Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. vol.24 no.3 Lima set./dic. 2017
http://dx.doi.org/10.15381/rpb.v24i3.13903
TRABAJOS ORIGINALES
A New Species of Dodecacius Schwarz (Coleoptera: Elateridae) from Madre de Dios, Peru
Una nueva especie de Dodecacius Schwarz (Coleoptera: Elateridae) de Madre de Dios, Perú
Paul J. Johnson
Insect Biodiversity Lab, Box 2207A, South Dakota State University, Brookings, South Dakota 57007, U.S.A. Email: paul.johnson@sdstate.edu
Abstract
Dodecacius Schwarz is reviewed, it includes two species known only from the eastern lower slopes of the Andes and adjacent Amazonia in southeastern Peru. Dodecacius paititi new species is described. Dodecacius testaceus Schwarz is treated as a new synonym of D. nigricollis Schwarz.
Keywords: taxonomy; endemic; Andes; Amazonia; species discovery.
Resumen
El género Dodecacius Schwarz es revisado, incluye dos especies conocidas solamente de las laderas orientales bajas de los Andes y la Amazonia adyacente en el sureste de Perú. Se describe la nueva especie Dodecacius paititi y Dodecacius testaceus Schwarz es considerado como un nuevo sinónimo de D. nigricollis Schwarz.
Palabras clave: taxonomía; endemismo; Andes; Amazonia; descubrimiento de especies.
Introduction
Dodecacius Schwarz (1902) was originally described with two species, D. nigricollis Schwarz and D. testaceus Schwarz, and each apparently represented by a single specimen. These species were the only ones listed in the genus since their description. Hyslop (1921) designated D. testaceus as the type species, and reiterated Schwarz’s (1902) suggestion that D. nigricollis may be the female and a color form of D. testaceus. Both specimens were collected at Chanchamayo, Peru. Since the original description of the genus and these two species, all of these taxa and specimens are rarely mentioned in the taxonomic literature, and generally remained unstudied. The recent discovery of an undescribed species attributable to Dodecacius allows this brief review.
The Coleoptera of the Andean eastern slopes, foothills and adjacent lowlands in southeastern Peru were sampled in the Beetles of Peru project (Chaboo 2015, Chaboo and Catenazzi 2015). The specimens reported below were collected at the Centro de Investigación y Capacitación Rio Los Amigos (CICRA), or Los Amigos Biological Station, along the Rio Madre de Dios, approximately 100 air kilometers west of Puerto Maldonado. The biological station is located adjacent to the Los Amigos Conservation Concession and the Manu-Tambopata Corridor between Manu National Park and Tambopata National Reserve. The area is an example of lowland Amazon rainforest with low topographic relief and high water tables near to the eastern foothills of the Andes. The new Interoceanic Highway traverses the region from Brazil to Puerto Maldonado, then to Cusco and three Pacific ports, and enables increased incursions and alterations to forest and riverine habitats.
A number of elateriform beetles were collected during the Beetles of Peru project. Many new country records, validations of original records, and undescribed species of Elateridae were obtained. A preliminary checklist of taxa for the country was produced (Johnson and Chaboo 2015) as a part of this work. Specimens representing undescribed species and new country records of Cerophytidae and Throscidae were also reported (Chaboo and Johnson 2015, Johnson and Chaboo 2016).
Here, reporting on these discoveries continues with the taxonomic treatment of unusual specimens. A new species of Dodecacius is described and a new synonymy is given.
Materials and Methods
All specimens of the new species described below are derived from sampling that was part of the Beetles of Peru project and are held at the Snow Entomological Museum (SEMC), University of Kansas, Lawrence, and were collected under Peru research permits No. 506-2011-AG-DGFFS-DGEFFS and No. 0159-2010-AG-DGFFS-DGEFFS to C.S. Chaboo. The holotype and half of the paratypes, plus unique specimens, will be repatriated to the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Peru (MUSM) by the SEMC under the permit agreement. Remaining duplicate specimens will be at the SEMC, except six paratypes retained by the author (PJJC).
The holotype of D. nigricollis was studied at the Senckenberg Deutsches Entomologisches Institut, Müncheberg, which contains the Otto Schwarz collection. The holotype of D. testaceus was not found and remains lost. No other specimens of either D. nigricollis or D. testaceus are known.
