Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Revista Peruana de Biología
versión On-line ISSN 1727-9933
Rev. peru biol. vol.26 no.1 Lima ene./mar. 2019
http://dx.doi.org/10.15381/rpb.v26i1.15907
TRABAJOS ORIGINALES
Morphological and cytogenetic description of a new species of Parasitylenchus Micoletzky, 1922 (Tylenchida, Allantonematidae) parasitizing Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae) in Argentina
Descripción morfológica y citogenética de una nueva especie de Parasitylenchus Micoletzky, 1922 (Tylenchida, Allantonematidae) parasitando Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae) en Argentina
Nora B. Camino* 1, 5, Sergio Rodríguez Gil 2, 5, Guillermo R. Reboredo 4, 5 and Sandra E. González 3, 5
1 Investigador CIC
2 Investigador CONICET
3 Personal de Apoyo CIC
4 CONICET
5 Centro de Estudio Parasitológicos y de Vectores, CEPAVE CCT La Plata CONICET, Facultad de Ciencias Naturales y Museo, UNLP. Boulevard 120 y 62, 1900 La Plata, Argentina.
Resumen
Se cita y describe por primera vez para Argentina Parasitylenchus pseusobifurcatus sp.n. (Tylenchida, Allantonematidae), parásito de adultos de Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae). Esta nueva especie está muy cercana a P. bifurcatus Poinar Jr. y Steenberg, 2012, por la característica de tener todas las hembras vermiformes y los machos inmaduros la punta del apéndice caudal bifurcada, pero se diferencian entre otros por el tamaño del gubernáculo. Se describe su fórmula cariotípica.
Palabras clave: Parasitylenchus pseusobifurcatus sp.n.; Coccinellidae; morfometría; citogenética; taxonomía.
Summary
Parasitylenchus pseusobifurcatus sp.n. (Tylenchida, Allantonematidae) is described for the first time for Argentina as a parasite of adults of Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae). The new species is closed to P. bifurcatus Poinar Jr. & Steenberg, 2012, by the characteristic of having the tail tip of all vermiform females and immature males bifurcated, but they differ by the size of the gubernaculum. Its karyotype formula is described.
Keywords: Parasitylenchus pseusobifurcatus sp.n.; Coccinellidae; morphometric; cytogenetic; taxonomy.
Introduction
The genus Parasitylenchus was erected by Micoletzky in 1922 and was originally proposed as a subgenus of Tylenchus. The type species P. dispar was described by Fuchs in 1915 and placed in the genus Tylenchus. Fuchs later described several species that are now included in the genus Parasitylenchus. They are, namely: P. ligniperdi (Fuchs 1929), P. morosus (Fuchs 1929), P. sulphurous (Fuchs 1929), P. chalcographi (Fuchs 1938), P. poligraphi (Fuchs 1938), P. pusilli (Fuchs 1938). The latter three species were described as subspecies of P. dispar. In addition to the species described by Fuchs, Wuelker (1923-1929) described P. hylastis and P. cossoni, the former species within the genus Tylenchus. P. scolyti was described by Oldham in 1930. Ruhm described P. grossmannae in 1954. For a more complete listing of the species in this genus the reader is referred to Wachek (1955) and Ruhm (1956). Later, in 2012 Poinar & Steenberg described another species P. bifurcates in Denmark.
In this study we found another species of the genus P. pseusobifurcatus sp.n., from Gran La Plata, Buenos Aires, Argentina. In the order Tylenchida, cytogenetically 40 genera have been analyzed, belonging to eight families, all of them parasitic species of agricultural plants. The order presents a wide diploid number range that varies between 2n = 10 and 2n = 56. All genera have a fairly stable number, mostly 2n = 16 or 2n = 18, except the genus Ditylenchus Filipjev, 1936, whose only species studied D. dipsaci (Kuhn, 1857) Filipjev, 1936, has a wide distribution of values of 2n that has a minimum of 24 and a maximum of 56 (Siddiqi 2000). In the genus Meloidogyne Göldi, 1892, the chromosomes are of the holocinetic type, and they do not present a chromosome with a localized centromere (Márquez-Corro et al. 2017, Paliulis et al. 2012). From the order Tylenchida, no species has been cytogenetically analyzed, being the first study of the chromosomes of tylenchid nematodes parasite of insects.
