Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. vol.26 no.1 Lima ene./mar. 2019
http://dx.doi.org/10.15381/rpb.v26i1.15906
TRABAJOS ORIGINALES
A new species of Hexamermis Steiner, 1924 (Nematoda, Mermithidae) parasitizing Epilachna paenulata (Germar, 1824) (Coleopera, Coccinellidae) in Argentina
Una nueva especie de Hexamermis Steiner, 1924 (Nematoda, Mermithidae) parásito de Epilachna paenulata (Germar, 1824) (Coleopera, Coccinellidae) en Argentina
Guillermo R. Reboredo 2,3 y Nora B. Camino* 1,3
1 Investigador CIC
2 CONICET
3 Centro de Estudio Parasitológicos y de Vectores, CEPAVE CCT La Plata CONICET; Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata (UNLP), Argentina.
Abstract
Hexamermis bonaerensis sp. n. a parasite of Epilachna paenulata (Germar, 1824) (Coleoptera, Coccinellidae) from Argentina is described. It is characterized by having the amphids medium sized, rounded oval shaped, the vagina muscularized and slightly protruding with a descending branch forming a loop before joining the uterus. It presents three rows of genital papillae: the ventrolateral in one row with thirteen papillae; the ventral row with two single, three pairs and two single preanal papillae, and with five pairs postanal papillae.
Keywords: nueva especie; Hexamermis; parásito; Coleoptera; taxonomía.
Resumen
Se describe a Hexamermis bonaerensis sp. n. un parásito de Epilachna paenulata (Germar, 1824) (Coleoptera, Coccinellidae) en Argentina. Se caracteriza por tener los anfídios de tamaño mediano y de forma oval redondeada. La vagina es musculosa y ligeramente protuberante, con una rama descendente que forma un asa antes de unirse al útero. Presenta tres hileras de papilas genitales: la ventrolateral en una hilera con trece papilas; la hilera ventral con dos papilas preanales simples, tres pares y dos preanales únicas, y con cinco pares de papilas postanales.
Palabras clave: new species; Hexamermis; parasite; Coleoptera; taxonomy.
Introduction
The beetle Epilachna paenulata (Germar, 1824) (Co-leoptera, Coccinellidae) is a South American coleoptera insect distributed in Brazil, Paraguay, Uruguay and Argentina. These beetles produce damages in the crops of Curcubitaceas, zucchini, watermelon, melon and pumpkin. Larvae and adults attack producing loss of foliage, flowers and cuticle of fruits. A study of the natural enemies of E. paenulata from Gran La Plata revealed the presence of nematodes. Many species of nematodes are parasites of various bark beetles in the neotropical region inhabiting the haemocel, specially from the order Tylenchida. Only three mermithid nematodes were reported parasitizing coccinellid insects: Christie in 1936 found Agamermis decaudata Cobb, Steiner & Christie, 1923; Diesing in 1851 described a new species Mermis coccinellae, and v. Linstow (1898) found Mermis nigrescens Dujardin, 1842.
The life cycle of the mermithid nematodes involves three main stages: egg, juvenile parasite and free-living adult. The juvenile parasite locates the first larval stages of the host insect and it is located in the hemocoel where it begins to parasitize it. At the end of this period, it emerges from its host killing it, and thus reaching the adult state of free life, which copulates and lay in the external environment. This behavior locates them as parasitoids (Wise de Valdes, 2006, 2007).
Since the parasitism results in the death of the host, they can be considered as biological control agent on insect plague E. paenulata. The genus Hexamermis Steiner, 1924, has been extensively studied in plagues of agriculture (Camino & Stock, 1989; HernándezCrespo & Santiago-Alvarez, 1997; Poinar Jr. & Chang, 1985; Wouts, 1981). At present, seven species have been isolated from Argentina, H. cochlearius Stock & Camino, 1992a, H. ovistriata Stock & Camino, 1992b, both in acridids; H. macrostoma Camino & Stock, 1994, a parasite of crickets; H. hortensis Camino & Stock , 1989, in Lepidoptera, noctuids; H. gracilis de Villalobos & Camino, 1998, H. distinctus Camino & Marino, 2007, and H. paranaense Achinelly & Camino, 2008, parasitizing white grub.
This study describes this nematode parasite and discusses aspects of its development. In addition, we report the first presence of Hexamermis in Epilachna paenulata from Argentina, and we described H. bonaerensis sp.n.