Morphological terms and concepts generally follow Calder (1996) and Lawrence et al. (2010). Integument color terms are based on Nichols and Schuh (1989). Measurements were made with an ocular micrometer at 0.1 and 0.01 mm increments between 10–50 magnifications. Body length was measured from the anterior margin of the frons to the elytral apices, and width was measured across the elytral humeri. The ocular index of Campbell and Marshall (1964) and Fender (1972), a ratio of frons width and head width inclusive of the eyes, was calculated to two decimal places. Antennomere length ratios were calculated for flagellomeres 2–12 as measured along the lateral midline from antennomere base to apex, and values were rounded to one decimal place. Ramus length was measured from the oblique basal angle to apex. Pronotal length is along the midline from anterior margin to the posterior margin at the antescutellar emargination, and width is at the widest point at midlength. Tarsomere lengths were measured from base to apex, values rounded to two decimal places, and given as a ratio string. Label data are generally presented verbatim, except dates are standardized to the dd.mm.yyyy format, with the month in lower case Roman type.
Systematics
Family Elateridae Leach, 1815
Genus Dodecacius Schwarz, 1902
Dodecacius Schwarz
Dodecacius Schwarz 1902: 153, 1907: 2; Hyslop 1921:
641, 1923: 157; Schenkling 1927: 3; Stibick 1979: 176;
Gaedike 1985: 90
Type species: Dodecacius testaceus Schwarz 1902: 154,
designated by Hyslop 1921: 641
Dodecanius Blackwelder 1944: 280 (misspelling)
The genus was described as new with D. nigricollis and D. testaceus included. It was originally assigned to Campylides of Candèze (1863, 1891) and compared to Euthysanius LeConte due to the strongly arched prosternal process and a pectinate antenna of 12 antennomeres. Schwarz (1907) listed only these original two species and reassigned the genus to Plastoceridae. Hyslop (1923), Schenkling (1928) and Blackwelder (1944) also placed the genus in Plastoceridae. Crowson (1955) recognized the structural inconsistencies within Plastoceridae and subsequently (Crowson 1972) restricted the family to include only Plastocerus Schaum, based on P. angulosus (Schaum) without remarking on the disposition of other genera formerly assigned to the family. Stibick (1979) reassigned Dodecacius to his Aplastini in Aplastinae. Lawrence (1981) had Plastoceridae, in part, in Elateridae, and in part equated the family with Cebrionidae. Gaedike (1985) incorrectly listed D. nigricollis in the previously re-organized Phylloceridae. Neither Lawrence and Newton (1995) or Costa et al. (2010) noted Dodecacius while placing associated genera in Cebrioninae, but not in a tribal arrangement. Johnson (2002) divided the American cebrionines between Cebrionini and Aplastini. Dodecacius and Octinodes Candèze are the only genera of Aplastini from South America, as the other described cebrionine genera Musopsis Chevrolat and Stenocebrio Solervicens are assigned to Cebrionini.
Schwarz (1907) defined Plastoceridae as having "6-7 Segmenten" in the males and "5 oder 6" in the female. These "Segmenten" are ventrites in the modern terminology. These ventrite counts were repeated by Costa et al. (2010). Historical ventrite counts of seven are undoubtedly based on misinterpretations of extruded sternites 8 and 9 that are normally recessed, but may become extruded depending on the specimen killing and preservation methods used. Both D. nigricollis and a new species have five ventrites as is typical of other Elateridae, which is also the case in both males and females of Neotropical Octinodes. In contrast, the males of Aplastus, Euthysanius, and Californian Octinodes have small lateral ventrite remnants beneath the metacoxae. If these remnants are counted then there are six ventrites observable on male Californian aplastines. The brachypterous females of Euthysanius species clearly have six ventrites, with the basal ventrite forming a complete sclerite across the abdomen. There are five ventrites on species of the cebrionine Scaptolenus LeConte and Selonodon Latreille. The basal ventrite (sternite 2) remnants on Californian aplastines can be seen due to the relatively flexible abdomen with a flexible and extensible thoracoabdominal membrane and the first ventrite loosely fitted against the metaventrite. In Dodecacius and Neotropical Octinodes species the ventrite 1 is rigid and tightly fitted to the posterior margin of the metaventrite and metacoxae, and does not expose a large basal membrane or sternite 2 remnant.
