Introduction
Ecologically-sound management plans for the grass páramos of the northern Andes depend on an understanding the responses of vegetation to fire (Bremer et al. 2019, Keating 2007). Tussock grasses dominate these ecosystems and have been directly linked to fire behaviour, since they provide the majority of fuel (Ramsay & Oxley 1996), and drive the responses of other plant species in post-fire vegetation dynamics (Laegaard 1992). It has even been suggested that changes in tussock grass physiognomy might provide biological indicators of time since last fire in these landscapes (Zomer & Ramsay 2018). Thus, the physiognomic responses of tussock grasses to fire and post-fire recovery processes could provide a useful tool for the understanding and management of grass páramo ecosystems. Grass páramos extend along the Andes from Venezuela to the north of Peru, generally above 3000 m in altitude (Hofstede et al. 2014). They are characterised by their high biodiversity and endemism (Luteyn 1999, Rangel Ch 2000) and provide various ecosystem services, such as water provision, climate regulation, and carbon storage in the soil (Cleef 2008). Furthermore, the páramos are culturally important to Andean people and extensive livestock grazing in these grasslands represents an important economic resource (Hofstede et al. 2003).
Grass páramos are burned periodically (Hofstede 1995, Horn & Kappelle 2009). Fires are mainly caused by people, but might also occasionally start naturally (Horn & Kappelle 2009, Keating 2007, White 2013). Although fires happened at the early Holocene, before people arrived in the Andes (Di Pasquale et al. 2008), these natural fire regimes changed once people had arrived (Buytaert et al. 2006, Villota et al. 2014). Nowadays, fires are set by farmers to encourage new green shoots for their livestock and as part of the process of converting the grassland into cultivation (Cleef 2008, Mena et al. 2001, Premauer & Vargas Ríos 2004). The dominant tussocks of the páramo burn easily since they are mostly composed of dead leaves which form a flammable fuel (Ramsay & Oxley 1996). For these reasons, grass páramos are considered a fire-dependent ecosystems (Horn & Kappelle 2009). This indicates that some species in these landscapes depend on fires to reproduce and grow, and changes to usual range of fire regimes would be expected to result in different community structures. Post-fire increases in light and space promote the recruitment of new individual plants of some species (Laegaard 1992, Ramsay 1999, Ramsay & Oxley 1996). Competitive exclusion by tussock grasses has been proposed for a range of páramo species, such as Espeletia giant stem rosette plants (Laegaard 1992), Puya giant basal rosette plants (Garcia-Meneses & Ramsay 2014), and several herbs and prostrate shrubs (Laegaard 1992, Ramsay 1999, Ramsay & Oxley 1996). Community structure changes after burning (Ramsay & Oxley 1996, Sklenář & Ramsay 2001) and local fire regimes play an important role in determining plant communities in the páramo (Ramsay 2014). It has also been shown to affect the genetic diversity of Puya hamata plants and seeds (Rivadeneira et al. 2020).
Thus, fire is an agent of change which modifies ecological processes in the páramo (Horn & Kappelle 2009). It affects vegetation height and density, which in turn affects soil exposure to sunlight, rain and wind, and potentially increases soil erosion (Camargo-García et al. 2012). It might also increase run-off, changing local hydrological cycles (Poulenard et al. 2001), with the consequent loss of organic material and nutrients (Vargas Ríos et al. 2002). After fire, páramo vegetation begins a successional recovery cycle (Vargas-Ríos 1997), driven by the interaction of species with different reproductive strategies, plant growth forms, and edaphoclimatic factors (Bedoya Zuluaga 2014, Ramsay 2001b).
