Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista Peruana de Biología
On-line version ISSN 1727-9933
Rev. peru biol. vol.26 no.3 Lima Aug. 2019
http://dx.doi.org/10.15381/rpb.v26i3.16775
TRABAJOS ORIGINALES
Geographic distribution analysis of the genusXenodacnis (Birds: Thraupidae) using ecologicalniche modeling
Análisis de la distribución geográfica del género Xenodacnis (Aves: Thraupidae) utilizando el modelado de nicho ecológico
Juan M. Aguilar ORCID: 0000-0002-3186-7438
Universidad del Azuay, Cuenca, Ecuador.
Abstract
Xenodacnis is a monotypic thraupid genus restricted to the tropical high Andes of Peru and Ecuador. Its only species, X. parina has a large discontinuous distribution from central Ecuador to southern Peru. To date, three subspecies are recognized, all separated by geographical barriers that clouded promote allopatric events. The taxonomic affinities of the Ecuadorian population have not been assessed since its discovery in the 1970s at the Cajas National Park in Azuay province. I studied the environmental affinities between the distribution of the described subspecies and the Ecuadorian population bias ecological niche modeling. I found a distinctive ecological niche in the distribution of each of the analyzed populations and also for the southern Arequipa population. These different environmental niche conditions come apart by deep Andean valleys playing a role as geographical barriers for the isolation of these populations that need further taxonomic analysis.
Keywords: High Andes; biogeography; isolation; niche modeling; Xenodacnis parina.
Resumen
Xenodacnis es un género de traupido mono típico restringido a los altos Andes tropicales de Perú y Ecuador. Su única especie, X. parina tiene una distribución extensa pero discontinua desde el centro Ecuador hasta el sur de Perú. A la fecha se reconocen tres subespecies, todas separadas por barreras geográficas que pudieron promover eventos alopátricos. Las afinidades taxonómicas de la población de ecuador no se han analizado desde su descubrimiento en los años 70 dentro del Parque Nacional Cajas en la provincia del Azuay. Yo estudié las afinidades ambientales entre las distribuciones de las subespecies descritas y la población en Ecuador mediante modelos de nicho ambiental. Encontré diferentes condiciones ambientales en los nichos de cada una de las poblaciones analizadas y también para la población sureña de Arequipa. Estas diferencias ambientales están separadas por profundos valles Andinos que cumplen el roll de barreras geográficas para el aislamiento de estas poblaciones que necesitan un próximo análisis taxonómico.
Palabras clave: Altos Andes; biogeografía; aislamiento; modelos de nicho; Xenodacnis parina.
Introduction
The thraupid genus Xenodacnis (Cabanis 1873) is a high Andean specialist that occurs from central Ecuador through southern Peru, in elevations between 3,000 and 4,400 m; it has a specialized diet on small insects and extra floral nectar gleaned from beneath the leaves of Gynoxys shrubs, mainly within Polylepis woodland patches (Ridgely & Greenfield 2001, Aguilar & Iñiguez 2015). It is placed in a clade of montane forest specialists with several genera formerly classified in the ‘finch’ family Emberizidae, including Phrygilus, Idiopsar, Diuca, Haplospiza, and Acanthidops (Campagna et al. 2011, Barker et al. 2012, Burns et al. 2014). Xenodacnis has a single species, the Tit-like Dacnis, X. parina; moreover, some authors consider that the genus is composed by at least two different species (del Hoyo & Collar 2018).
Xenodacnis was described in 1873 by Jean Louis Cabanis, the type locality being "Maraynioc", Junín department, east central Peru (Mlíkovský 2010). Later, Bond y de Schauensee (1939) described a different species from the northern Andes of Peru: X. petersi, with two subspecies: X. p. petersi (Yánac, Ancash department, west central Peru), and X. p. bella (Atuén, Amazonas department, northern Peru). In a revision of the genus, Zimmer (1942) and later Zimmer y Mayr (1943) established size and some plumage characters (i.e., bright streaks in male foreparts) as diagnosable characters, and suggested that X. petersi merit species status, but were later lumped under a single species by de Schauensee (1966), a treatment followed by all subsequent authors (Paynter 1970, Ridgely & Tudor 2009, Hilty 2011). Currently, X. parina is considered as a single species with three subspecies: X. parina parina, X. p. bella and X. p. petersi by most authorities (Clements et al. 2017, Remsen et al. 2017), but del Hoyo y Collar (2018) recently resurrected species status for the petersi group. Yet, the taxonomy of X. parina has not been thoroughly revised to date.