Male Dodecacius species are immediately recognizable in the Andean and Amazonian faunas with their pectinate antennae having 12 antennomeres, while the antennae of Octinodes species have 11 antennomeres. Female specimens of either genus have not been seen or reported, but by analogy with other aplastines they may be expected to have serrate antennae.
Dodecacius nigricollis Schwarz
Dodecacius nigricollis Schwarz 1902: 154; Schwarz 1907: 2; Hyslop 1921: 641; Schenkling 1927: 3; Gaedike 1985: 90
Dodecanius nigricollis, of Blackwelder 1944: 280
Dodecacius testaceus Schwarz 1902: 154 new synonym
Specimen studied. HOLOTYPE, labeled: "Chancham[a] yo / Peru K. Lange / Schwarz det. / nigricollis Schw. / Coll. Schwarz / Holotypus" (SDEI).
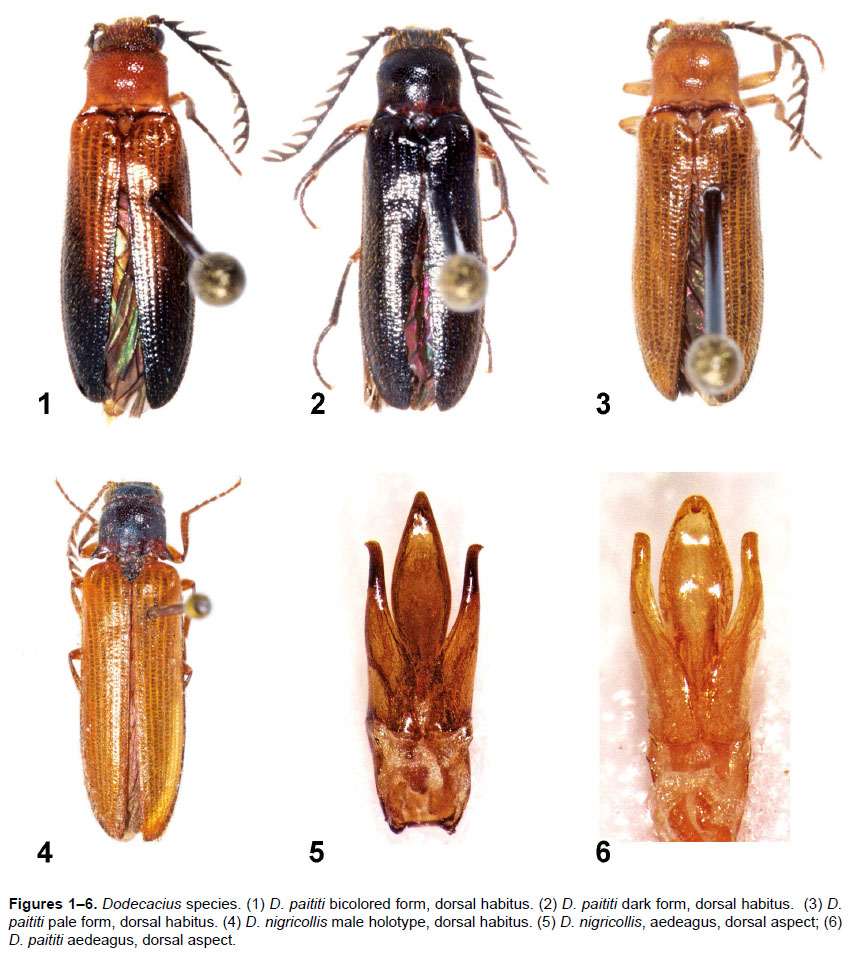
Taxonomic Notes. Otto Schwarz obtained his specimens of D. nigricollis (Figs. 4–5) and D. testaceus from Carl (or Karl) Friedrich Lange, a German industrialist and insect collector in Annaberg, presently Annaberg-Buchholz, Germany. No evidence was found to indicate that Lange visited or collected in Peru (Lamas 1980; G. Lamas in litt. to D. Silva). It is probable that Lange purchased these specimens from the O. Staudinger & A. Bang-Hass natural history dealership in Dresden. One of their collectors was Carl Oswald Schunke, a resident collector in La Merced, Chanchamayo, who supplied specimens from throughout the Chanchamayo Valley and other nearby localities.