Since the ecological effects of fire are complex, it is important to know the local fire history and fire regime of an ecosystem (Bowman et al. 2011, Ramsay 2014). However, it is difficult to determine the characteristics of a fire regime (such as frequency, intensity, severity, regularity, extent). Some of these characteristics depend on historical contingency, such as the weather conditions during the fire event, the amount of fuel which built up between fires, and human intervention (Horn & Kappelle 2009, Ramsay 1999). Building up an adequate picture of fire regimes in páramo ecosystems is difficult because of the time and resources needed to carry out such investigations in mountain environments (Garcia-Meneses & Ramsay 2014). In areas with a high proportion of cloud-free days, satellite imagery can provide a means to estimate extent and severity of páramo fires (Borrelli et al. 2015), but good quality remote sensing is unreliable in many páramo regions. Nevertheless, with páramo regions showing relatively rapid climate change attempts are being made to control and monitor fires, especially in protected areas (Armenteras et al. 2020).
Tussock grasses are dominant in most páramos (Hofstede et al. 2003). They are keystone species and ecosystem engineers, because the microclimate they provide influences greatly patterns of biodiversity in these high-altitude ecosystems. Tussock grasses survive fires because their buds are protected within the tussock bases, though fragmentation of tussock bases often occurs (Laegaard 1992, Ramsay & Oxley 1996, Rodríguez-Beltrán & Vargas-Ríos 2002). After fire, new green shoots appear, but gradually the tussocks accumulate dead leaves as the vegetation canopy becomes taller and denser (Laegaard 1992). In turn, changes to the physical size and structure of these dominant plants is likely to be a key driver in determining successional patterns in other species, as well as performing important soil engineering functions (Fernandez Monteiro et al. 2011). Although the general patterns of survival and recovery in páramos tussock grasses have been described (for example, Laegaard 1992), there have been no detailed quantitative studies of these plants’ physiognomy in relation to time since fire.
This study assesses the changes in tussock grass physiognomy (tussock cover, tussock basal area, tussock number, tussock height, and the proportion of dead leaves) in relation to time since fire. Alongside these measurements, we also evaluated fine-scale light conditions at the soil surface. With these data, we consider the importance of tussocks in determining post-fire recovery of páramo vegetation. We also discuss the potential of tussock grasses as indicators of time since fire in the páramos.
Material and methods
Study Areas.- This study was carried out in northern Ecuador, in the tussock- and Espeletia-dominated páramos of the Reserva Ecológica “El Ángel” (plus its buffer zone) and the Reserva Biológica La Bretaña (Table 1).
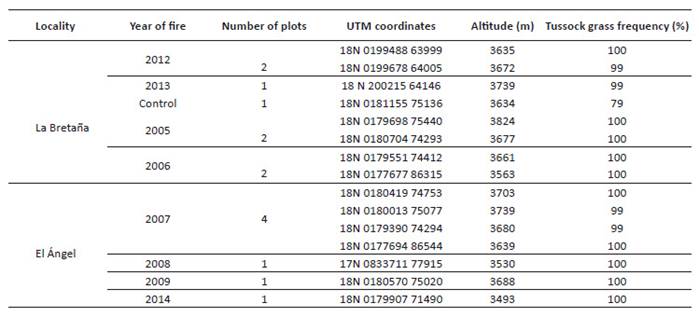
Table 1 Location and basic characteristics of 15 paramo grassland plots in northern Ecuador. Tussock grass frequency indicates the number of 1 m2 quadrats (of 100) which included tussock grass. The control plot had not been burned since at least 1975.
These areas provided sites of known time since fire. For El Ángel, Valdospinos Navas (2008) identified a range of sites that had been burned since 2000 to that date, and subsequent fires have been registered locally by Ministry of Environment rangers (Bustos Insuasti 2008). The terrain made access difficult to many of the recorded fires in the centre of the park and far from roads, so we selected 14 of the more accessible burned areas, and surveyed them in March-May 2015. For La Bretaña, we used páramo fire records of the fire brigade at San Pedro de Huaca, Carchi. The study areas were burned across a range of dates, from 2000 - 2014, giving us a range of times since fire of 0.5 - 10 y at the time of survey. We also surveyed an area which had not been burned for at least 40 y, as a form of unburned control.