Material and methods
Adult coccinelids insects were collected by hand from September to April 2016/2017 on plant species Cucurbita maxima var. zapallito (Carrière) Millán, 1947 (round green zucchini), at the locality near Gran La Plata, Colonia Urquiza (34°96'72"S, 58°04’96"W). The beetles were put in individual plastic containers. In total, 260 adults of Epilachna paenulata (Germar, 1824) (Coleopera, Coccinellidae) were sampled.
The coccinellids adults were kept individually in dishes with food (round green zucchini leaf) and water at room temperature. Living nematodes were emerged from adult host, and then they were rearing in Petri dishes with sand and distilled water at 22° C ± 2. The adult nematodes were killed by placing them in distilled water at 60°C for 2 min. They were first fixed in 50% TAF solution in water for 48 hours, and then into pure TAF (Poinar 1975). The longitudinal chord arrangement and the apical view of the head were prepared in glycerine jelly (Hooper, 1970). Measurement was made from live and fixed specimens with a micrometer on a Zeiss light microscope. The measures are of Holotype male and Allotype female ± standard deviation (minimum-maximum). All the specimens were used for photographing in Olympus BX51 microscope with Olympus DP71 camera.
Results
Family Mermithidae Braun, 1883
Genus Hexamermis Steiner, 1924
Hexamermis bonaerensis sp. nov.
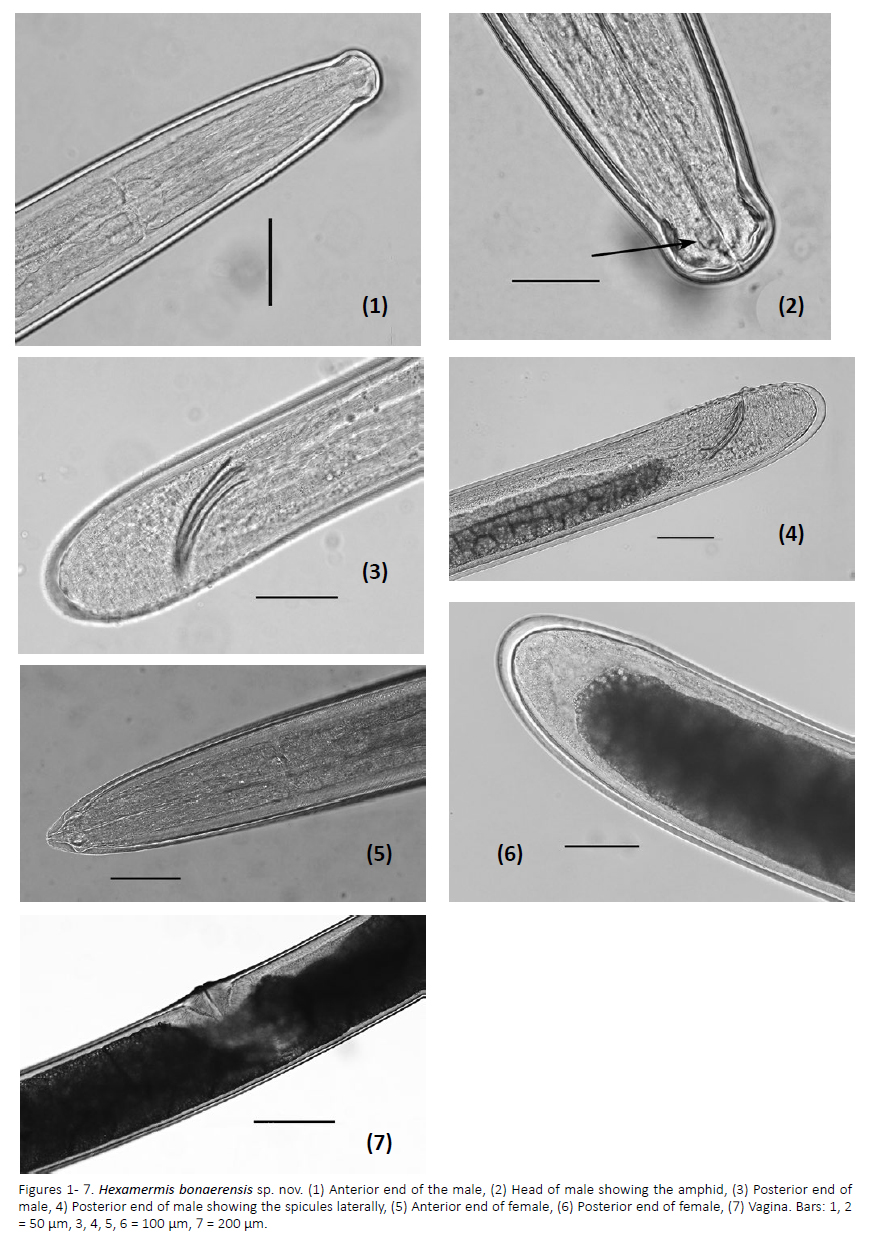
Figures 1-7.