Schwarz (1902) noted that his single specimen of D. nigricollis may be a female of D. testaceus, but did not indicate the sex of the latter. Schwarz’s suggestion was noted by Hyslop (1921), without correction. The holotype of D. nigricollis held in the SDEI was examined, dissected, and is male. Dodecacius testaceus was not listed by Gaedike (1985) and the species was absent from the SDEI collection in 2016. No information was found in collection records about it being lent, exchanged, or otherwise relocated (L. Behne, pers. comm.). Additional specimens potentially representing these two nominal species were not seen in other collections. Based on these two specimens being collected at the same locality, Chanchamayo, Peru, and the essentially identical descriptions separating the nominal species on variable color traits, D. testaceus is here treated as a new synonym of D. nigricollis. The choice of priority is based on the apparent loss of the D. testaceus holotype and is proposed in accordance with ICZN (1999) Art. 24.2.2 and Recommendation 24A.
Dodecacius paititi Johnson, new species
Diagnosis. This species of Dodecacius is immediately recognized by its pectinate antenna with 12 antennomeres with short rami on a hypognathous head, and slender legs with filiform tarsi. It differs from D. nigricollis by a smaller size, a shorter and proportionately wider pronotum, more coarsely punctured elytral striae, and aedeagal morphology.
Description. Genus characters as given by Schwarz (1902, 1907). Body (Figs. 1–3) 8.1–9.9 mm long, 2.4–2.9 mm wide across humeri; elongate, subparallel, dorsum shallowly to moderately convex. Integument of body and elytra variably testaceous, rufotestaceous, to partially to entirely dark infuscate. Pubescence testaceous to dark, matching the integumental ground color. Head and pronotum densely, subumbilicately to umbilicately punctured, separated by <0.2X own diameter; elytral striae with large serial punctures.
Head hypognathous, evenly convex on vertex, shallowly convex between eyes, becoming depressed anteriorly. Frontal margin truncate, coplanar with labrum. Ocular index 70–80. Antenna with 12 antennomeres, pectinate; antennomere 2 short, subglobular; antennomers 3–11 ramate; antennomere 12 elongate-oval, slightly compressed; antennomere 2–4, 7, 12 length ratio 1.0:2.2:3.0, 3.4, 4.2. Antennomeres 6–11 subequal in length to antennomere 5. Antennomere 3–5, 10 ramus length ratio 0.9:1.0:1.3, 1.2.
Pronotum transverse, length 0.74X width, anterior margin shallowly arcuate; disc moderately convex, densely umbilicately punctured, punctures becoming smaller and denser laterally and posteriorly. Lateral margins ecarinate, evenly rounded. Hind angle strongly divergent, ecarinate dorsally, evenly rounded laterally. Prosternum short, anterior margin truncate, intercoxal process strongly arched, disc between coxae depressed to shallowly impressed. Legs slender, segments narrow; tarsi filiform, densely set with stout setae; metatarsomere length ratio 1.00:0.57:0.43:0.39:1.26.
Elytral striae of large, shallow serial punctures. Intervals very narrow, irregularly sinuate around strial punctures. Apices divergent, separately rounded.
Abdominal ventrites 5, subequally densely and shallowly punctured; ventrite 5 broadly rounded apically, with oblique and elongate sublateral shallow impressions.
Aedeagus (Fig. 6) with median lobe broadly elliptical, narrowing apical, apex strongly deflexed, broadly rounded. Paramere shallowly sinuate laterally, strongly sinuate against median lobe; apices broadly rounded, ventrolaterally denticulate. Basal piece trapezoidal.
Female unknown.
Type Material. HOLOTYPE, male labeled: PERU, Madre de Dios, CICRA Field Stn, garden, 12.56940°N, 70.10100°W, 260 m, 2-9.ix.2010, MJ Endara, malaise trap, PER 10-09-MAT014 / SEMC1096871 (MUSM via SEMC).