Ridgely (1980) provided the first report of X. parina from southern Ecuador, in Cajas National Park, Azuay province. He did not determine the subspecies identity for this population, but suggested that it might represent an undescribed form, resembling the geographically closest X. p. bella, but the taxonomic status of the Ecuadorian population is still uncertain (Ridgely & Greenfield 2001, del Hoyo & Collar 2018).
The northern distribution of X. parina is interrupted by the dry North Peru Low, a depression of the Andean cordillera that starts at the Jubones River valley in Ecuador and ends at the Huancabamba River valley in Peru (Weigend 2002, 2004). In central Peru the Andean valleys split the described subspecies; the nominal X. p. parina from the eastern Andean ridges and X. p. petersi from the western cordillera, this last subspecies is separated from the northern Peruvian X. p. bella by Marañon River. It has been postulated that these barriers for species dispersal, acted as drivers of allopatric speciation in birds (Vuilleumier 1969, Parker et al. 1985, Gutiérrez-Pinto et al. 2012, Winger & Bates 2015, Hazzi et al. 2018). However, in allopatry, species with specialized diets (e.g. Xenodacnis), may have conserved morphological traits that reflect ecological adaptations (Winger & Bates 2015), making it difficult to assess the degree of morphological variation for some taxa. Consequently, morphologically cryptic species are often lumped under a single species with presumed widespread distributions (Cabot & de Vries 2009, Lara et al. 2012, Avendaño et al. 2015), as might be the case for allopatric populations of Xenodacnis.
Environmental niche models have been useful to define Andean bird distributions (Jiguet et al. 2010, Tinoco et al. 2009); and have shown non-overlapping distribution in closely related high Andean species with apparently little differences in niches occupied (Jiguet et al. 2010). In order to analyze the distribution of X. parina, I performed an environmental analysis to assess the taxonomy of this isolated, cryptic bird species complex (Gill 2014, Sangster 2014).
Material and methods
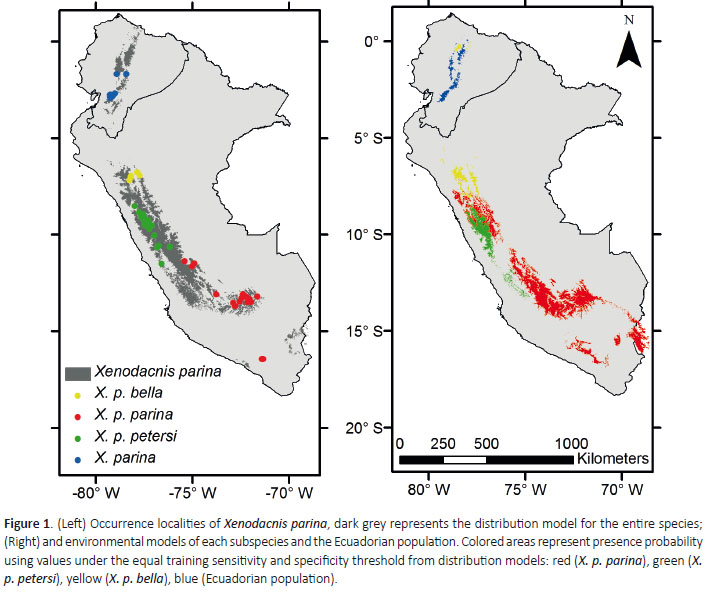
To explore environmental niche and predict the geographic ranges of X. parina described subspecies of Peru and the Ecuadorian population; occurrence localities were obtained from online resources (eBird 2015), published literature, fieldwork and unpublished records. Environmental niche models were based on 19 bioclimatic variables, obtained from WorldClim (http://www. worldclim.org), which included seasonality, averages and extremes in temperature and precipitation across South America at a resolution of 30 sec (Hijmans et al. 2005). To avoid spatial autocorrelation, occurrences in localities closer than 5 km were excluded. Niche models were obtained using the maximum entropy algorithm as implemented in Maxent 3.3.3k (Phillips et al. 2006). For all models, we used default parameter settings. To test model performance, we evaluated if 30% of randomly selected points are predicted by 10 replicate bootstrap models performed with remaining fraction of data, obtaining a maximum possible test value of the area under the ROC curve (Test AUC). Binary maps of presence and absence of suitable habitat conditions were based on the mean values of equal training sensitivity and specificity threshold from models (Phillips et al. 2006). One environmental niche model was obtained for the species as a whole, one for each described subspecies from Peru, and one for the Ecuadorian isolated population.