Material and methods
Insect.- Adult coccinellid insects were collected by hand from September 2016 to April 2017 on plant species Cucurbita maxima var. zapallito (Carrière) Millán, 1947 (round green zucchini), at a locality near Gran La Plata, Colonia Urquiza (34°96'72S, 58°0496W). The beetles were distributed in individual plastic containers. In total, 260 adults of Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae), were sampled and dissected. The coccinellids adults were dissected in Petri dishes filled with distilled water under a stereomicroscope.
Nematodes.- Living nematodes were removed from the hemocel of adult host, and then they were killed by placing them in distilled water at 60 °C for 2 minutes. They were fixed in 50% TAF solution in water for 48 hours and then into pure TAF (Poinar 1975). All the specimens were used for photographing in Olympus BX51 microscope with Olympus DP71 camera. Measurements of common nematode body features were performed on 16 fixed nematode specimens of each nematode stage of life cycle.
Cytogenetic.- For cytogenetic studies the females were placed in distilled water for 30 minutes and then fixed in Carnoy's solution (3 parts of pure ethyl alcohol and 1 part of acetic acid). The cytogenetic preparations were made by squash and stained with 45% acetic orcein (Rodríguez Gil et al. 2009). The representative cells of each stage were photographed with an Olympus microscope with digital camera DP 71, with the program DP controller 3.3.1.292 and the images were processed with the programs GIMP version 2.8.16 and inks-cape gnu general public license, version 2.
Results
Tylenchida Thorne, 1949
Sphaerularioidea Lubbock, 1861
Allantonematidae Pereira, 1931
Genus Parasitylenchus Micoletzky, 1922
Parasitylenchus pseusobifurcatus sp.n.
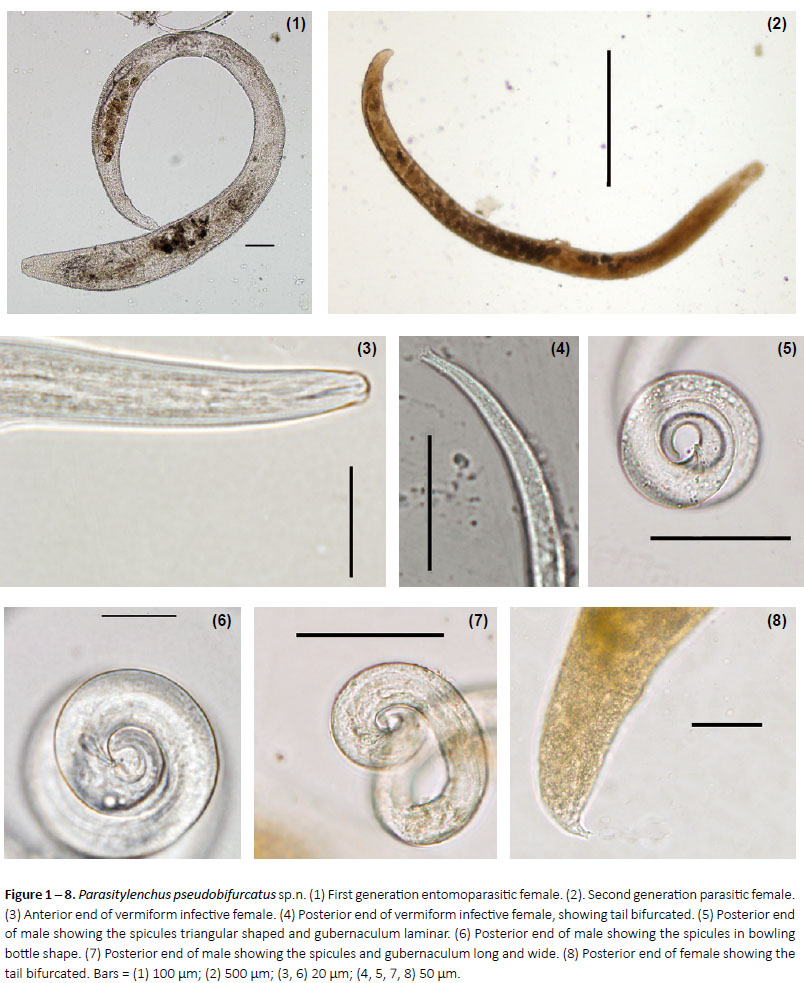
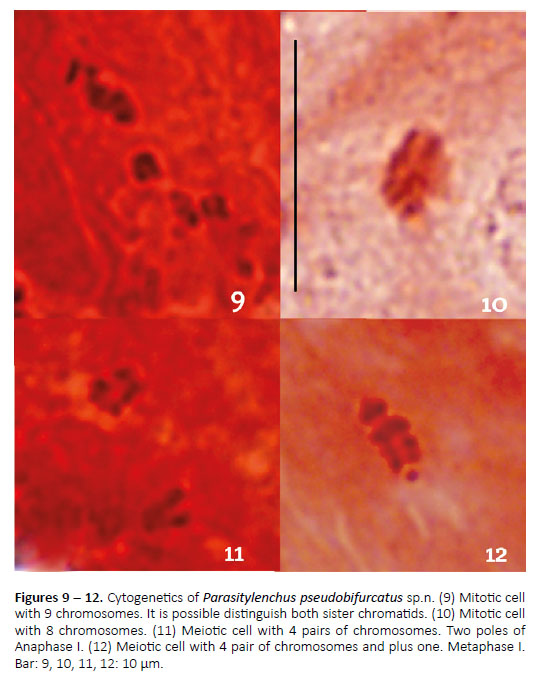
Figures 1 – 12