Description: Color of trophosome white in postparasitic juveniles. Short nematodes. Cuticle with crisscross fibres. Female: head homocephalic, pointed (Fig. 5). Six cephalic papillae surrounding the mouth. Amphids medium sized, rounded oval shaped, located posterior to head papillae, amphidial opening pocket-shaped (Fig. 2). Mouth central and terminal. Six hypodermal chords: lateral one with two rows of cells, dorsal, ventral and subventral chords with only one row of cells. Vulva a longitudinal slit. Vagina "J" shaped, orientated at 90º to the longitudinal body axis and protruding (Fig. 7). Anterior portion of the vagina muscularized, with a descending branch forming a loop before joining the uterus. Without tail appendage. Male: head conoid shaped (Fig. 1). Spicules pairs, medium sized, slightly curved and not sculpture, tip of the spicules pointed (Figs. 3, 4). Three rows of genital papillae: the external rows with thirteen papillae situated in a single line; ventral row with seven preanal papillae arranged as followed: two single papillae, three pairs of papillae, and two single papillae, and five pairs postanal papillae, the adanal papillae absents. Tail without appendage (Fig. 6).
Male (n=12): body length: 3.20 mm ± 2.34 (2.80– 3.42), width of the head at the level of cephalic papillae: 67.64 µm ± 3.455 (62–70), width of the body at the level of nerve ring: 136 µm ± 11 (112–145), greatest width of the body: 170.62 µm ± 28 (148–212), width of the body at cloaca: 182 µm ± 21 (166–216), distance from the head to the nerve ring: 352.36 µm ± 37 (256–388), length of amphidial pouch: 20.62 µm, width of amphidial pouch: 18 µm, length of spicules: 176.42 µm ± 12 (156– 190), width of spicules: 11 µm.
Female (n=13): body length: 8.2 mm ± 4.42 (7.88– 10.50), width of the head at the level of cephalic papillae: 88 µm ± 6 (67–92), width of the body at the level of nerve ring: 152 µm ± 22 (114–176), greatest width of body: 247 µm ± 26 (198–272), width of the body at vulva: 280 µm ± 34 (212–297), distance from head to the nerve ring: 360 µm ± 42 (325–410), width of the body at posterior end of the trophosome: 211 µm ± 25 (196–236), length of vagina: 611.75 µm ± 45 (576– 665), width of vagina: 48 µm ± 10 (32–58), length of amphidial pouch: 24 µm, width of amphidial pouch: 22 µm, V%: 48 ± 2 (48–52).
Type host: Adults of Epilachna paenulata (Germar, 1824) (Coleopera, Coccinellidae); The collection was made at midday in a farm near La Plata city, during the summer of 2016/2017. The insect was found over round green zucchini´s flowers.
Type locality: Colonia Urquiza (34°96'72"S, 58°04’96"W), Gran La Plata, Buenos Aires province, Argentina.
Type material: deposited in Helminthological Collection of the Museo de Ciencias Naturales de La Plata. Holotype: NM200598. Paratypes deposited in Nematological Collection of CEPAVE nº 00379
Etymology: The name of the species refers to the locality Buenos Aires province
Discussion
Hexamermis bonaerensis sp. nov. is placed in the genus Hexamermis to possess a horn-shaped vagina, amphids separated from the lateral cephalic papillae (Rubstov, 1978). Postparasitic juveniles of Hexamermis differ from Agamermis Cobb, Steiner & Christie, 1923 by the tail tip appendage always preserved after final molt (Hernández-Crespo & Santiago-Álvarez, 1997).
Hexamermis bonaerensis sp. n. shares the morphology of its vagina ("J" shaped) perpendicular to the longitudinal body axis with: H. albicans von Siebold, 1848; H. arvalis Poinar & Gyrisco, 1962; H. brevis Hagmeier, 1912; H. cathetospiculae Poinar & Chang, 1985; H. cavicola Welch, 1963; H. cochlearius; H. dactylocercus Poinar & Linares, 1985; H. distinctus; H. elongata Kaiser, 1977; H. gracilis; H. glossinae Poinar et al., 1981; H. hortensis; H. incisura Kaiser, 1977; H. lineata Kaiser, 1977; H. macrostoma; H. microamphidis Steiner, 1925; H. ovistriata, H. paranaense and H. serenensis Hernández-Crespo & Santiago-Alvarez, 1997.
