versión On-line ISSN 1727-9933
Rev. peru biol. vol.20 no.2 Lima ago. 2013
TRABAJOS ORIGINALES
Zooplankton composition in five oxbow lakes from the Upper Juruá River, Acre State, Brazil
Composición del zooplancton en cinco lagos de la parte alta del río Juruá, Estado de Acre, Brasil
Maria José Alencar dos Santos1, Erlei Cassiano Keppeler1, Lisandro Juno Soares Vieira2, Alzenira Jacob Serra1 and Antonio Sergio Ferraudo3
1 Federal University of Acre, Forest Campus – Water analyses and Microbiology laboratories, Cruzeiro do Sul, AC, Brazil. CEP 69980-000. Email Erlei Cassiano Keppeler: erleikeppeler@gmail.com
2 Federal University of Acre, Rio Branco Campus - Center for Biological Sciences and Nature, BR 364 km 04 - Industrial District - Rio Branco, Acre, Brazil. 69915-900
3 São Paulo State University -Faculty of Agriculture and Veterinary Sciences -Jaboticabal, SP, Brazil. CEP 14870-000
Abstract This work was conducted in five oxbow lakes located between Cruzeiro do Sul and Rodrigues Alves counties (Acre State, Brazil), to provide additional information about the composition of zooplankton assemblages in the Upper Juruá River. Samples were collected from May 2009 to May 2010, and fixed with 4% formalina. The numeric density (ind.m-3) was obtained from subsequent sub-samples (1 mL). The study recorded 19 zooplankton families. Rotifers showed higher species richness (81 species), followed by cladocerans (3 species) and various forms of copepods and other organisms. Higher zooplankton means of numeric density was found in Novo Lake, with rotifers (1879 ind.m-3), cladocerans (207 ind.m-3), copepods (870 ind.m-3). Diversity and numeric density were similar to other Neotropical aquatic ecosystems.
Keywords: Rotifera, diversity, numerical density, oxbow lake, Neotropical.
Resumen
Este estudio se realizó en cinco lagos, situados entre Cruzeiro do Sul y Rodrigues Alves, y proporcionan información sobre la composición de las comunidades del zooplancton de la parte alta del Río Juruá. Se realizaron muestreos mensuales desde mayo de 2009 a mayo de 2010. El material se recolecto con una red de plancton con abertura de malla de 55 µm de malla. Fueron observadas 19 familias zooplancton. Los rotíferos tuvieron la mayor riqueza de especies (81), seguido por los cladóceros (3) y copépodos y otros organismos. La mayor densidad promedio se observó en el lago Novo, con 1879 ind.m-3 de rotíferos, 207 ind.m-3 de cladóceros, y 870 ind.m-3 de copépodos. La diversidad y la densidad numérica fueron similares a otros ecosistemas acuáticos del Neotrópico.
Palabras clave: rotíferos, diversidad, densidad numérica, lago marginal, neotropical.
The inland waters of the world present, in total, about 1570 species of rotifers (Segers, 2007b), 620 species of Cladocera (Forró et al. 2008) and 2814 species of Copepoda (Boxshall and Defaye, 2007). In Brazil, 800 species of Rotifera (Souza-Soares et al. 2011), 142 of Cladocera (Smith et al. 2011) and 124 of Copepoda (Silva & Matsumura-Tundisi 2011, Matsumura-Tundisi & Tundisi 2011) have been recorded. In the Amazon, a total of 300 species of these groups have been found (Robertson & Hardy 1984, Keppeler & Gomes 2012).
Organisms are distributed heterogeneously in space and time, interacting with abiotic and biotic factors, and these interactions depend on contingencies (Scheiner & Willig 2008). This distribution is associated with severe costs and benefits, such as the occurrence of predation, optimal feeding and environmental conditions (Iglesias et al. 2007). These interactions can structure local communities (Cottenie et al. 2003) of zooplankton, especially in lakes (José de Paggi & Devercelli 2011).
River dynamics, including the formation and extinction of oxbow lakes, are associated with the dynamic work of the rivers (hydro-sedimentation). This way, erosion processes are continuous, although within the limits of the floodplain, and these lakes present high biodiversity, including rare and endemic species (Wantzen et al. 2008).
The present diversity of zooplankton species may be directly related to the large spatial heterogeneity of the floodplains and to seasonal changes in water level fluctuation. These two characteristics favor the occupation of different floodplain habitats, which remain isolated or connected according to the season, thereby providing an exchange of species between the water systems (Lansac-Tôha et al. 2004).