Description: Primary heterosexual female: develops from preadult fertilized female that enter into the host, in its body cavity, obese sausage-shaped curving ventrally, with round, wrinkled ends. Cephalic region usually overgrowns by body expansion. Stylet with minute basal knobs. Vulva subterminal. Uterus filling most of body, with ovary convoluted in anterior region. Ovoviviparus. Juveniles develop into bisexual forms in host´s body cavity. Secondary small heterosexual female, less obese and in larger numbers than primary females. Yellowishbrown body. Stylet similar to that in primary female. Excretory pore near stylet base. Ovary with two flexures. Tail short and conoid. Male in host´s body cavity smaller than secondary female but not obese. Cephalic region continuous. Excretory pore at the level of nerve ring. Testis outstretched. Tail tapering to a rounded tip. Bursa absent. Spicules cephalated, in bowling bottle shape or triangular. Gubernaculum present, the same size as the spicule and polymorphic. The tip of the caudal appendage of all vermiform females (Fig. 8) and immature females is bifurcated (Fig. 4).
First generation entomoparasitic females (Fig. 1): develops from preadult fertilized female that invades host´s, obese, sausage-shaped curving ventrally, with round, wrinkled ends. Cuticle annulated. Vulva subterminal, uterus filling most of body cavity, with ovary convoluted in anterior region, ovoviviparous.
Second generation parasitic females (Fig. 2): ovoviviparous. The subsequent generations of parasitic (swollen, gravid) females tend to be shorter than the first generation forms. Young parasitic females of all generations have a relative long tail and long vulva-tail measurement. As they mature, the tail shortens, the vulva becomes more posterior in position, the stylet becomes faint, the pharyngeal glands become atrophied and the excretory pore, nerve ring, anus and vulva are more difficultto locate. The ovary is usually reflexed 2-3 times in the parasitic females.