Paratypes labeled as holotype, except as noted: SEMC 1096856, 1096857, 1096858, 1096859, 1096863, 1096864, 1096865, 1096866, 1096873, 1096875 (10, MUSM ); 1096877, 1096878, 1096880, 1096881, 1096883, 1096948, 1096951, 1096952, 1096954, 1096956 (10, SEMC), 1096958, 1096960, 1096966, 1096987 (4, PJJC); 9-16. ix.2010, PER10-09-MAT-015 / SEMC 1097850, 1097854, 1097856 (3, MUSM), 1097859, 1097862) (2, SEMC); 16-23. ix.2010, PER10-09-MAT-016 / SEMC 1097461, 1097467, 1097485, 1097487, 1097496 (5, MUSM), 1097888, 1097891, 1097893, 1097902, 1097912 (5, SEMC), 1097920 (1, PJJC); 18-25.x.2010, PER10-10-MAT-020 / SEMC 1061787 (1, MUSM); 25.x-1.xi.2010, PER10-10-MAT-021 / SEMC 1097073, 1097074, 1097075, 1097078, 1097080 (5, MUSM), 1097082, 1097085, 1097089, 1097090 (4, SEMC), 1097091 (1, PJJC).
Etymology. The species epithet "paititi" is after the mythical lost Incan city of Paititi that was supposedly located in southeastern Peru or adjacent northeastern Bolivia. The name is here treated as a noun in apposition.
Taxonomic notes. There is considerable variation in integumental color of D. paititi. Of 52 specimens examined 27 (52%) are entirely testaceous, nine (17%) are entirely dark infuscate, and 16 (31%) have varying degrees of both colors. Of the total specimens, two (4%) have bicolored pronota, four (8%) have the apical quarter of the elytra infuscate, and 10 (19%) have about one-third to two-thirds of the elytra infuscate.
The 12-"segmented" pectinate antenna of Dodecacius species is unique for click beetles in South America. Species of Octinodes that also occur in the region have pectinate antennae, but with 11 antennomeres. The species of Euthysanius from California, Arizona and Utah, are the only other elaterids in the Americas with 12 antennomeres. Species of Aplastus LeConte, also in the Californian region, have 11 antennomeres.
Distribution. Dodecacius paititi new species is presently known only from the type locality in Madre de Dios, at 12.56940°N, 70.10100°W.
Acknowledgements
My thanks to Caroline S. Chaboo for inviting me to review the elateroid beetles from her Beetles of Peru project, and to the Amazon Conservation Association (ACA) and their staff at the Centro de Investigacíon y Capacitación Rio Los Amigos (CICRA) for support and assistance to Chaboo. Zack Falin, University of Kansas Biodiversity Institute and Natural History Museum, is thanked for loans of specimens obtained under Peru research permits No. 506-2011-AG-DGFFS-DGEFFS and No. 0159-2010-AG-DGFFS-DGEFFS issued to Chaboo. Gratitude is extended to Stephan Blank and Lutz Behne for hosting my visit to the Senckenberg Deutsches Entomologische Institut (SDEI), Müncheberg, and generously allowing me free access to the beetle collection and other facilities. Andrew Liston, Marko Prous, Andreas Teager, Grit May, Eckhard Groll, and Editha Schubert of the SDEI are each thanked for their generous assistance and friendship during my visit. Editha Schubert is also thanked for her assistance in seeking biographical information on Carl Lange. Diana Silva and Gerardo Lamas, MUSM, generously shared historical information on Chanchamayo, and two anonymous reviewers provided helpful corrections and additions to a late manuscript draft. The author is grateful to the Ernst Mayr Grant Committee, Museum of Comparative Zoology, Harvard University for generously supporting travel to the SDEI. This publication is a product of the Insect Biodiversity Lab at South Dakota State University.
