peru. Further testing of the starch is currently under way to assessing its potential for industrial applications.
Protein, lipid content, and fatty acid composition of seeds.- The protein and fat content of seeds from 9 locations sampled are shown in Table 4. The fat content in Piura was notably higher than that in Tumbes and Lambayeque, while the amount of protein tended to be higher inTumbes with respect to the other 2 departments (data not shown). However, the fact that only 1 accession from Tumbes and Lambayeque was evaluated does not allow drawing definitive conclusions. The range of fat content in all accessions (22.22 - 39.37%) falls within the values reported for other oily seeds, in between peanut (36 - 56%) and cotton (15 - 22%), and very similar to safflower (25 - 40%). The protein percentage (21.37 - 29.06%) is somewhat lower than that reported for soja (35 – 44%), and comparable to flax (20 – 24%) and sunflower (18 – 25%) (López Bellido 2002).
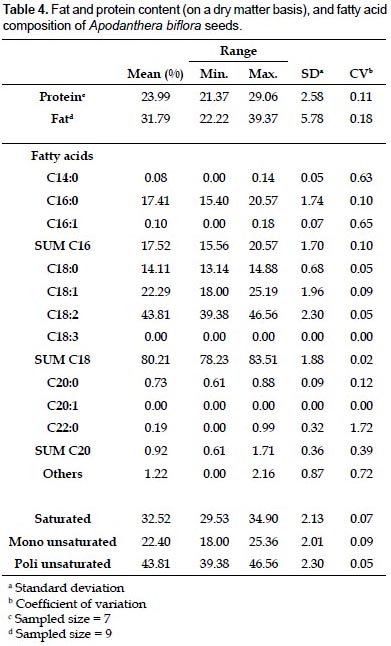
The fatty acid composition as determined by gas chromatography is shown in Table 4. There was a slight predominance of polyunsaturated over saturated fatty acids, whereas the monounsaturated were clearly the less abundant. When examining the individual fatty acid content, the percentage of linoleic acid (43.81%) stands out, followed by oleic (22.29%), palmitic acid (17.41%), and stearic acid (14.11%). The percentage of other fatty acids was below 1%, and therefore considered not significant. The overall composition of the oil is comparable to that of cotton (http://www.scientificpsychic.com/fitness/fattyacids1. html), with a strong dominance of n-6 over n-3 fatty acids. As a matter of fact, no linolenic acid could be detected in the oil of any of the accessions analyzed.
While the fat and fatty acid composition are compatible with the use of the A. biflora seed-derived oil for human consumption, the short life of the mature fruit in wild specimens makes difficult to build up sufficient starting material for an industrial approach.
Concluding remarks.- Because of its chemical composition, the root of A. biflora emerges as a useful resource for food security in arid areas such as the dry forest of northwestern Peru; in addition, its starch content opens the possibility of industrial applications whose viability is currently being assessed. The seed-derived oil also shows interesting characteristics for human consumption as a food additive, although the short life of the mature fruit curtails its production at an industrial scale. Domestication efforts might prove useful to overcome this limitation. In that regard, our investigations have revealed a phenotypic and genetic diversity in the population studied that is promising for the future development of a domestication program.
Acknowledgements
We thank Aníbal Calderón, Jenny Rojas, David de Las Casas, Luis Espinoza, Adán Yarlequé, Lucinda Prado, Yolmer Gutiérrez, Adan Zeña, Alberto Valdera, José Cedillo, Dante Alemán, the Comunidad Campesina José Ignacio Távara Pasapera (Chulucanas, Morropón), the Empresa Comunal de Servicios Agropecuarios "Cucungara" S.R.L. (Catacaos, Piura), and the Asociación para la conservación de la Biodiversidad y Desarrollo Sustentable de los Bosque Secos del Morante (ACBIODESA, Catacaos, Piura) for their assistance in the field labors. We also thank the Laboratory of Quality Control at Alicorp S.A. for their valuable assistance in the analysis of fatty acids, the Peruvian Ministry of Agriculture for authorizing sample collection (Permit N° 0058-2009-AG-DGFFS-DGEFFS), and the herbarium of the Universidad Peruana Cayetano Heredia where the specimens collected are preserved. This work was supported by the Programa de Ciencia y Tecnología (FINCyT), Contrato N°002-FINCyT-PIBAP-2009.
Literature cited
Achu M.B., E. Fokou, C. Tchiégang, M. Fotso & F.M. Tchouanguep. 2005. Nutritive value of some Cucurbitaceae oilseeds from different regions in Cameroon. African Journal of Biotechnology. (4): 1329-1334.