Hexamermis albicans, a parasite of orthopterans, dermapterans, coleopterans, dipterans, hymenopterans and lepidopterans, is separated by the arrangement of the genital papillae: 4‒8 preanal and 5‒9 postanal.
Hexamermis arvalis, a parasite of orthopterans and lepidopterans, can be distinguished by having a vagina situated parallel to the longitudinal body axis and in the arrangement of the genital papillae: 3 to 6 irregular and discontinuous rows.
Hexamermis brevis, a parasite of coleopterans, are greater than the body width at the cloaca. Also preanal papillae extended previously along the entire length of the spicules and placed in several rows to triplets. The spicules are very short (50‒130 µm).
Hexamermis cathetospiculae, found in lepidopterans, is distinguished by the size of the spicules (289‒334 µm) and the genital papillae having three broken (double) rows, lateral double rows containing 20–32 papillae each, extending previously past the cloacal opening, but only half-length the spicules, median double row of 20–25 papillae extending previously almost the lateral papillae.
Hexamermis cavicola, a parasite of lepidopterans, is separated by the presence of irregular rows of genital papillae (eight anal and ten postanal) and female head is tapered more sharply than that of any other of the genus.
Hexamermis cochlearius, a parasite found in orthopterans, can be distinguished by having spicules slightly curved with a concavity in the internal face of the tip forming a receptaculum. Six rows of genital papillae; a double row of ventrolateral papillae, the external row with 10 papillae and the internal one with 6; median ventral rows with a single preanal papilla, two single ones situated in each side of the anus and 7 pairs of postanal papillae.
Hexamermis dactylocercus, found in homopterous, has a tail appendage on the postparasitic, a digit-like appendage on the tails of the adults and the papillae arrangement in 6 or 7 broken rows.
H. distinctus parasite of scarabaeid insect can be separated by the small amphids, the arrangement of the genital papillae in three double rows, the ventral row with four pairs and four single preanal papillae, and with eight pairs and two singles postanal papillae.
Hexamermis elongata, with unknown host, differs in the size of the spicules (180 µm) and the genital papillae have 4 rows: the lateral ones with 6‒15 papillae, and the median rows with 8 preanal and 4‒17 postanal papillae.
Hexamermis glossinae, a parasite of tsé-tsé fly, differs from all other species of Hexamermis in the small amphids located on the lateral head papillae; spicules slightly curved, roughly equal in length to the body diameter at the cloaca, male with three double rows of genital papillae; vagina straight.
Hexamermis gracilis, a parasite of coleopterans, is different by having the male three rows of genital papillae with the ventrolateral divided in two rows, the external with 8 papillae and the internal with 4; the ventral row with 13 preanal papillae and 14 postanal.
Hexamermis hortensis, found in lepidopterans, is distinguished by the number and arrangement of the male's genital papillae with 8 surrounding the anus, double row of 6 ventrolateral preanal and double row of 15 ventrolateral papillae.
Hexamermis incisura, with unknown host, differs from H. paranaense n. sp. by the size of the spicules (110 µm), the genital papillae arrangement (11 lateral; the median rows with 8 preanal and 9 postanal) the small amphids situated behind the cephalic papillae and thin at the beginning.
Hexamermis lineata, a parasite of coleopterans, is distinguished in the size of spicules (185‒250 µm), the arrangement of the genital papillae: 8‒25 lateral and 8‒17 postanal in the median rows and by the small amphids situated behind the cephalic papillae.
Hexamermis macrostoma, a parasite found in orthopterans, is separated from H. paranaense n. sp. by the stoma size and the genital papillae arrangement in the male, with four preanal rows with 30 papillae and three postanal rows with 24.
Hexamermis microamphidis, found in hemipterans and lepidopterans, can be separated by having shorter spicules, with a length smaller than body diameter at anus level, and the very small amphids.
Hexamermis ovistriata, a parasite of orthopterans, differs from the new species in the arrangement of the genital papillae in the males; six rows: double row of lateral papillae, the external with thirteen papillae and the internal one with twelve irregularly arranged; ventral row with fourteen preanal papillae: a single one at the beginning of the spicules, three pairs, a single one, and three pairs; eighteen postanal papillae: two triplets, five pairs ending with two single ones. Eggs with three longitudinal lines of striations.