The Juruá river presents in its course several oxbow lakes that are lakes shaped like a horseshoe. According to Junk (2001), the channel of the river acts as axis of migration, local dispersal routes and as refuge for organisms in the period of low waters. The lower courses of very large rivers often represent a fairly homogeneous area with a low number of habitats specific to lotic species that depend partly, on the input of organic matter from the floodplain. The degree to which these water level fluctuations influence the lake ecosystems depends deeply on the morphology of the lake, so that shallow lakes or with large shallow margins are the most sensitive.
This paper presents new information on the species richness and numeric density of zooplankton assemblages in five oxbow lakes of the Upper Juruá River.
Material and methods
Study area.- The five lakes studied are oxbow lakes connected to the Juruá river by side channel spillways, located in the municipalities of Cruzeiro do Sul and Rodrigues Alves, Acre State, Brazil (Fig. 1). Novo Lake (7°4401S; 72°3756W ) has a length of 1690 m and maximum width of 120 m, Verde Lake (7°5002S; 72°3821W) has a length of 3370 m and maximum width of 130 m, St. Elias Lake (7°4619S; 72°3637W ) has a length of 2730 m and maximum width of 140 m, Moju Lake (7°4851S; 72°3637W ) has a length of 5040 m and maximum width of 180 m, and the Cigana Lake (7°3415S; 72°3757 W ) has a length of 2820 m and maximum width of 170 m.
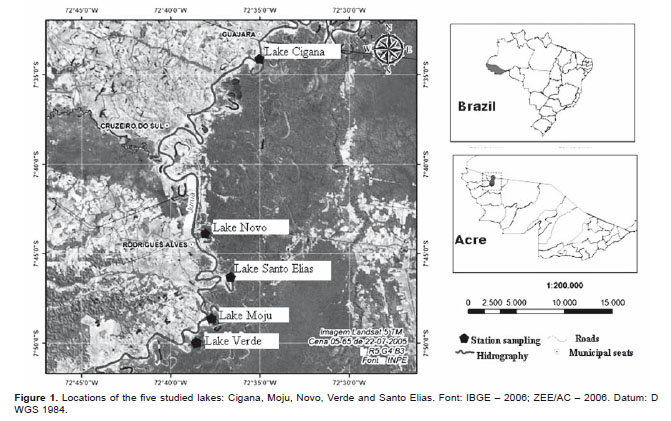
Methods.- Samples were collected trimonthly between May 2009 and May 2010, in littoral and pelagic regions of each lake. However, was carry out at the beginning of the samplings a t-test analysis between the two zones (pelagic and littoral), as there was no statistical significance (p <0.05), and a low number density, we chose to consider only a collection point.
Each zooplankton sample was obtained by the filtration of 200 liters of water in a plankton net (55 μm mesh-size), followed by fixation with 4% formalin.
Species richness of the zooplankton assemblage was determined using optical microscopy, and species identification was based on Koste (1978), Koste and Robertson (1983), Elmor-Loureiro (1997) and Korovchinsky (1992). The frequency of occurrence (FO) was calculated as FO = O x 100/TO, O is the number of occurrences of the category in analysis, and TO is the total number of occurrences of all categories (Zar 1996).
For determining numerical densities (ind.m-3), ten subsequent subsamples (1 mL) were collected with a pipette and analyzed in Sedgewick Rafter Counting Chambers, in which were counted at least 80 individuals from each sample. In samples with low density, individuals were counted in full. Data quantitative were expressed as mean and standard deviation.
This study was conducted with authorization of the Chico Mendes Institute for Biodiversity-ICMBio, Biodiversity Information System (SISBIO), under the license number 13435-2. The collected material is deposited in the collection of the Federal University of Acre.
Statistical Analysis.- The characterization regarding the composition of the five lakes was performed using hierarchical cluster analysis, adopting the Euclidean distance as the coefficient of similarity between the lakes and Ward's algorithm as a method of connection between the groups and the component analysis, resizing the initial set into smaller ones and preserving the variance considered relevant. The new dimensions are formed by the eigenvectors generated from eigenvalues of the original covariance matrix (Hair et al. 2005). All analyzes were processed in the statistical software Statistica, version 7.0, by Statsoft (Statsoft 2004).