Literature cited
Blackwelder R.E. 1944. Checklist of the coleopterous insects of Mexico, Central America, The West Indies, and South America. United States National Museum Bulletin, Part 2, 185: 189–341. DOI: http://dx.doi.org/10.5479/si.03629236.185.2 [ Links ]
Calder A.A. 1996. Click beetles: Genera of Australian Elateridae (Coleoptera). Collingwood, Australia: CSIRO Publishing. Candèze E.C.A. 1863. Monographie des Élatérides, tome quatrieme. Mémoires de la Société royale des sciences de Liege 17:1–534, 6 pls. DOI: http://dx.doi.org/10.5962/bhl.title.47120 [ Links ]
Candèze E.C.A. 1891. Catalogue Méthodique des Élatérides connus en1890. Liége, Belgium: H. Vaillant-Carmanne. DOI: http://dx.doi.org/10.5962/bhl.title.47119 [ Links ]
Campbell J.M. & J.D. Marshall. 1964. The ocular index and its application to the taxonomy of the Alleculidae (Coleoptera). The Coleopterists Bulletin 18: 42. DOI: http://dx.doi.org/10.1649/829.1 [ Links ]
Chaboo C.S. 2015. Beetles (Coleoptera) of Peru: A survey of thefamilies. Part I. Overview. Journal of the Kansas Entomological Society 88 (2): 135-139. DOI: http://dx.doi.org/10.2317/0022-8567-88.2.135 [ Links ]
Chaboo C.S. & A. Catenazzi. 2015. Beetles of Peru: Biogeography.Journal of the Kansas Entomological Society 88 (2): 140–143.DOI: http://dx.doi.org/10.2317/kent-88-02-140-143.1 [ Links ]
Chaboo C.S. & P.J. Johnson. 2015. Beetles (Coleoptera) of Peru: A survey of the families. Cerophytidae Latreille, 1834. Journalof the Kansas Entomological Society 88 (2): 273. DOI:http://dx.doi.org/10.2317/kent-88-02-273-273.1 [ Links ]
Costa C., J.F. Lawrence & S. Policena Rosa. 2010. Elateridae Leach, 1815, pp. 75-103. In Leschen, R.A.B., R.G. Beutel, J.F. Lawrence (editors), Coleoptera, Beetles. Vol. 2: Morphologyand Systematics (Elateroidea, Bostrichiformia, Cucujiformiapartim). Kristensen, N.P. and R.G. Beutel (eds), Handbookof Zoology. Arthropoda: Insecta. De Gruyter, Berlin, Germany. DOI: http://dx.doi.org/10.1515/9783110911213.75 [ Links ]
Crowson R.A. 1955. The Natural Classification of the Families of Coleoptera. Nathaniel Lloyd, London, UK. Crowson R.A. 1972. A review of the classification of Cantharoidea (Coleoptera), with the definition of two new families,Cneoglossidae and Omethidae. Revista de la Universidad de Madrid 21 (82): 35–77. [ Links ]
Fender K.M. 1972. Some new and little known species of Malthini from the southwestern United States (Coleoptera: Cantharidae). The Coleopterists Bulletin 26 (2): 43–52. DOI: http://dx.doi.org/10.2307/2421804 [ Links ]
Gaedike H. 1985. Katalog der in den Sammlungen der Abteilung Taxonomie der Insekten des Institutes für Pflanzenschutzforschung, Bereich Eberswalde (ehemals Deutsches Entomologisches Institut), aufbewarhrten Typen – XXIII (Coleoptera: Rhipiceridae, Cebrionidae, Elateridae, Eucnemidae, Throscidae, Chelonariidae, Buprestidae, Phylloceridae, Dicronychidae, Dascillidae, Helodidae, Dryopidae,Georyssidae, Heteroceridae, Dermestidae, Byrrhidae). Beiträge zur Entomologie 35 (1): 13–96. DOI: http://dx.doi.org/10.1002/mmnd.4810350423 [ Links ]
Hyslop J.A. 1921. Genotypes of the elaterid beetles of the world. Proceedings of the U.S. National Museum 58 (2353): 621–680.DOI: 10.5479/si.00963801.2353.621 [ Links ]
Hyslop J.A. 1923. Present status of the coleopterus family Plastoceridae. Proceedings of the Entomological Society of Washington 25 (7–8): 156–160. DOI: http://dx.doi.org/10.1093/besa/17.3.160 [ Links ]