Aguirre Z., R. Linares-Palomino & L.P. Kvist. 2006. Woody species and vegetation formations in seasonally dry forests of Ecuador and Peru. Arnaldoa. (13): 324-346.
AOAC. 1980. Official methods of Analysis of AOAC, 13AOAC (1980). Official methods of Analysis of AOAC, 13th edition. Washington D.C, USA. Association of Official Analytical Chemists.
Arazate-Fernández A.M. & G.N. Grenón-Cascales. 2002. Contribución al conocimiento del pepinillo silvestre (Melothria pendula L.). Ciencia Ergo Sum. (9): 78-86.
Brack A. 1999. Diccionario Enciclopédico de plantas útiles del Perú. Cusco, Perú. Editorial Bartolomé de las Casas.
Bussmann R.W. & D. Sharon. 2006. Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture. Journal of Ethnobiology and Ethnomedicine. (2): 47-64.
Bussmann R.W. & D. Sharon. 2009. Shadows of the colonial past-diverging plant use in Northern Peru and Southern Ecuador. Journal of Ethnobiology and Ethnomedicine. (5): 4-20.
Charles A.L., K. Sriroth, & T. Huang. 2005. Proximal composition, mineral contents, hydrogen cyanide and phytic acid of 5 cassava genotypes. Food Chemistry. (92): 615-620.
Clescerl L.S., A.E. Greenberg, & A.D. Eaton, eds. 1999. Standard Method for the Examination of Water and Wastewater, 20th edition.
DeVeaux J.S. & E.B. Shultz. 1985. Development of Buffalo Gourd (Cucurbita foetidissima) as a semiaridland starch and oil crop. Economic Botany. (39): 454-472.
Ferreyra R. 1983. Los tipos de vegetación de la costa peruana.Anales Jardín Botánico de Madrid. (40): 241-256.
James C.S. 1999. Analytical chemistry of foods. Frederick, MD, USA. Aspen Publishers, Inc.
Jiménez F.S. 2005. Características nutricionales de la arracacha (Arracacia xanthorrhiza) y sus perspectivas en la alimentación. Red Peruana de Alimentación y Nutrición (r-PAN).
Jørgensen P.M. & S. León, eds. 1999. Catalogue of the vascular plants of Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden. 75. St. Louis, MO, USA: Missouri Botanical Garden.
Lisinska G. & W. Leszczynski. 1989. Potato Science and Technology. London, New York. Elsevier Science Publishers Ltd.
López-Bellido L. 2002. Cultivos Industriales. Barcelona, España. Mundi-Prensa.
Luna W.A. & J.A. Mera. 2006. Producción de dextrinas de yuca a partir de almidón nativo en la rallandería Todoyuca ubicada en el corregimiento Pescador (municipio de Caldono, Cauca). BEng Thesis, Universidad del Cauca, Colombia.
Macbride J.F. 1937. Cucurbitaceae Flora of Peru. Field Museum of Natural History, Botanical Series. (13)6/2: 321-383.
Mostacero L.J., F.C. Mejía & O.T. Gamarra. 2002. Taxonomía de las fanerógamas útiles del Perú. Concytec. (1): 538-539.
Pennington R.T., D.E. Prado, & C.A. Pendry. 2000. Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography. (27): 261-273.
Svenson H.K. 1946. Vegetation of the coast of Ecuador and Peru and its relation to that of the Galapagos Islands. II Catalog of plants. American Journal of Botany. (33): 427-498.
USDA, National Nutrient Database for Standard Reference, Release 25 (online). Basic Report, Nutrient data for 11134, Cassava, raw. <http://ndb.nal.usda.gov/ndb/foods/show/2892> [acceso: 29/10/2012]
*Autor para correspondencia.
Facultad de Ciencias y Filosofía, Universidad Peruana Cayetano Heredia. Avenida Honorio Delgado 430, San Martín de Porres, (Apartado Postal 4314, Lima 100), Perú.
Email Daniel Clark: daniel.clark@upch.pe
Email Maribel Tupa: trdu7@yahoo.es
Email Andrea Bazán: maryandrea17@hotmail.com
Email Lily Chang: lily.chang@upch.pe
Email Wilfredo Gonzáles: wilfredo.gonzales@upch.pe
Presentado: 17/02/2012
Aceptado: 11/08/2012
Publicado online: 10/11/2012