To analyze differences in environmental conditions between the Ecuadorian population and all Peruvian subspecies, the climate envelope of each group was characterized by extracting the bioclimatic values at each occurrence locality and synthesizing eight non-correlated variables in a principal component analysis (PCA). The first two components of the analysis were plotted to illustrate multivariate space of the environmental niche, with 50% confidence ellipses for each subspecies and the Ecuadorian population. Finally, we analyzed the correlations between environmental distances and geographical distances among subpopulations using a Mantel test.
Results
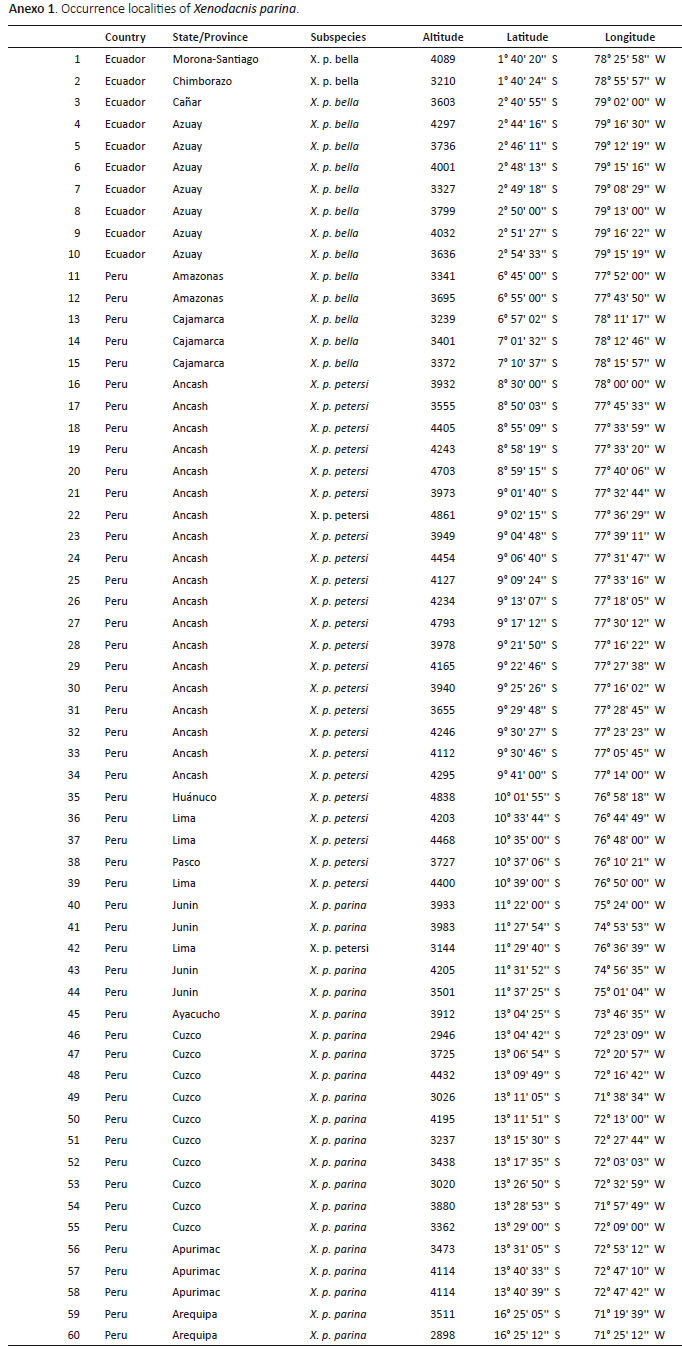
A total of 60 occurrence localities of Xenodacnis were obtained after data clean and avoiding spatial autocorrelation: 20 records of X. p. parina, 25 of X. p. petersi, 5 of X. p. bella and 10 from Ecuador (Anexo 1). The predicted distribution for the entire species (n = 60, ETSS = 0.249, TestAUC = 0.984) showed a somewhat continuous distribution in Peru, but an entire isolation for the Ecuadorian population (Fig. 1). The suitable environmental conditions for the Ecuadorian population are also restricted to this country (n =10, ETSS = 0.432, Test AUC = 0.998 sd=0.002), but the model predicts areas extending north where it has notbeen recorded (Fig 1). Distribution models for the Peruvian subspecies overlap mostly in west-central Peru (Fig1). Models showed that suitable conditions for X. p. parinaand X. p. petersi are not found in Ecuador (n= 20, ETSS =0.307, Test AUC = 0.986 sd=0.003), but are widely distributed across the Peruvian Andes, with a large overlap inan area were only X. p. petersi has been positively recorded (n = 25, ETSS = 0.302, Test AUC = 0.997 sd= 0.001); the southern Arequipa records have also proven differentenvironmental conditions in the model obtained for the species. Model for X. p. bella showed that environmental conditions for this subspecies are marginally predicted innorthern Ecuador, in an area were no Xenodacnis has been recorded (n = 5, ETSS = 0.587, Test AUC = 0.997 sd=0.001)(Fig. 1).