H. paranaense found in white grub, is different in the small amphids, three rows of genital papillae, the ventrolateral divided in two rows with eight papillae in the outer row and with six papillae in the inner one, the ventral row with four pairs and one single preanal papillae, and with two pairs, a triplet, one pair, a single and one pair postanal papillae, the tip of the spicules rounded.
Hexamermis serenensis, a parasite of orthopterans, can be distinguished by its long size, the high number of papillae (96–134) in the male distributed in 6–7 broken rows and the morphology of the tail which is conical.
Hexamermis bonaerensis sp. nov. is characterized by: i) amphids medium sized, rounded oval shaped, amphidial opening pocket-shaped, ii) anterior portion of the vagina muscularized and slightly protruding with a descending branch forming a loop before joining the uterus, and iii) three rows of genital papillae: the ventrolateral in one row with thirteen papillae; the ventral row with two single, three pairs and two single, the adanal papillae are absents.
Acknowledgements
We would like to thank to Lic Luis Giambelluca for photograpied the material. Many thanks are expressed to Manuela Reboredo for her careful review of the English language.
Roles:
GRR, NBC: conducted the sampling; NBC, GRR: processed the material in the laboratory; NBC, GRR: wrote the manuscript; NBC, GRR: revised and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethical / legal aspects:
This work did not incur any ethical or legal problems.
Funding:
The present work was carried out thanks to the financing of the Comisión de Investigaciones Científicas de la provincia de Buenos Aires, CIC and of the funds of the Incentive Program of the Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata (UNLP).
Citación:
Reboredo G.R & N.B. Camino. 2019. A new species of Hexamermis Steiner, 1924 (Nematoda, Mermithidae) parasitizing Epilachna paenulata (Germar, 1824) (Coleopera, Coccinellidae) in Argentina. Revista peruana de biología 26(1): 021 026 (Febrero 2019). doi: http://dx.doi.org/10.15381/rpb.v26i1.15906
Publicación registrada en Zoobank/ZooBank article registered:
LSIDurn:lsid:zoobank.org:pub:9F5EA90D-381D-4FC8-8F0F-86B9E1D2F014
Acto nomenclatural/nomenclatural act:
LSIDurn:lsid:zoobank.org:act:35252E81-2E50-4F12-94DF-FC3617971627
Literature cited
Achinelly, M.F. & Camino, N.B. 2008. Hexamermis paranaensis new species (Nematoda, Mermithidae): a parasite of Diloboderus abderus (Coleoptera, Scarabaeidae) in Argentina. Iheringia, série Zoologia, 98: 460-463. [ Links ]
Camino, N.B. & Marino, H.A. 2007. Hexamermis distinctus n. sp. (Nematoda, Mermithidae) parasitizing Diloboderus abderus Sturm, 1826 (Coleoptera, Scarabaeidae) in Argentina. Bolletino del Museo Civico di Storia Naturale di Venezia, 58: 3-6. [ Links ]
Camino, N.B. & Stock, S.P. 1989. Hexamermis hortensis sp. n. (Nematoda: Mermithidae) parásita de larvas de Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) en Argentina. Revista Ibérica Parasitología, 49: 329-333 [ Links ]
Camino, N.B. & Stock, S.P. 1994. Hexamermis macrostoma n. sp. (Nemata: Mermithidae) parasitizing the cricket Gryllodes laplatae (Orthoptera: Gryllidae) in Argentina. Fundamental and applied Nematology, 17(5):397-399 [ Links ]
Christie, J.R. 1936. Life history of Agamermis decaudata, a nematode parasite of grasshoppers and other insects. Journal of Agricultural Research 52: 161-178 [ Links ]
de Villalobos, L.C. & Camino, N.B. 1998. Una nueva especie del género Hexamermis Steiner, 1924 (Nematoda: Mermithidae) parásita de larvas de Diloboderus abderus Sturm (Coleoptera: Scarabaeidae) en Argentina. Iheringia, serie Zoologia, 84:79-82. [ Links ]
