Introduction
Chitin is one of the most abundant biopolymers in nature after cellulose and is found in crustacean exoskeletons, insects and fungal cell walls. It is a polysaccharide consisting of β-1,4-linked N-acetyl-D-glucosamine, that in natural tissues is associated mainly to proteins and minerals, but also to lipids and pigments (Dun et al. 2019; El Knidri et al. 2018). It has been observed that the content of chitin vary according to the source and the species, from which this biopolymer has been recovered. It was reported that in Crangon crangon shrimp waste, protein content ranges from 10 to 38%, minerals from 31 to 44% and chitin from 24 to 46% (M. Bajaj et al. 2011). Chitin and its deacetylated derivative chitosan, have a commercial value and are highly demanded due to their biocompatibility and biodegradability capacity, which makes them applicable in medicine, agriculture, environmental protection, food processing, cosmetics, pharmaceuticals, textile industries and biotechnological products (Arbia et al. 2013; Prameela et al. 2010; El Knidri et al. 2018).
The annual international trade of crustaceans was 5129421 t in 2016 (FAO 2018). The increasing amount of waste generated from industrial processing of hydrobiological resources (exoskeletons of shrimp, prawn, crab, and other crustacean) has become an environmental problem. Exoskeleton and cephalothorax of some crustacean species such as shrimp or prawn, are wasted, although they contains chitin, proteins and pigments that could have an important commercial value. The amount recovered of those components depend on processing conditions, species, seasonal variations, etc. (Duarte de Holanda and Netto, 2006; Rodde et al. 2008; Xu et al. 2008; Al Sagheer et al. 2009; Palpandi et al. 2009; Wang et al. 2011).
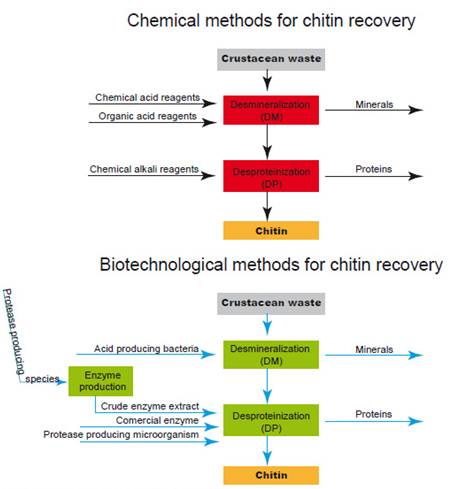
Chitin recovery from crustacean waste requires two main processes, demineralization (DM) and deproteinization (DP) in order to separate the biopolymer from proteins and minerals to which it is associated in natural tissues. Industrial production of chitin involves chemical methods with the use of an alkali such as sodium hydroxide (NaOH) to remove proteins and hydrochloric acid (HCl) to remove minerals (Fig. 1). Although those methods have been commercially viable, they represent an environmental cost that needs to be addressed. As an alternative, research efforts have been made to contribute to replace chemical procedures by biotechnological ones which are environmentally friendly as shown in Figure 1 (Arbia et al. 2013; Ghorbel-Bellaaj et al. 2012; Liu et al. 2014; Francisco et al. 2015; Bashandy et al. 2016; Sedaghat et al. 2017; Zhang et al. 2017; Hamdi et al. 2017; Castro et al. 2018; Dun et al. 2018; Liu et al. 2020). Comparatively high cost of their manufacturing process, has been reported as a disadvantage for the use of chitin in some industries (Sini et al. 2007), however, a biological method to recover this biopolymer seems to be low cost and feasible to scale it up to industrial level (Dun et al. 2018).
Moreover, it has been detected the amino acid composition of the fermentation broth obtained after the bioprocesses, in order to give a full use of the crustacean waste (Liu et al. 2020). It also is necessary to take into consideration variations in the protein and mineral content between the exoskeletons of different species but also within the same species, particularly because they could be determinant to set up processes conditions.
This mini-review compiles some of the most updated published information on biotechnological processes applied to chitin recovery from crustacean waste.