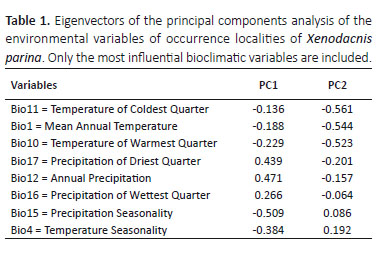
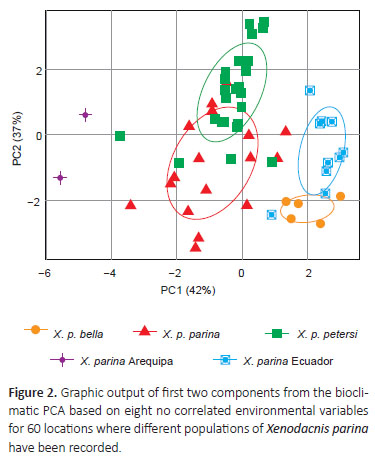
The first two components from the PCA of bioclimatic variables explained 79% of variance. First component had high loadings on precipitation means and seasonality values. The second component had the highest loadings in temperature variables, especially temperature of coldest quarter (Table 1). Overall, the population of Ecuador occupies a climatic envelope characterized by higher minimum annuals and less variation in seasonal temperatures, accompanied by higher annual precipitation (Fig. 2). Because data from Arequipa did not occupy the predicted distribution for the entire species, we separated data from this southern X. p. parina population for the following PCA. The results of the Mantel test also revealed correlation between environmental and geographical distance (r= 0.35, p= 0.001).
Discussion
These results show isolation accompanied by variation of environmental niche conditions. These environmental differences suggest a reduction in the ability to move across Andean barriers and highlighted the differences between biogeographical regions in the Andes of Peru and Ecuador (Wang & Bradburd 2014, Hazzi et al. 2018), reviling also differences for the Arequipa southern population. The Ecuadorian population inhabits shrubby paramo characterized by higher precipitation and higher temperature, when compared with Peruvian high Andean ecosystems, the X. p. bella populationin northern Peru is found in the Jalca biome (Ochoa-Tocachi et al. 2018), whereas western and eastern described subspecies from central Peruvian Andes inhabit more xeric puna ecosystem, which is drier, colder, and more seasonal (Luteyn 1999, Tovar et al. 2013); the Arequipa population also inhabits lower precipitation means and different seasonality values (Fig. 2), and is isolated by the Apurimac River Valley (Hazzi et al. 2018). Both, the reduced ability to disperse across a major geographical barrier and isolation by environment are drivers of speciation in the tropical Andes (Wang & Bradburd 2014, Smith et al. 2014, Winger & Bates 2015).