Males (Figs. 5, 6, 7): pharyngeal glands atrophied; tail tip angular-truncate, showing bifurcation in juveniles; spicules straight, in bowling bottle shape or triangular, with wide base. The gubernaculum long can assume several shapes, laminate and in bowling bottle shape. Usually it is straight, however it may be bent upward, or rarely appear double. Unfortunately the small size of this structure hinders further analysis. The bursa is absent
Vermiform (infective) female (Figs. 3, 4): the cuticle is very thick, and ridged both transversely and longitudinally. It has an obtuse head with the lip region somehow constricted. The straight stylet lacks basal knobs or thickenings and the dorsal and subventral gland openings are located within three stylet lengths from the base of the stylet. The excretory pore is fairly posterior and opens opposite to or posterior to the nerve ring. The hemizonid is located just posterior to the nerve ring. The pharyngeal glands may extend to the tip of the ovary. The uterus and ovary are fairly short until the female mates, at which time the uterus expands with circular sperm cells. The great majority of vermiform females inside the host have mated. The tail tip of all vermiform females and immature males is notoriously bifurcated.
Type host: Adults of Harmonia axyridis (Pallas, 1773) (Coleoptera, Coccinellidae). The collection was made at midday in a farm near La Plata city, during the summer of 2016/2017. The insect was found over round green zucchini´s flowers.
Type locality: Colonia Urquiza (34°96'72S, 58°0496W), Gran La Plata, Buenos Aires province, Argentina.
Type material: deposited in Helminthological Collection of the Museo de Ciencias Naturales de La Plata. Holotype: NT20058. Paratypes deposited in Nematological Collection of CEPAVE nº 00378
The prevalence observed was 25%. The number of nematodes per H. axyridis was ranged from five to eighty-two
Cytogenetics results.- In the three types of females analyzed, stages of mitotic metaphases and meiotic anaphases were observed with two chromosomal complements. In cells with mitosis we observed 8 and 9 chromosomes (Figs. 9, 10); cells with meiosis presented four bivalents chromosome and others cells with four bivalents plus a univalent chromosome. (Figs. 11, 12). The chromosomes of this species are very tiny: the largest is 1.60 microns in metaphase. In meiotic metaphase, both homologous chromosomes are paired along their longitudinal axis, pretending not to have a localized centromere. No figures that denote the presence of chiasmas were observed. In mitotic metaphase, the largest chromosome usually has a break at one end that gives it an "L" appearance; both arms are separated at an approximate 45 degree angle.
Discussion
Current species described only two of the genus P. bifurcatus Poinar Jr. & Steenberg, 2012, from Denmark, and P. coccinellinae Iperti & van Waerebeke, 1968, from France, are parasitizing Coccinellidae, coincidentally to Harmonia spp. Most of the species of the genus are para sites of Curculionidae and a few of Drosophila. P. pseudobifurcatus sp. n. is very similar to P. bifurcatus by having the bifurcated vermiform infective female and immature male tail. The excretory pore opening at the level of the nerve ring, but it differs from our new species in the presence of the bursa. The gubernaculum is shorter. The male is smaller than our new species. And to P. coccinellinae seems to be our new species by the absence of the bursa, but it can be separated by the excretory pore near stylet base. The tail is wide, short and pointed. Iperti and van Waerebeke (1968) described the young female that it would seem to be the infective female, as having a short stylet (4–5 µm) with basal thickenings that are lacking in P. bifurcates and in P. pseudobifurcatus sp. n. They also show the spicule in lateral view as curved with the base separated, having our new species spicule in bowling bottle shape. They did not give a complete description of the vermiform infective female. They described two fe-males, the young female world seems to be the infective and the other one is a gravid female, and a male. Also the measurements are incomplete so we do not incorporate P. coccinellinae into Table 1.