These survey areas ranged in elevation from approximately 3500 - 3750 m (Table 1). Grazing by cows and llamas did occur, and although livestock density was generally low, animals did tend to concentrate grazing activity in areas less than 2 y after fire.
The vegetation in these areas was typical of páramos in northern Ecuador and southern Colombia, dominated by Calamagrostis intermedia (J. Presl) Steud. tussock grasses and Espeletia pycnophylla Cuatrec. giant stem rosettes. Other tussocks are present in smaller numbers: grasses of the genera Agrostis, Bromus, Calamagrostis, Cortaderia, Festuca; and the sedges Rhynchospora ruizana Boeck. and Uncinia phleoides (Cav.) Pers.
The data presented here reflect the response of the Calamagrostis species. In general, most Espeletia plants survive fires, but lose some of their marcescent leaves on the stem, which are not replaced (Zomer & Ramsay 2018). Thus, the effect of fires on Espeletia plants is to open up space and light levels nearer the ground. Although new Espeletia plants can germinate and occupy the new space, this process is impeded by the growth of tussock grasses (Laegaard 1992). In addition, Espeletia plants tend to burn as “independent” fire plants; the fire is mostly propagated by the tussock grasses. Thus, the tussock grasses’ response to fire and post-fire development is more important to understanding the community dynamics of páramos where both species are co-dominant. South of northern Ecuador, Espeletia is absent from the grass páramos and tussocks alone dominate (Hofstede et al. 2003).
Field measurements.- In each fire site, we established a 50 x 2 m plot, divided into 100 x 1 m2 quadrats. The presence or absence of tussock grasses in each quadrat was recorded. Vegetation height was measured in each 1 m2 quadrat using the drop-disc technique (Stewart et al. 2001), with a 20 cm diameter disc, 190 g in weight, allowed to fall to rest on top of grass tussocks (or other vegetation if no tussocks were present, but excluding Espeletia plants).
In five locations within the plot, located at 5 m, 15 m, 25 m, 35 m y 45 m on its longest axis, detailed drawings of the tussock bases within a 1 m² area were made at a scale of 1:10 and with a precision of ± 5 cm. In these same locations, representative leaf material from the tussocks was cut and arranged with leaves in parallel on a flat surface. A matt black plastic sheet with a 19 x 10 cm window was placed over the leaf material and secured flat. A high-resolution (12.1 MP) photograph of the leaf material exposed by the window was taken from approximately 1 m distance for later analysis. This was used later to estimate the proportions of live and dead leaves within the tussock.
Light at ground level was determined by measuring the percentage of incident photosynthetically active radiation (PAR) using a SunScan Canopy Analysis System with BF2 Beam Fraction Sensor (Delta-T Devices Ltd, Cambridge, UK). The sunscan probe was held at ground level underneath the vegetation at 1 m intervals along the length of the plot (50 in total). Each reading consisted of 64 light measurements of the percentage of total light (above the canopy) reaching the sensors on the ground. The mean percentage of the light above the canopy which reached ground level was identified for each set of SunScan readings.
Subsequent analysis was based on these mean values (n= 50 for each plot).
Image and data analysis.- Drawings of tussock bases within 1 m2 areas were scanned and the number of tussocks, the area of each tussock, and the total area of tussocks within each 1 m2 was derived from these images using ImageJ (Schneider et al. 2012). Tussock basal area and crown area have been shown as useful predictors of above-ground biomass in tropical alpine tussock grasslands (Oliveras et al. 2014).
We also used ImageJ to extract percentages of live, green leaves (settings: hue 40 - 90, saturation 0 - 255 and brightness 60 - 255) and dead, straw-coloured leaves (settings: hue 5 - 40, saturation 0 - 255 and brightness 60 - 255) from the dark background in our field photographs of tussock leaf samples.
Statistical analyses were performed with R (R Core Team 2018). Linear regressions were performed using a two-tailed test with reduced major axis (RMA or Model II) regression in R using the package “lmodel2” (Legendre 2018). For dead leaf accumulation with time since fire, an exponential rise to maximum curve was fitted.