It seems plausible that an ancient Xenodacnis spread north from the older central Andes from Peru as other high Andean birds (Chesser 2000, Gutiérrez-Pinto et al. 2012, Valderrama 2014, Benham et al. 2015), colonizing new high Andean ecosystems reaching north of the North Peru Low (Weir 2009, Tobias et al. 2014, Winger & Bates 2015), during the Miocene (3.4 Ma; Weir & Schluter 2008). This colonization event might have been followed by allopatry 2.7 Ma, when the northern Andes in Ecuador had already reached modern elevations (Gregory-Wodzicki 2000), and active Andean drainage systems had already shaped the North Peru Low (Garzione et al. 2008). The colonization of Xenodacnis may have followed a high Andean common fashion of expansion and isolation with deep Andean valleys playing an important role in the differentiation of populations (Gutiérrez-Pinto et al. 2012, Valderrama 2014, Benham et al. 2015, Hazzi et al. 2018).
All information presented is a contribution to assess the distribution and taxonomy of Xenodacnis; results obtained from environmental niche analysis indicates that X. parina as a hole species occupy different environmental conditions across a large tropical high Andean distribution, supporting the current taxonomy of the genus, in which X. p. petersi in a polytypic group and X. p. parina is monotypic (Clements et al. 2018); however, the northern and southern populations, from Ecuador and Arequipa, are not yet described lineages, geographically isolated with particular environmental conditions that need further taxonomic analysis.
Citación:
Aguilar J.M. 2019. Geographic distribution analysis of the genus Xenodacnis (Birds: Thraupidae) using ecological niche modeling. Revista peruana de biología 26(3): 317 - 324 (Septiembre 2019). doi: http://dx.doi.org/10.15381/rpb.v26i3.16775
Literature cited
Aguilar J.M., & X. Iñiguez. 2015. Hábitos alimentarios de Xenodacnis (Xenodacnis parina) en los páramos del sur del Ecuador. Ornitología Neotropical 26:211-217. [ Links ]
Avendaño J.E., A.M. Cuervo, J.P. López-O, N. Gutiérrez-Pinto, A. Cortés-Diago, & C.D. Cadena. 2015. A new species of tapaculo (Rhinocryptidae: Scytalopus) from the Serranía de Perijá of Colombia and Venezuela. Auk 132:450-466. https://doi.org/10.1642/AUK-14-166.1 [ Links ]
Barker F.K., K.J. Burns, J. Klicka, S.M. Lanyon, & I.J. Lovette. 2012. Going to extremes: contrasting rates of diversification in a recent radiation of new world passerine birds. Syst. Biol. 62:298-320. https://doi.org/10.1093/sysbio/sys094 [ Links ]
Benham P.M., A.M. Cuervo, J.A. McGuire, & C.C. Witt 2015. Biogeography of the Andean metaltail hummingbirds: Contrasting evolutionary histories of treeline and habitatgeneralist clades. Journal of Biogeography. https://doi.org/10.1111/jbi.12452 [ Links ]
Bond J., & R.M. de Schauensee. 1939. Description of new species and subspecies of Xenodacnis. Notulae Naturae 40: 1-2. [ Links ]
Burns K.J., A.J. Shultz, P.O. Title, N.A. Mason, F.K. Barker, J. Klicka, S.M. Lanyon, & I.J. Lovette. 2014. Phylogenetics and diversification of tanagers (Passeriformes: Thraupidae), the largest radiation of Neotropical songbirds. Molecular Phylogenetics and Evolution 75: 41-77. https://doi.org/10.1016/j.ympev.2014.02.006 [ Links ]