Temporal analysis using curve tests for all lakes were conducted in order to find the best fit for them.
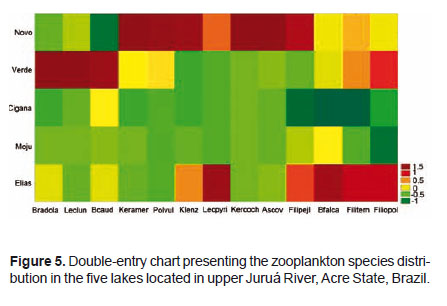
Table 1 shows the results regarding the zooplankton composition in the lakes Novo, Verde, Cigana, Moju and St. Elias. The five lakes presented 81 species of rotifers, three species of cladocerans, and various forms of copepods and other organisms. The zooplankton composition of the five lakes presented 19 families (Fig. 2). Rotifers predominated and were distributed in 15 families (Table 1): the most frequent genera of Rotifera in this study were Lecane and Brachionus.
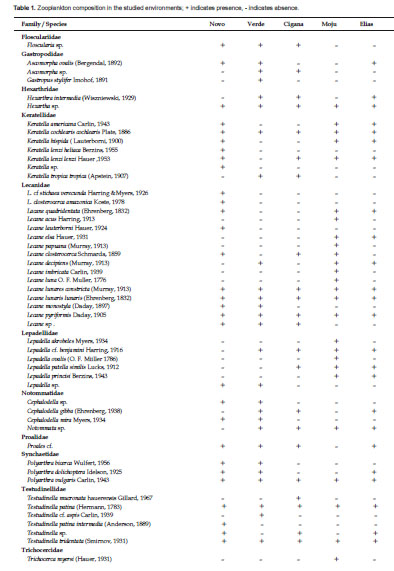
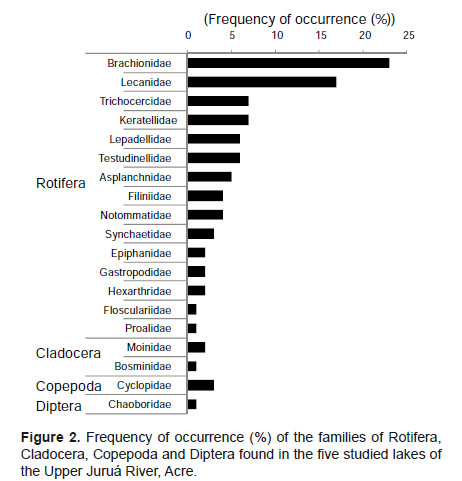
Cladocerans were represented only by Bosminidae (1 sp.) and Moinidae (2 spp.) and occurred in all lakes, except Moina sp., that did not occur in the Novo Lake, and Bosminopsis deitersi, absent in the lakes Cigana and Moju.
The rotifers Asplanchna cf. herrickii, Brachionus budapestenen sis, Keratella lenzi heliaca, Keratella sp., Lecane cf. stichae verecunda, L. closterocerca amazonica, L. lauterboni, and Testudinella tridentata were recorded only in the Novo Lake; Asplanchna brightwelli, Platyas quadricornis, Gastropus stylifer, Testudinella cf. aspis, and Trichocerca chattoni, only in the Verde Lake; Testudinella mucronata hauerensis occurred only in the Cigana Lake, while Epiphanes sp., Lecane acus, L. papuana, L. imbricata , L. luna, Lepadella acrobeles, L. ovalis, Trichocerca myersi, and T. bidens occurred only in the Moju Lake. Cladocerans appeared in more than one lake, but Moina cf. minuta was the only species present in all lakes. As for copepods, only the larval forms (nauplius and copepodite) were frequent and appeared in the five studied environments.
This study found five occurrences new to the Acre State: Asplanchna cf. herricki (Novo Lake), Anuraeopsis coelata (Lakes Novo and Verde) Testudinella cf. aspis (Verde Lake), Floscularia sp. (Lakes Novo, Verde and Cigana), and Lepadella acrobeles (Moju Lake).
As for frequency of occurrence, the most frequent species in the Novo Lake was Brachionus falcatus (100%), followed by Notomata (75%). On the other hand, in the Verde Lake the most frequent species was Brachionus caudatus (75%). In the Cigana Lake, the most frequent species were Notomata (75%), Trichocerca similis (75%), Polyarthra vulgaris (100%), and young forms of copepodites. In the Moju Lake, the most frequent species was Brachionus caudatus (100%), Brachionus dolabratus , and Brachionus falcatus (75%). In St. Elias Lake, the most frequent species were Brachionus caudatus (100%), Brachionus dolabratus (100%), Notomata (100%), Polyarthra vulgaris (100%) and Brachionus falcatus (75%).