Deproteination and demineralization of crustacean waste bioprocesses
To separate chitin from proteins and minerals to which it is associated in waste natural tissues, two steps need to be made, deproteinization (DP) and demineralization (DM). Microorganisms and proteolytic enzymes (enzymatic extracts or purified enzymes) have been used to separate proteins and minerals from the tissues. Those bioprocesses can be performed in two separate steps (to remove proteins and to remove minerals) or in one step to remove both simultaneously.
The efficiency of both bioprocesses, depends on the species, carbon source, pH (initial and during fermentation), volume of inoculum, temperature, among others (Prameela et al. 2010; Gortari & Hours 2013; Liu et al. 2020). Biotechnological bioprocesses have shown advantages and disadvantages; it has been remarked the need for the development of new methods to produce high quality chitosan with an improved degree of deacetylation (El Knidri et al. 2018).
Table 1 shows the results reported by some authors between years 2009 and 2020, to achieve chitin recovery with different operational conditions, most of which are similar, however the table also shows observed differences, which are relevant if the bioprocesses are to be scaled to pilot and industrial level; those factors are species used and bioreaction times achieved, among others.
The studies discussed in the present review can only be partially compared among them due to the use of crustacean waste that comes from different species, therefore they have different protein and minerals content, which may have an impact on the processing time.
Table 1: Overview for chitin recovery by biotechnological methods.
| Deproteinization (DP) | Demineralization (DM) | Species | Bioprocess Time (hours/day) | Results | Authors | Country | |
|---|---|---|---|---|---|---|---|
| 1 | 4% glucose concentration, 37 °C, initial pH 6.5, inoculum level 6% | 5% glucose concentration, 37 °C, initial pH 6.5, final pH 3.4, inoculum level 4%. | Lactobacillus rhamnoides, Bacillus amyloliquefaciens (BA01) | 48 h/84 h | DP 96.8% DM 97.5% | Liu et al. 2020 | China |
| 2 | 3% (w/v) shrimp waste, 37°C, 150 rpm | - | Brevibacillus parabrevis TKU046 | 4 d | DP 95% | Doan et al. 2019 | Taiwan Vietnam |
| 3 | 50 °C, 5% (w/v) crayfish shell waste, 5% (w/v) glucose, proteinase K, 10% inoculum. | Bacillus coagulans | 48 h | DP 93% DM 91% | Dun et al. 2018 | China | |
| 4 | 15% sucrose and 85% crab biomass. | Lactobacillus plantarum sp. | 60 h | DP 95.3% DM 99.6% | Castro et al. 2018 | Mexico | |
| 5 | 5% glucose, 180 rpm, 30 /37 °C | Serratia marcescens db11 Lactobacillus plantarum | 6 d | DP 87.19% DM 89.59% | Chakravarty et al. 2018 | USA | |
| 6 | Sucrose (10% w/w), 30 °C | Lactobacillus brevis Rhizopus oligosporus | 120 h/72 h | DP 96% DM 66.5% | Aranday-García et al. 2017 | Mexico Japan | |
| 7 | 50°C, E/S ratio of 5U/mg, shrimp shells, crab shells and pH 8. | - | Portunus segnis | 3 h | DP 84.7%, 91.06% | Hamdi et al. 2017 | Tunisia |
| 8 | Shrimp shell waste 5% (w/v), 20% glucose, 50 °C and 100 rpm. | Pseudomonas aeruginosa | 6 d | DP 92% DM 82% | Sedaghat et al. 2017 | Iran | |
| 9 | pH10, 60°C and E/S ratio of 10 U/mg | - | Bacillus safensis S406 | 3 h | DP 93% | Mhamdi et al. 2017 | Tunisia |