ICZN 1999. International Code of Zoological Nomenclature, 4th Edition. The International Trust for Zoological Nomenclature, London, UK. DOI: http://dx.doi.org/10.5962/bhl.title.50608 [ Links ]
Johnson P.J. 2002. Elateridae Leach 1815, pp. 160-173. In Arnett, R. H., Jr., Thomas, M. C., Skelley, P. E. & Frank, J. H. (eds.),American Beetles, volume 2. Polyphaga: Scarabaeoideathrough Curculionoidea. Boca Raton, Florida: CRC Press. DOI: http://dx.doi.org/10.1201/9781420041231 [ Links ]
Johnson P.J. & C.S. Chaboo. 2015. Beetles (Coleoptera) of Peru: A survey of the families. Elateridae Leach, 1815. Journal of the Kansas Entomological Society 88 (2): 269–272. DOI: http://dx.doi.org/10.2317/kent-88-02-269-272.1 [ Links ]
Johnson P.J. & C.S. Chaboo. 2016. First record of the beetle family Throscidae (Insecta: Coleoptera), a new species of Aulonothroscus Horn, and new species records to the fauna of Peru / Primer registro de la familia de escarabajos Throscidae(Insecta: Coleoptera), una nueva especie de AulonothroscusHorn y tres nuevos registros de especies para la fauna de Perú.Revista Peruana de Biologia 23 (3): 237–242. DOI: http://dx.doi.org/10.15381.rpb.v23i3.12858 [ Links ]
Lamas G. 1980. Introduccion a la historia de la entomología en el Peru. II. Periodo de los viajeros, colectores y estudiosos especializados. Revista Peruana de Entomología 23 (1): 25–31. [ Links ]
Lawrence J.F. 1981. Coleoptera, pp. 482–553, 1107. In Parker, S.P. (ed.), Synopsis and Classification of Living Organisms. NewYork, New York: McGraw-Hill Book Company [ Links ]
Lawrence J.F., R.G. Beutel, R.A.B. Leschen, & A. Ślipiński. 2010. Glossary of morphological terms, pp. 9-20. In Leschen,R.A.B., R. G. Beutel, and J. F. Lawrence (volume eds.),Coleoptera, Beetles, Volume 2: Morphology and systematics (Elateroidea, Bostrichiformia, Cucujiformia partim).In Kristensen, N.P., and R.G. Beutel (eds.) Handbook of Zoology, Arthropoda: Insecta. Berlin/New York: Walter de Gruyter. [ Links ]
Lawrence J.F. & A.F. Newton Jr. 1995. Families and subfamilies of Coleoptera (with selected genera, notes and references, and data on family-group names), pp.779–1006. In Pakaluk, J. and S. A. Ślipiński (eds.). Biology, Phylogeny, and Classification of Coleoptera: Papers Celebrating the 80th Birthday of Roy A. Crowson. Muzeum i Instytut Zoologii PolskaAkademia Nauk, Warszawa. [ Links ]
Nichols, S.W., & R.T. Schuh. 1989. The Torre-Bueno glossary ofentomology. New York, New York: The New York Entomological Society. DOI: http://dx.doi.org/10.1017/s0007485300046022 [ Links ]
Schenkling S. 1927. Plastoceridae. Pars 93. Coleopterorum Catalogus.Vol. 11. Berlin, Germany: W. Junk 7 p. [ Links ]
Schwarz O. 1902. Dodecacius nov. gen Elateridarum aus Peru.Deutsche Entomologische Zeitschrift, 1902 (Heft 1):153–155. [ Links ]
Schwarz O. 1907. Coleoptera. Fam. Plastoceridae,Fasc. 50, pp. 1–10, 1 pl. In: P. Wytsman, Genera Insectorum.Bruxelles, Belgium: V. Verteneuil & L. Desmet. [ Links ]
Stibick J.N.L. 1979. Classification of the Elateridae (Coleoptera):Relationships and classification of the subfamilies and tribes.Pacific Insects 20 (2–3): 145–186. [ Links ]
Publicación registrada en Zoobank/ZooBank article registered:
urn:lsid:zoobank.org:pub:CF42CC9C-F496-4B4F-9C1A-FBB413A43E02
Acto nomenclatural/nomenclatural act: urn:lsid:zoobank.org:act:84A545F1-FAF8-42C1-83DA-C9D90CA0CA39
Permisos de colecta: Research permits No. 506-2011-AG-DGFFS-DGEFFS and No.0159-2010-AG-DGFFS-DGEFFS
Presentado: 07/03/2017
Aceptado: 26/08/2017
Publicado online: 28/10/2017