The Novo Lake showed the highest density of organisms, with average and standard deviation of 1879±3166 ind.m-3 of Rotifera, Cladocera (207±412 ind.m-3), Copepoda (870±44 ind.m-3) and Chaoboridae (0 ± 1.0 ind.m-3). The Verde Lake presented highest density of Rotifera (1357±1917 ind.m-3), followed by Cladocera (275 ± 550 ind.m-3), Copepoda (106 ± 212 ind.m-3) and Chaoboridae (94 ± 187 ind.m-3). Lake St. Elias presented Rotifera as the highest density (1040 ± 687 ind.m-3), followed by Copepoda (41±73 ind.m-3) and Cladocera (3 ± 6 ind.m-3).The Moju Lake totaled 1687±201 ind.m -3 of Rotifera, Copepoda (14±13 ind.m3) and Cladocera (5 ± 10 ind.m-3) ind.m3), Copepoda (13.0 ± 12 ind.m-3), and Chaoboridae (20 ± 28). The Cigana Lake had the lowest density of individuals, with Rotifera being the highest density (407 ± 108 ind.m3) followed by Copepoda (10 ± 8), Cladocera (4 ± 5 ind.m-3) and Chaoboridae (12 ± ind.m-3).
The species with the largest number of individuals were the rotifers Keratella cochlearis, Polyarthra vulgaris and Trichocerca similis.
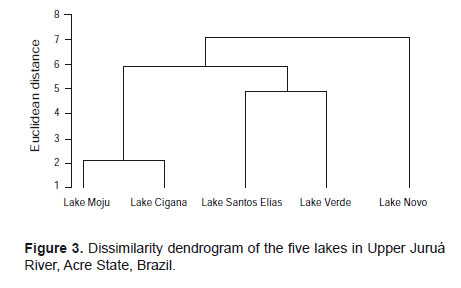
The dendrogram in Figure 3 shows a group structure of the studied lakes, considering the most abundant species.
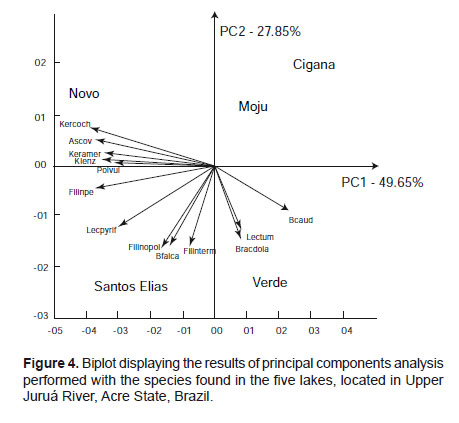
The species with the highest discriminatory power in the first principal component were Keratella lenzi (-0.99), Filinia pjeleri (-0.95) and Ascomorpha ovalis (-0.91), located to the left of the biplot graph (Fig. 4), while the species discriminated in the second principal component were Notomata (-0.92) and Filinia opoliensis (-0.82) (Figs. 4 and 5).
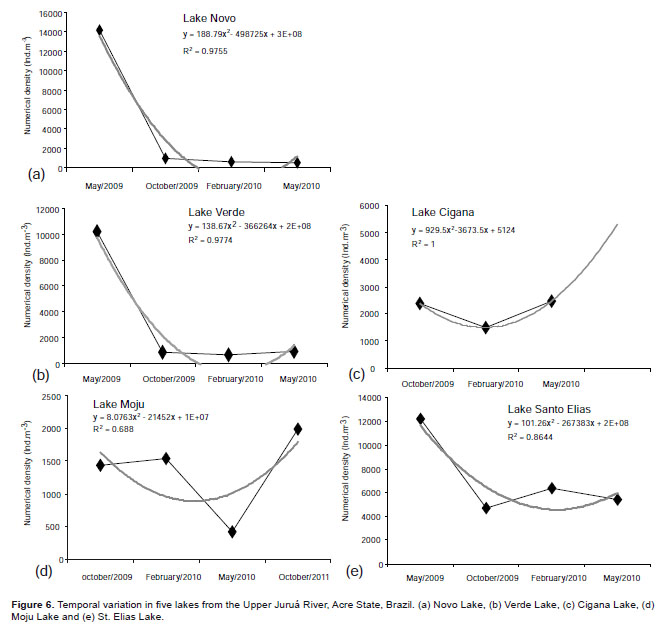
The temporal variation of each lake is shown in Figure 6 (a, b, c, d, e). It is noted that there showed pattern of variation, fitting a polynomial curve.