Results
Total basal area of tussocks reduced with time since fire (RMA regression, R 2=0.432 p=0.015; Fig. 1A), as did tussock number (R 2=0.562 p=0.002; Fig. 1B). Mean individual tussock basal area did not show a trend with time since fire (R 2=0.127 p=0.233; Fig. 1C). The mean height of tussocks increased with time since fire (R 2=0.568 p=0.002; Fig. 1D), while the mean cover by tussocks <20 cm tall fell (R 2=0.725 p<0.001; Fig. 1E). For each variable, the means differed for sites of similar time since fire.
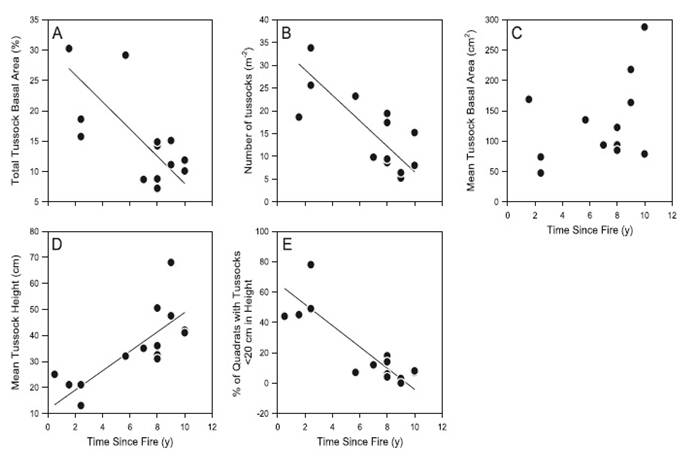
Figure. 1 Relationship between time since fire and tussock grass variables A. Total basal cover of tussock grass (line equation, y = -2.24x + 30.43). B. The number of grass tussocks per m2 (y = - 2.80x + 34.61). C. Mean tussock basal area (y = 15.75x + 19.35). D. Median height of tussocks (y= 3.73x + 11.51). E. Proportion of 100 quadrats with tussock height <20 cm (y = -7.00x + 65.87). Figs A-C do not include the plot burned 0.5 years before the survey, because tussocks had not clearly formed so soon after the fire.
For most sites within the first seven years after fire, the median amount of light reaching the ground below the vegetation canopy varied on average between 25 and 60%, though the variance within plots was high (Fig. 2).
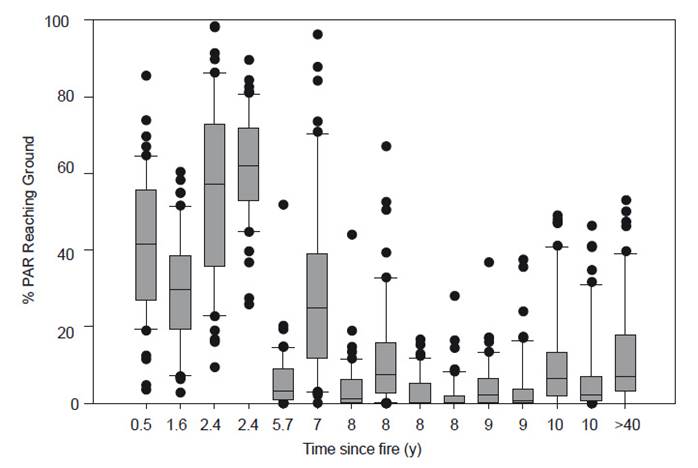
Figure. 2 Median proportion of PAR reaching the ground below the vegetation canopy in 15 paramo grassland plots in northern Ecuador, including one plot not burned for at least 40 years. At intervals of 1 m along each 50 m plot, 64 simultaneous PAR readings were taken and the mean calculated. The median (horizontal line), 25% and 75% quartiles (boxes), 10% and 90% quartiles (error bars), and outliers (circles) are shown for these 50 means from each plot.