| 10 | 33% w/v shrimp shell waste, 50% (v/v), pH 6.2, 125 rpm, 35 °C. | Lactobacillus plantarum | 72 h | DP 99% DM 87% | Neves et al. 2017 | Brazil | |
| 11 | Sucrose 5% (w/v), shrimp shell waste (12.5%, w/v), | Bacillus subtilis | 7 d | DP 97% DM 82% | Gamal et al. 2016 | Egypt | |
| 12 | 2% shrimp shell powders, 15 % glucose, 35 °C. | Serratia marcescens B742, Lactobacillus plantarum ATCC 8014 | 6 d | DP 94.5% DM 93.0% | Zhang et al. 2016 | China USA | |
| 13 | 7.75 U/mg A21, 60 °C; 10 U/mg S. scrofa, 50 °C. | - | Bacillus mojavensis A21 Scorpaena scrofa | 9 h | DP 96% | Younes et al. 2016 | Tunisia France |
| 14 | E/S ratio of 55U/g, pH7 and 37°C. | 25°C and shells-lactic acid ratio of 1:11 (w/w) | Streptomyces griseus | 3 h/ 20 min | DP 91.1% DM 98.6% | Hongkulsup et al. 2016 | UK |
| 15 | Crustacean waste 18g/L, 10g/L glucose, initial pH 7, 40°C and 150 rpm. | Bacillus subtilis and Bacillus licheniformis | 24h | DP 84%, 74.2% DM 55%, 60% | Pachapur et al.2015 | Canada | |
| 16 | 5% glucose and 5% cassava starch | Lactobacillus plantarum strains T1 and L137 | 7 d | DP 84.4% DM 83% | Francisco et al. 2015 | Philippines | |
| 17 | 5% (w/v) shrimp shell waste, 10% (w/v) glucose, 10% (v/v) inoculum, 37 °C and 100 rpm. | Pseudomonas aeruginosa, Serratia marcescens, Bacillus pumilus | 6 d | DP 74.76%, DM 76.46%, | Sedaghat et al. 2015 | Iran | |
| 18 | 5% (w/v) shrimp shell waste, 5% (w/v) glucose, and initial pH 7, 37°C. | Bacillus pumilus A1, B. mojavencis A21, B. licheniformis NH1, B. cereus BG1, B. amyloliquefaciens An6 and B. subtilis A26 | 5 d | DP 94 % DM 80% | Hajji et al.2015 | Tunisia | |
| 19 | Shrimp waste 15g, 45°C, E/S ratio of 5 U/mg | - | Bacillus mojavensis A21 and Balistes capriscus | 3h | DP 77%, 78% | Younes et al. 2014 | Tunisia France |
| 20 | 30 °C, 180 rpm. | Bacillus licheniformis 21886 Gluconobacter oxydans DSM-2003 | 168h | DP 87% DM 93.5% | Liu et al. 2014 | China | |
| 21 | 171.37 g/L sugars, 32°C, 4.84 g shell, 100 mL of fermentation medium. | Lactobacillus helveticus | 254.38h | DP 78% DM98% | Arbia et al. 2013 | Algeria France | |
| 22 | Shrimp shell concentration of 70 g/L, glucose 50 g/L, pH of 5.0, 35 °C. | Bacillus pumilus A1 | 6 d | DP 94% DM 88% | Ghorbel-Bellaaj et al. 2013 | Tunisia | |
| 23 | 55°C, pH 7.8-8, aeration 2.3 vvm, 275 rpm. | 30 °C, 50 rpm | Lactobacillus acidophilus FNCC 116 Bacillus licheniformis F11.1 | 96,60 h | DP 95.37% DM 97.69% | Junianto et al. 2013 | Indonesia |
| 24 | Inoculum 5%, shrimp head waste 10g/80mL, 30°C, 180 rpm, initial pH 10. | - | Bacillus licheniformis OPL-007 | 2 d | DP 85.3% | Mao et al. 2013 | China |
| 25 | 10% (w/w) shrimp head, 5% glucose, 1.2% (v/v) inoculum size, 42 °C, initial pH of 5.0. | Streptococcus thermophilus | 64 h | DP 93.59% DM 92% | Mao et al. 2013 | China | |
| 26 | 2% shrimp shell powders. | 2% shrimp shell powders, 15% glucose. | Serratia marcescens B742 Lactobacillus plantarum ATCC 8014 | 4,2 d | DP 94.5% DM 93% | Zhang et al. 2012 | China USA |
| 27 | 30°C, 120 rpm, 50g/L sugar cane molasses, 66.7 g/L crustacean wastes from crab. | Lactobacillus sp. B2 | 120 h | DM 88% DP 56% | Flores-Albino et al. 2012 | México | |
| 28 | 30°C,180 rpm, 20g/L date syrup, 5% of inoculum | Lactobacillus plantarum | 6 d | DM 45% DP 54% | Khorrami et al. 2012 | Iran | |