Discussion
Rotifera was the dominant phylum in the five lakes studied. The most frequent families in this study were Lecanide, Lepadellidae, Keratellidae, Testudinellidae and Trichocercidae. These families were also present in the studies by Pecorari et al. (2006) and José de Paggi and Devercelli (2011), in the floodplain of the Middle Paraná River, and lakes of Amazonia (Robertson & Hardy 1984).
The most common genus of Rotifera in this study was Lecane, which was likewise the most diverse genus in the Santa Fe Lake, Argentina (José de Paggi & Devercelli 2011), and in the study by Oliveira et al. (2010) in the small Preto River and in an artificial pond in Cruzeiro do Sul (Acre), proving it to be a genus with wide geographic distribution, occurring in tropical and subtropical regions.
The genus Brachionus (Brachionidae), considered endemic in the Neotropics, as well, the most frequent in three urban lakes in João Pessoa (PB) (José de Paggi & Paggi 2007). The most frequent species found by this research were Brachionus falcatus, Brachionus dolabratus, Notomata sp., and Polyarthra vulgaris. In general, they did not appear to be restricted to only one of the lakes, occurring commonly in oxbow lakes, as in lakes Pirapora and Amapá (Keppeler 2003, Keppeler & Hardy 2004). Brachionus falcatus also has records in the Amazon rivers, such as the Solimões, Amazonas, White, Negro, Tapajós, Tocantins, and Nhamundá (Robertson & Hardy 1984). Brachionus dolabratus was recorded in the rivers Solimões, Madeira and Tapajós (Robertson & Hardy 1984)
Asplanchna cf. herrickii and Anuraeopsis coelata are also mentioned in African tropical regions, Australia, the Neotropics, Nearctic, East and paleo-Arctic region (Segers 2007), being considered cosmopolitan. Testudinella cf. aspis has not been mentioned in the Neotropics, while Lepadella akrobeles was found in the Brígida River in Pernambuco (Melo Júnior et al. 2007).
The species with the largest number of individuals were Keratella cochlearis, Polyarthra vulgaris and Trichocerca similis, which also showed a high numerical density in the lakes Pirapora and Amapá (Keppeler 2003, Keppeler & Hardy 2004). These organisms were also abundant in other ecosystems of Brazil, such as the Alto Tietê River (Lucinda et al. 2004), a reservoir in Curitiba (Serafim-Júnior et al. 2010), and in sub-tropical and tropical areas, like the limnic ecosystems of Pernambuco (Melo Júnior et al. 2007), which are tropical.
The studied lakes, in general, showed distinct compositions, with only the Lakes Moju and Cigana showing high similarity. The Novo Lake showed a different pattern regarding the species found, since there is a division separating the lakes Moju and Cigana from St. Elias and Verde. This separation was also evident in principal component analysis considering the first two components, managing to retain 77.5% of the original variation. This difference in lakes is possibly associated with the migration of species from the river for the lakes or contrariwise.
Several species showed high discriminatory power: Keratella lenzi, Filinia pjeleri, Ascomorpha ovalis (factor 1) Notomata and Filinia opoliensis (factor 2). Keratella lenzi was also dominant in the oxbow lake Massacará, originated from the Paraopeba River, São Francisco Basin in Minas Gerais (Sampaio & López 2000), as well as Ascomorpha ovalis in the Sapucaí River, Furnas reservoir, also in Minas Gerais. Filinia pjeleri was also the dominant species in the Amapá Lake, Acre (Keppeler & Hardy 2004). In the same lake were also dominant Filinia opoliensis, which were also dominant in the Brôa Reservoir (Rodriguez & Matsumura-Tundisi 2000).
The richness and density of zooplankton species in the studied lakes is equivalent to what is usually observed in other tropical and sub-tropical systems, showing the wide geographical distribution presented by the Rotifera.