The cytogenetic study of the Tylenchida order isvery scarce and fragmentary: of the approximately30,000 species taxonomically described as plant parasites (Siddiqi 2000), less than 100 are the speciesfrom which something of their chromosomes is known.This is the first cytogenetic contribution since thereare no previous data of tylenchid nematodes parasitesof insects (Eisenback & Triantaphyllou 1991; Triantaphyllou & Hirschmann 1980). The morphology of thechromosomes shows that they would not present a localized centromere, ie holokinetic chromosomes (Márquez-Corro et al. 2017). We describe for the first timetwo karyotypes compatible with the presence of a sexual chromosome (Adamson 1989). The haploid chromosome number described for P. pseudobifurcatus sp. n. would be the lowest of the tyilenchid nematodes andwould be close to the plant parasitic nematodes, with genus Helicotylenchus Steiner, 1945, that presents n = 5 chromosomes and to Meloidodera (Chitwood, Hannon& Esser, 1956), which has description of species thatalso have n = 5 chromosomes (Triantaphyllou & Hirschmann 1980).
Conclusions
The genus Parasitylenchus is characterized by having three types of adults inside the host: a first generation parasitic female, a second generation parasitic female and a male. The eggs produced by the first generation females develop into juveniles that mature to adults,mate and keep eggs and juveniles inside the host. Thejuveniles from the second generation females exit the host, killing it, and the final molt and mating occurs inthe environment where the infective females find new hosts. As parasitism results in the reduction of the fatbody and partial or complete atrophy of the reproductive organs of the host that compromises its fecundity,it can therefore be considered as an agent of biologicalcontrol to management abundant populations of this invasive species. Although P. pseudobifurcatus sp. n. isan insect parasite that kills its host, it does not belong toentomopathogenic nematodes, which kill its host withthe help of an associated bacterium, relatively quickly(between 24 and 48 hours after infection). Thus, weconsider our new species as a parasite that castratesand slowly kills its host, co-existing both a time necessary for the development of the nematode. Future genomic studies will help to determine how closely relatedthese populations are.
Acknowledgements
We would like to thank to Lic. Luis Giambelluca for photograpied the material. Many thanks are expressed to Manuela Reboredo for her careful review of the English language.
Roles:
NBC, GRR, SEG: conducted the sampling; NBC, GRR, SEG, SRG: processed the material in the laboratory; NBC, SRG: wrote the manuscript; NBC, GRR, SEG, SRG: revised and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethical / legal aspects:
This work did not incur any ethical or legal problems.
Funding:
The present work was carried out thanks to the financing of the Comisión de Investigaciones Científicas de la provincia de Buenos Aires, CIC and of the funds of the Incentive Program of the Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata (UNLP).