| 29 | E/S rate of 7.75 U/mg, 60 °C and pH 9. | - | Bacillus mojavensis A21. B. subtilis A26 B. licheniformis NH1 B. licheniformis MP1, Vibrio metschnikovii J1 and Aspergillus clavatus ES1 | 6 h | DP 88.5 % | Younes et al. 2012 | Tunisia France |
| 30 | 15% glucose, 37°C. | Lactobacillus acidophilus SW01 | 168 h | DP 97.4%, DM 97.7% | Duan et al. 2012 | China | |
| 31 | 5% (w⁄v) shrimp shell waste, 5% (w⁄v) glucose, initial pH 7.0, inoculum 1.5% (v/v), 30 °C, 200 rpm. | Pseudomonas aeruginosa A2 | 7 d | DP 90% DM 92% | Ghorbel-Bellaaj et al. 2012 | Tunisia | |
| 32 | 10% (w/v), bacterial starter 5%, 35 °C. | Lactobacillus plantarum | 96 h | DP 94% DM 92%, | Pacheco et al. 2011 | Mexico | |
| 33 | 55 °C, 250 rpm, 2.5 vvm aeration. | 30 °C, 50 rpm. | Bacillus licheniformis F11.1, Lactobacillus acidophilus FNCC116 | 60, 48h | DP 79.61% DM 88.65% | Wahyuntari et al. 2011 | Indonesia |
| 34 | 37 °C. | HCl | Erwinia chrysanthemi mutant | 16h | DP 95% DM 99% | Giyose et al. 2010 | South Africa |
| 35 | 5% Inoculum, 15% glucose. | Natural probiotic (milk curd). | 72h | DP 89% DM 69% | Prameela et al. 2010 | India | |
| 36 | Shell waste (10%, w/v), 15% glucose (w/v), and initial pH 8.8. | Lactococcus lactis Teredinobacter turnerae | 7 d | DP 90.2% DM 98.3% | Aytekin and Elibol 2010 | Japan Turkey | |
| 37 | Ratio shells /water 1:2 (w/v), 40 °C. | Acid treatment, 25 °C. | Bacillus cereus SV1 | 9 h | DP 88.8% DM 99% | Manni et al. 2010 | Tunisia |
| 38 | 55 °C, 2 vvm, 500 rpm. | Bacillus licheniformis strains F11.1, F11.2, F11.3 and F11.4 | 60 h | DP 84% DM 96.4% | Hoffmann et al. 2010 | Germany | |
| 39 | 3% shell waste, pH 7.0, 37 °C, and 200 rpm. | Bacillus cereus and Exiguobacterium acetylicum | 7 d | DP 97.1%, 92.8% DM 95.0%, 92.0% | Sorokulova et al. 2009 | USA | |
Biotechnological methods to extract chitin from crustaceans are effective and environmentally friendly. However, one of the most relevant requirements still to be achieved, when compared with chemical methods, is reduction of processing time for total DP and DM. Some authors have reported that DP and DM takes altogether between 2 to 7 days (Sorokulova et al. 2009; Aytekin & Elibol 2010; Ghorbel-Bellaaj et al. 2012; Khorrami et al. 2012; Zhang et al. 2012; Mao et al. 2013; Ghorbel-Bellaaj et al. 2013; Hajji et al. 2015; Sedaghat et al. 2015; Francisco et al. 2015; Zhang et al. 2016; Rawia et al. 2016; Sedaghat et al. 2017; Chakravarty et al. 2018; Doan et al. 2019). Some other authors have reported processing time between 24 to 254 hours (Hoffmann et al. 2010; Prameela et al. 2010; Wahyuntari et al. 2011; Pacheco et al. 2011; Duan et al. 2012; Flores-Albino et al. 2012; Mao et al. 2013; Junianto et al. 2013; Arbia et al. 2013; Liu et al. 2014; Pachapur et al. 2015; Mhamdi et al. 2017; Aranday-Garcia et al. 2017; Neves et al. 2017; Liu et al. 2020).
It is remarkable to have reduced bioprocessing time to 3 to 16 hours (Manni et al. 2010; Giyose et al. 2010; Younes et al. 2012; Younes et al. 2014; Hongkulsup et al. 2016), bearing in mind that some conditions such as the use of commercial enzymes, processing times, and the possibility of applying different microorganisms from those already studied (Table 1), need to be evaluated before attempting to scale the bioprocess up.