Species richness was different between the lakes, because of the combination of a number of factors and conditions. This could have been due to differences in morphology, considering size and width. This influences the migration of species from rivers to lakes and vice versa. Another factor that contributed to species richness was the low presence of Chaoboridae, zooplankton predators. This was observed in the steady dominance of small rotifers.
The organisms are not only heterogeneously distributed in space, but also in time. Each lake had its peculiarities, presenting a distinct composition and dominance. Only 7% of all organisms occurred in 75 to 100% of the samples, which is possibly associated with species migration from the river to the lake.
Acknowledgement
The authors thank the Brazilian Ministry of Fisheries and Aquaculture (Senate budget amendment), the Secretary of Agriculture of the State of Acre, Cruzeiro do Sul office, for their support on collections, and to technician William Monteiro Ayache for his support for carry out samplings in the field. Scholarship to the students were provides by CNPq/UFAC for the develop of work.
Boxshall G.A. & D. Defaye. 2007. Global diversity of copepods (Crustacea, Copepoda) in Freshwater. Hydrobiologia 595 (1): 195 – 207. [ Links ]
Cottenie K., E. Michels, N. Nuyten & L. Meester. 2003. Zooplankton metacommunity structure: regional vs. Local processes in highly interconnected ponds. Ecology, 84(4): 991-1000. [ Links ]
Elmor-Loureiro L.M.A. 1997. Manual de identificação de Cladóceros límnicos do Brasil. Brasilia, Universa. [ Links ]
Forró L., N.M. Korovchinsky, A.A. Kotov et al. 2008. Global diversity of freshwaters of cladocerans (Cladocera;. Crustacea) in freshwater. Hydrobiologia 595(1): 195–207. [ Links ]
Hair J.F., R.E. Anderson, R.L. Tatham, et al. 2005. Análise Multivariada de Dados. 5. ed. Porto Alegre, Bookman. [ Links ]
José de Paggi S. & J. Paggi, 2007. Zooplankton. In The Middle Paraná River: Limnology of a Subtropical Wetland. (M.H. Iriondo, Paggi, J. C. Parma, M. J., Orgs.). Berlin, Springer-Verlag Heidelberg, p. 229-249. [ Links ]
José de Paggi, S, & Devercelli, M. 2011. Land use and basin characteristics determine the composition and abundance of the microzooplankton. Water Air Soil Pollut, 218(1/4): 93-108. [ Links ]
Keppeler E.C. 2003. Estudo comparativo da composição do zooplâncton de dois excossistemas laxcustres da Amazônia Sul-Ocidental. Acta Scientiarum. Biological Sciences (Online). 25 (2): 467-477. [ Links ]
Keppeler E.C. & E.R. Hardy. 2004. Abundance and composition of Rotifera in an abandoned meander lake (Lago Amapá) in Rio Branco, Acre, Brazil. Revista Brasileira de Zoologia 21 (2): 233-241. [ Links ]
Keppeler E.C. & B.M. Gomes. 2012. Deforestation in Amazonia and its consequences for ecosystems and biodiversity. In Annual Conference of the society for tropical ecology: Islands in land - and seascape: The Challenges of Fragmentation (Society for tropical ecology Org.), Erlangen, http://www.gtoe-conference.de/download/gtoe2012AbstractBook.pdf . Acesso em 02/02/2012.