Cabanis J. 1873. Ueber Xenodacnis parina nov. gen. et spec. des Berliner Museums, von C. Jelski in Peru entdeckt. Journal für Ornithologie 21: 311-312. [ Links ]
Cabot J. & T. de Vries. 2009. A new subspecies of Gurney's Hawk Buteo poecilochrous. Bulletin of the British Ornithologists' Club 129: 149-164. [ Links ]
Campagna L., K. Geale, P. Handford, D.A. Lijtmaer, P.L. Tubaro, P. L. & S.C. Lougheed. 2011. A molecular phylogeny of the sierra-finches (Phrygilus, Passeriformes): extreme polyphyly in a group of Andean specialists. Molecular Phylogenetics and Evolution 61: 521-533. https://doi.org/10.1016/j.ympev.2011.07.011 [ Links ]
Chesser R.T. 2000. Evolution in the high Andes: the phylogenetics of Muscisaxicola ground-tyrants. Molecular Phylogenetics and Evolution 15: 369-380. https://doi.org/10.1006/mpev.1999.0774 [ Links ]
Clements J.F., T.S. Schulenberg, M.J. Iliff, D. Roberson, T.A. Fredericks, B.L. Sullivan, & C.L. Wood. 2018. The eBird/Clements checklist of birds of the world: v2018. Cornell Lab of Ornithology, Ithaca, New York. http://www.birds.cornell.edu/clementschecklist/download/ (accessed 22 November 2018). [ Links ]
del Hoyo J., & N. Collar 2018. Streaked Dacnis (Xenodacnis petersi), in: del Hoyo J., A. Elliott, J. Sargatal, D.A. Christie, & E. de Juana (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona. (retrieved from https://www.hbw.com/node/1344189 on 23 November 2018). [ Links ]
eBird. 2015. eBird: An online database of bird distribution and abundance. eBird, Cornell Lab of Ornithology, Ithaca, New York. http://www.ebird.org (accessed 10 July 2015). [ Links ]
Garzione C.N., G.D. Hoke, J.C. Libarkin, S. Withers, B. MacFadden, J. Eiler, P. Ghosh, & A. Mulch. 2008. Rise of the Andes. Science 320: 1304-1307. https://doi.org/10.1126/science.1148615 [ Links ]
Gill F. 2014. Species taxonomy of birds: which null hypothesis? Auk 131: 150-161. https://doi.org/10.1642/AUK-13206.1 [ Links ]
Gutiérrez-Pinto N., A.M. Cuervo, J. Miranda, J.L. Pérez-Emán, R.T. Brumfield, & C.D. Cadena. 2012. Non-monophyly and deep genetic differentiation across low-elevation barriers in a Neotropical montane bird (Basileuterus tristriatus; Aves: Parulidae). Molecular Phylogenetics and Evolution 64: 156-165. https://doi.org/10.1016/j.ympev.2012.03.011 [ Links ]
Gregory-Wodzicki K.M. 2000. Uplift history of the Central and Northern Andes: a review. Geological Society of America Bulletin 112: 1091-1105. https://doi.org/10.1130/0016-7606(2000)112<1091:UHOTCA>2.0.CO;2
Hazzi N.A., J.S. Moreno, C. Ortiz-Movliav, & R.D. Palacio. 2018. Biogeographic regions and events of isolation and diversification of the endemic biota of the tropical Andes. Proceedings of the National Academy of Sciences 115(31): 7985-7990. https://doi.org/10.1073/pnas.1803908115 [ Links ]
Hijmans R.J., S.E. Cameron, J.L. Parra, P.G. Jones, & A. Jarvis. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965-1978. https://doi.org/10.1002/joc.1276 [ Links ]
Hilty S.L. 2011. Family Thraupidae (tanagers). Pp. 46-329 in: del Hoyo J., A. Elliott, & D.A. Christie (eds). Handbook of the birds of the world, volume 16: Tanagers to New World blackbirds. Lynx Edicions, Barcelona. [ Links ]