Diesing, K.M. 1851. Systema helminthum. 2:588 pp. [ Links ]
Hagmeier, A. 1912. Beitrage zur Kenntnis der Mermithiden. 1. [ Links ]
Biologische Notizen und Systematische Beschreibung einiger alter and neuer Arten. Zoologische Jahrbücher 32: 521-612. [ Links ]
HernándezCrespo, P. & Santiago-Álvarez, C. 1997. Description of Hexamermis serenensis sp. n. (Nematoda: Mermithidae) a parasite of Dociostaurus macroccanus (Thunberg) (Orthoptera: Acrididae) in Spain.Fundamental and applied Nematology 20: 37-42 [ Links ]
Hooper, D.J. 1970. Handling, fixing and mounting nematodes. En Laboratory methods for work with plant and soil nematodes. Ed. Southey, J.F.M.A.F.F. Technology Bulletin 2, H.M.O.S. London. [ Links ]
Kaiser, H. 1977. Untersuchugen zur Morphologie, Biometrie, Biologie, und Systematik von Mermithidaen. Zoologische Jahrbücher Abteilung für Systematik 104: 20-71 [ Links ]
Poinar Jr., G.O. 1975. Entomogenous nematodes: a manual and host list of insect-nematode associations. Leiden, The Netherlands, Brill, 317 pp. [ Links ]
Poinar Jr., G.O. & Chang, P. 1985. Hexamermis cathetospiculae n. sp. (Mermithidae: Nematoda) a parasite of the rice atemborer, Tryporyza incertulas (Wlk.) (Pyralydae: Lepidoptera) in Malaysia. Journal of Nematology 17: 360-363. [ Links ]
Poinar Jr., G.O. & Gyrisco, G.G. 1962. A new Mermithid parasite of the alfalfa weevil, Hypera postica (Gyllenhal). Journal of Insect Pathology 4: 201-206. [ Links ]
Poinar Jr., G.O. & Linares, B. 1985. Hexamermis dactylocercus sp. n. (Mermithidae: Nematode) , a parasite of Aeneolamia varia (Cercopidae: Homoptera) in Venezuela. Revue of Nématologie 8: 109-111. [ Links ]
Poinar Jr., G.O., Mondet, B., Gouteux, J.P. & Laveissiere, C. 1981. Hexamermis glossinae sp. nov. (Nematoda: Mermithidae), a parasite of tse-tse flies in West Africa. Canadian Journal of Zoology 59: 858-861 [ Links ]
Rubstov, I.A. 1978. [Mermithida, Classification, importsnce and use]. Leningrad, "Nauka", 207 p. (In Russian).
Seibold, C.T.E. von 1848 Ueber die Fadenwurmer der Insekten. Entomologische Zeitschrift 9: 290-300 [ Links ]
Steiner, G. 1925. Mermithids parasitic in the tea bug (Helopeltis antonii Sign.). Mededell. Proefst. Thee 44: 10-16 [ Links ]
Stock, S.P. & Camino, N.B., 1992a. Hexamermis cochlearius sp. n. (Nematoda: Mermithidae) a parasite of Dichroplus elongatus Giglio-Tos (Orthoptera: Acridiidae) in Argentina. Nematologia mediterranea, 20:167-169. [ Links ]
Stock, S.P. y Camino, N.B., 1992b. Hexamermis ovistriata n. sp. (Nematoda: Mermithidae) a parasite of the grasshopper Staurorhectus longicornis Giglio-Tos (Orthoptera: Acridiidae) in Argentina. Fundamental and applied Nematology, 15(1): 15-18. [ Links ]
von Linstow, O.F.B. 1898. Das Genus Mermis. Archives Mikroskopischen Anatomie 66: 355-366. [ Links ]
Welch, H.E. 1963. Amphimermis bogongae sp. nov. and Hexamermis cavicola sp. nov. from the Australian bogong moth. Parasitology 53: 55-62 [ Links ]
Wise de Valdez, M. 2006. Parasitoid-induced behavioral alterations of Aedes aegypti mosquito larvae infected with mermithid nematodes (Nematoda: Mermithidae). Journal of Vector Ecology 31: 344-354. [ Links ]
Wise de Valdez, M. 2007. Predator avoidance behavior of Aedes aegypti mosquito larvae infected with mermithid nematodes (Nematoda: Mermithidae). Journal of vector Ecology 32: 150-153. [ Links ]
Wouts, W.M. 1981. On the identify of Hexamermis albicans (von Siebold, 1948). Systematic Parasitology 3: 127-128. [ Links ]
*Corresponding author
Email Nora Camino: nemainst@cepave.edu.ar
Email Guillermo Reboredo: grreboredo@yahoo.com.ar
Presentado: 29/06/2018
Aceptado: 16/02/2019
Publicado online: 30/03/2019