Korovchisky N.M. 1992. Sididae and Holopedidae (Crustacea: Daphniformes); Brackhys Publishers, Leiden. [ Links ]
Koste W. 1978. Rotatoria Die Rädertiere Mitteleuropas bergründet von Max Voigt-Monogononta. 2. Auflage neubearbeitet von Walter Koste. Berlin, Gebrüder Borntraeger. [ Links ]
Koste W. & B. Robertson. 1983. Studies of the Rotifera ( Phylum Aschelminthes), from a Central Amazonian Varzea lake, Lago Camaleão (Ilha da Marchantaria, Rio Solimões, Amazonas. Amazoniana, 8(2): 225-254. [ Links ]
Lansac-Tôha F.A., C.C. Bonecker, L.F.M. Velho, et al. 2004. Zooplankton in the Upper Paraná River floodplain: composition, richness, abundance and relationships with the hydrological level and the connectivity. In: Agostinho A.A., L. Rodrigues, L.C. Gomes, et al. (Orgs.) Structure and functioning of the Paraná River and its floodplain: LTER-Site 6 (PELD- Sítio 6). 1ª.ed. Maringá, EDUEM, p. 75-89. [ Links ]
Iglesias C., N.G. Goyenela, N. Mazzeo, et al. 2007. Horizontal dynamics of zooplankton in subtropical lake blanca (Uruguay) hosting multiple zooplankton predators and aquatic plant refuges. Hydrobiologia 584 (1):179-189. [ Links ]
Junk W.J. 2001. The flood pulse concept of large rivers: learning from the tropics. Verh. Internat. Verein. Limnol. 27(7):3950-3953. [ Links ]
Lucinda I., I.H. Moreno, M.G. Melão, et al. 2004. Rotifers in freshwater habitats in the Upper Tietê River Basin, São Paulo State, Brazil. Acta Limnol. Bras. 16 (3): 203-224. [ Links ]
Matsumura-Tundisi T. & J.G. Tundisi. 2011. Checklist dos Copepoda Calanoida de água doce do Estado de São Paulo, Brasil, Brasil. Biota Neotropica 11(suppl.1): 551-557
Silva W.M. da & T. Matsumura-Tundisi. 2011. Checklist of fresh-water free living Copepoda Cyclopoida from São Paulo State, Brazil. Biota Neotropica 11(suppl.1): 559-569 [ Links ]
Melo Júnior M., V.L. dos S. Almeida, S.M.N. Neumann-Leitão, et al. 2007. O estudo da arte da biodiversidade de Rotiferos planctônicos de ecos-sistemas límnicos de Pernambuco. Biota Neotropical 7 (3): 109-117. [ Links ]
Oliveira E.A., J.S. Freitas, E.C. Keppeler, et al. 2010. Zooplâncton de dois ecossistemas aquáticos rasos, em áreas de incidência do Anopheles darlingi, no noroeste do Estado do Acre. Ensaios e ciências. Ciências Biológicas, Agrárias e da saúde 14 (2): 71-81 . [ Links ]
Pecorari S., S. José de Paggi & J.C. Paggi. 2006. Assessment of the urbanization effects on a lake by zooplankton. Water resources. 33(6): 677-685. [ Links ]
Robertson B.A. & E.R. Hardy. 1984. Zooplankton of Amazonian lakes and rivers. In: H. Sioli (Org.) The Amazon Limnologie and landscape ecology of a mighty tropical river and its basin. Dordrecht, Dr. W. Junk Publishers, p. 337-352. [ Links ]
Rodriguez M.P. & T. Matsumura-Tundisi. 2000. Variation of density, species composition and dominance of Rotifers at a shallow tropical reservoir (Broa reservoir, SP, Brazil) in a short scale time. Revista Brasileira de Biologia, 60(1):1-9. [ Links ]
Sampaio E. V. & C. M. López. Zooplankton Community Composition and Some Limnological Aspects of an Oxbow Lake of the Paraopeba River, São Francisco River Basin, Minas Gerais, Brazil. Braz. Arch. Biol. Technol. 43(3):285-293. [ Links ]
Scheiner S.M. & M.R. Willig. 2008. A general theory of ecology. Theor Ecol 1: 21-28 [ Links ]
Segers H. 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa. 1564: 1-104p. [ Links ]
Serafim-Júnior M., G. Perbicher-Neves, A.R. de Brito, et al. 2010. Variação espaço – temporal de Rotifero em um reservatório eutrofizado no sul do Brasil. Iheringia, Sér. Zoología, 100 (3): 233- 241. [ Links ]
Segers H. 2007. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595 (1): 45 – 59. [ Links ]
Souza-Soares F., J.G. Tundisi, & T. Matsumura-Tundisi. 2011. Checklist de Rotifera de agua doce do Estado de São Paulo, Brasil. Biota Neotropica, 11(1a):1-25 [ Links ]
Statsoft. 2004. Statistica, version 6.0. Statsoft Company. [ Links ]
Zar J.H. 1996. Biostatistical analysis. Third editions Prentice-Hall International Editions, New Jersey. 662p.
Wantzen K.M., W.J. Junk & K.O. Rothhaupt. 2008. An extension of the floodpulse concept (FPC) for lakes. Hydrobiologia, 613:151-170. [ Links ]
Presentado: 18/06/2013
Aceptado: 08/10/2013
Publicado online: 09/12/2013
 Citado por SciELO
Citado por SciELO  Similares en
SciELO
Similares en
SciELO  uBio
uBio 



