In order to reduce processing times and improve the effectiveness of biotechnological methods to recover chitin from crustacean waste, microbial species employed in DP and DM, have taken a relevant role. Some species of bacteria have been studied, as Bacillus spp. which have a high proteolytic capacity (Liu et al. 2020; Doan et al. 2019; Dun et al. 2018; Mhamdi et al. 2017; Gamal et al. 2016; Younes et al. 2016; Pachapur et al.2015; Sedaghat et al. 2015; Hajji et al. 2015; Younes et al. 2014; Liu et al. 2014; Ghorbel-Bellaaj et al. 2013; Junianto et al. 2013; Mao et al. 2013; Younes et al. 2012; Wahyuntari et al. 2011; Manni et al. 2010; Hoffmann et al. 2010; Sorokulova et al. 2009). Similarly, another genus highly used is Lactobacillus, not only due to its high proteolytic activity, but also to its capacity to produce lactic acid, which has allowed to perform DP and DM in a single step. The acid produced by Lactobacillus spp. inhibits the growth of undesirable competitive bacteria (Castro et al. 2018; Chakravarty et al. 2018; Aranday-García et al. 2017; Neves et al. 2017; Zhang et al. 2016; Francisco et al. 2015; Arbia et al. 2013; Junianto et al. 2013; Zhang et al. 2012; Flores-Albino et al. 2012; Khorrami et al. 2012; Duan et al. 2012; Wahyuntari et al. 2011; Pacheco et al. 2011; Aytekin & Elibol 2010). Lactobacillus has also been used to demineralize crustacean waste only (Liu et al. 2020).
Additionally, other proteolytic bacterial species with relevant results on crustacean waste DP and DM have been studied, such as Pseudomonas, Serratia, Streptomyces, beside others (Doan et al. 2019; Chakravarty et al. 2018; Sedaghat et al. 2017; Zhang et al. 2016; Hongkulsup et al. 2016; Sedaghat et al. 2015; Liu et al. 2014; Mao et al. 2013; Zhang et al. 2012; Ghorbel-Bellaaj et al. 2012; Giyose et al. 2010; Aytekin and Elibol 2010; Prameela et al. 2010; Sorokulova et al. 2009).
Furthermore, in the search of improving DP and DM, the fungi Rhizopus oligosporus has also been considered (Aranday-García et al. 2017); and crude extracts from eukaryotes tissues as Portunus segnis (blue crab), Balistes capriscus (gray triggerfish) and Scorpaena scrofa (red scorpionfish) (Hamdi et al. 2017; Younes et al. 2014; Younes et al. 2016).
The price of crustacean waste is comparable to that of wood waste (Mao et al. 2016). On the other hand, conventional exoskeletons treatment based on the use of acid and base, although convenient and effective, is expensive and damaging to the environment. Biotechnological methods seem to be encouraging, but still in need of innovation that allows the technology to move into large-scale production, having to improve long processing times in order to obtain pure chitin.
At the moment, the challenge is to innovate towards environmentally sustainable technologies to transform crustacean waste into products such as biopolymers, pigments (astaxanthin), calcium, peptides, and protein hydrolysates, among others that have a potential high market value.
Conclusions
Biotechnological extraction of chitin from crustaceans waste has been achieved by different strategies. Some of them are, the treatment with microbial acid fermentation for demineralization (DM) and deproteinization (DP); microbial proteases fermentation for DP; and direct use of proteolytic enzymes for DP.
Treatments of crustacean waste with crude enzyme extracts, have achieved the fastest processing times, however, previous steps to produce the enzyme extracts may represent an increase in operational costs that needs to be taken into account before scaling the process up.
Microbial fermentation has shown a potential for deproteinization and demineralization, keeping in mind that processing times still need to be reduced, in order to scale the bioprocess up.
The utilization of crustacean waste needs to be further investigated to the aim of adapting laboratory-scale biotechnological methods for industrial scale extraction of chitin from crustacean waste and its derivative products. The challenge now is to develop a bioprocess that is commercially and environmentally cost/effective viable.












 uBio
uBio