Citación:
Camino N.B., S. Rodríguez Gil, G.R. Reboredo and S.E. González. 2019. Morphological and cytogenetic description of a new species of Parasitylenchus Micoletzky, 1922 (Tylenchida, Allantonematidae) parasitizing Harmonia axyridis (Pallas, 1773) (Coleopera, Coccinellidae) in Argentina. Revista peruana de biología 26(1): 027 -032 (Febrero 2019). doi: http://dx.doi.org/10.15381/rpb.v26i1.15907
Literature cited
Adamson M. L. 1981. Development and transmission of Gyrinicola batrachiensis (Walton, 1929) Adamson, 1981 (Pharyngodonidae: Oxyuroidea). Canadian Journal of Zoology 59: 1351–1367. https://doi.org/10.1139/z81-188. [ Links ]
Adamson M.L. 1989. Evolutionary biology of the Oxyurida (Nematoda): biofacies of a haplodiploid taxon. Advances in Parasitology 28:175-228. https://doi.org/10.1016/S0065-308X(08)60333-4 [ Links ]
Eisenback J.D., & H.H. Triantaphyllou. 1991. Root-knot nematodes: Meloidogyne species and races- Pp. 191-274, In W.R. Nickle, ed. Manual of Agricultural Nematology. Marcell Dekker: New York. [ Links ]
Fuchs G. 1915. Tylenchus dispar curvidentis m. und Tylenchus dispar cryphiyli m. Zoologischer Anzeiger 45: 195-207. [ Links ]
Fuchs G. 1929. Die Parasiten einiger Bussel-uncl Borkeiikafer. Zeitschrift fur Parasitenkunde 2: 248-285. [ Links ]
Fuchs G. 1938. Neue Parasiten und Halbparasiten bei Borkenkafem undeinige andere Nematoden. II. Zoologische Jahrbucher 70. Systematisch 291-380 [ Links ]
Iperti G. & van D. Waerebeke. 1968. Description, biologie et importance d’une nouvelle espèce d’Allantonematidae (Nematoda), parasite des coccinelles aphidiphages: Parasitylenchus coccinellinae n. sp. Entomophaga 13: 107-119 https://doi.org/10.1007/BF02371781
Márquez-Corro J.I., M. Escudero & M. Luceño. 2017. Do holocentric chromosomes represent an evolutionary advantage? A study of paired analyses of diversification rates of lineages with holocentric chromosomes and their monocentric closest relatives. Chromosome Research 1–14. https://doi.org/10.1007/s10577-017-9566-8 [ Links ]
Micoletzky H. 1922. Die freilebenden Erdnematoden mit besonderer Beruek-sielitigung der Steiermark und der Bukowina, zugleich mit einer Revisionsamtlicher nicht mariner, freilebender Nematoden in Form von Genus Beschreibungen und Bestimmungsschlusseln. Archiv für Naturgeschichte 87A 9: 1-650 [ Links ]
Oldham J.N. 1930. On the infestation of Elm Bark Beetles (Scolytidae) by a nematode Parasitylenchus scolyti n. sp. Journal of Helminthology 8: 239-248. [ Links ]
Paliulis L.V., I.F. Korf & S.W.L. Chan. 2012. Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Research 20: 579–593. https://doi.org/10.1007/s10577-0129292-1 [ Links ]
Poinar G.O.Jr. & T. Steenberg. 2012. Parasitylenchus bifurcatus n. sp. (Tylenchida: Allantonematidae) parasitizing Harmonia axyridis (Coleoptera: Coccinellidae). Parasites & Vectors 5: 218. https:// doi.org/10.1186/17563305-5-218 [ Links ]
Ruhm W. 1954. Einigc neue Ipidenspezifische Nematodenartem. Zoologischer Anzeiger 153: 9-10 [ Links ]
Ruhm W. 1956. Die Nematoden der Ipiden. Parasitologische Sehrifteiireihe 6: 148. [ Links ]
Siddiqi M.R. 2000. Tylenchida parasites of plants and insects. 2nd ed. Wallingford, UK, CABI Publishing, 833 pp. [ Links ]
Triantaphyllou A.C. & H. Hirschmann. 1980. Cytogenetics and morphology in relation to evolution and speciation of plant-parasitic nematodes. Annual Review of Phytopathology 18: 333–359. [ Links ]
Wuelker G. 1923. Uber Fortpflanzung und Entwicklung von Allantonema undverwandten Nematoden. Erg. Fortsehr. Zool. 5: 389-507.. 1929. Bemerkungen zur Arbeit von G. Fuehs. Die Parasiten einigerEussel und Borkenkafer. Zeit Parasitenk. 2: 286-290 [ Links ]
* Autor para correspondencia
Email NC: nemainst@cepave.edu.ar
Email SRG: sergio.rodriguezgil@gmail.com
Email GR: grreboredo@yahoo.com.ar
Email SG: san.gonzalez.san@gmail.coM
Presentado: 05/07/2018
Aceptado: 13/02/2019
Publicado online: 30/03/2019