del Hoyo J., & N.J. Collar. 2018. Streaked Dacnis (Xenodacnis petersi). In: del Hoyo J., A. Elliott, J. Sargatal, D.A. Christie, & E. de Juana (eds.) Handbook of the birds of the world alive. Lynx Edicions, Barcelona. https://www.hbw.com/node/1344189 (accessed 7 December 2018). [ Links ]
Jiguet F., M. Barbet-Massin, & P-Y. Henry. 2010. Predicting potential distributions of two rare allopatric sister species, the globally threatened Doliornis cotingas in the Andes. Journal of Field Ornithology 81: 325-339. https://doi.org/10.1111/j.1557-9263.2010.00289.x [ Links ]
Lara C.E., A.M. Cuervo, S.V. Valderrama, D. Calderón-F., & C.D. Cadena. 2012. A new species of wren (Troglodytidae: Thryophilus) from the dry Cauca River Canyon, northwestern Colombia. Auk 129: 537-550. https://doi.org/10.1525/auk.2012.12028 [ Links ]
Luteyn J.L. 1999. Paramos: a checklist of plant diversity, geographical distribution and botanical literature. Memoirs of the New York Botanical Garden 84: 1-278. [ Links ]
Mlíkovský J. 2010. Types of birds in the collections of the Museum and Institute of Zoology, Polish Academy of Sciences, Warszawa, Poland. Part 4: varia, addenda and conclusions. Journal of the National Museum (Prague), Natural History Series 179 (6): 47-92 [ Links ]
Ochoa-Tocachi B.F., W. Buytaert, & J. Antiporta. 2018. High-resolution hydrometeorological data from a network of headwater catchments in the tropical Andes Scientific Data volume 5, Article number: 180080. https://doi.org/10.1038/sdata.2018.80 [ Links ]
Parker T.A., T.S. Schulenberg, G.R. Graves, & M.J. Braun. 1985.
The avifauna of the Huancabamba region, northern Peru, pp. 169-197 in: Buckley P.A., M.S. Foster, E.S. Morton, R.S. Ridgely, & F.G. Buckley. (eds.) Neotropical ornithology. Ornithological Monographs 36, American Ornithologists' Union, Washington D.C. https://doi.org/10.2307/40168282
Paynter R.A. (ed). 1970. Check-list of birds of the world 13 (Emberizinae, Catamblyrhynchinae, Cardinalinae,Thraupinae, Tersininae). Harvard University Press, Cambridge. [ Links ]
Phillips S.J., R.P. Anderson, & R.E. Schapire. 2006. Maximum entropy modeling of species geographic distribu tions. Ecological Modelling 190: 231-259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 [ Links ]
Remsen J.V., J.I. Areta, C.D. Cadena, A. Jaramillo, M. Nores, J.F. Pacheco, J. Pérez-Emán, M.B. Robbins, F.G. Stiles, D.F. Stotz, & K.J. Zimmer. 2017. A classification of the bird species of South America. American Ornithologists' Union, Washington. http://www.museum.lsu.edu/~Remsen/SACCBaseline.html (accessed 22 November 2017). [ Links ]
Ridgely R.S. 1980. Notes on some rare or previously unrecorded birds in Ecuador. American Bird 34: 242-248. [ Links ]
Ridgely R.S., & P. Greenfield. 2001. The birds of Ecuador: status, distribution and taxonomy. Cornell Univ. Press, Ithaca. [ Links ]
Ridgely R.S., & G. Tudor. 2009. Birds of South America. Passerines. Helm Identification Guides, London. [ Links ]
Sangster G. 2014. The application of species criteria in avian taxonomy and its implications for the debate over species concepts. Biol. Rev. 89: 199-214. https://doi.org/10.1111/brv.12051 [ Links ]
Correspondencia:
Juan M. Aguilar: maguilar@uazuay.edu.ec
Departamento de posgrados, Universidad del Azuay, Av. 24 de Mayo 7-77 y Hernán Malo, Código postal: 981, Cuenca, Ecuador.
Presentado: 18/12/2018
Aceptado: 12/02/2019
Publicado online: 30/09/2019