For sites older than seven years after fire, these light levels were consistently low (mostly <5%) and varied much less. The mean light levels reaching the ground showed a decline with time since fire (R 2=0.709, p<0.001; Fig. 3A). This is reflected in a sharp increase in the number of quadrats with < 20% of above-canopy light levels reaching the ground (R 2=0.745, p<0.001; Fig. 3B).
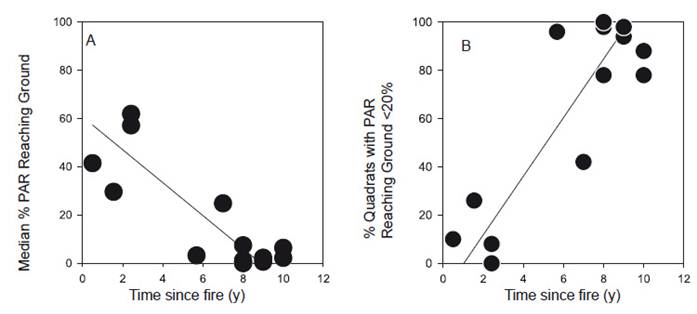
Figure. 3 Relationship between time since fire and the proportion of PAR reaching the ground below the vegetation canopy in 14 paramo grassland plots in northern Ecuador. A. Median of 50 means from each plot (line equation, y = 60.74-6.84x). B. Proportion of quadrats with <20% PAR reaching the ground (y = 12.16x - 12.51).
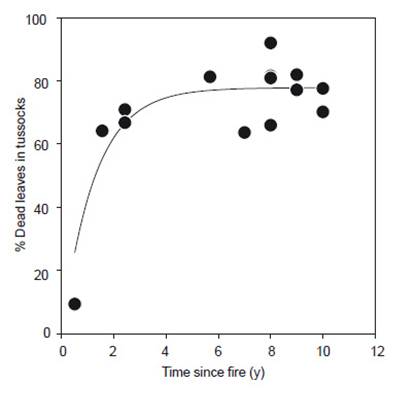
The number of dead leaves in tussocks was lowest during the first year (about 10%) but the proportion of dead leaves increased rapidly to around 60 - 70% by two years after fire, and consistently 60 - 80% afterwards (R 2=0.792 p<0.001; Fig. 4).
Discussion
Tussock grasses often survive fires, since their buds are protected from lethal fire temperatures within the tussock bases (Laegaard 1992, Ramsay & Oxley 1996). Fire mortality has been reported, but it is patchy at a fine scale, resulting in the fragmentation of the original tussocks and a smaller basal area (Laegaard 1992, Suárez & Medina 2001). During the first 1 - 2 y after a fire, páramo tussocks rapidly increase in numbers and basal area, in a tussock-building phase (Laegaard 1992), driven by the expansion of surviving tussock fragments in the absence of competition and the appearance of new seedlings in open areas (Ramsay 2001a, Ramsay & Oxley 1996, Vargas-Ríos 1997). Despite this overall increase in tussock number and cover, leaf mortality is high during this early phase, suggesting a rapid turnover of short-lived leaves (Ramsay 2001a, Ramsay & Oxley 1996). Unfortunately, our study did not have sufficient resolution of registered fire sites within 1 - 2 y after a fire to describe adequately this tussock- building phase.
Our study showed clear reductions in both the number of tussocks and basal area in relation to time since fire. These results are consistent with a competitive phase in post-fire tussock succession, which starts 1 - 2 y after the fire (Ramsay 1999, Ramsay 2001a, Smith & Young 1987, Velásquez-Romo 2000). Between approximately 2 to 10 y after fire, our study recorded an approximate halving of both basal area and the number of tussocks per unit area. A study of tussock grass responses to time since fire in an Australian grassland found that tussocks senesced by 11 y after fire, and subsequent fires did not immediately return the tussocks to a more vigorous state, leading the authors to conclude that ≤5 y fire intervals were necessary to maintain tussock dominance (Morgan & Lunt 1999). While our study did not indicate a canopy “collapse”, Zomer and Ramsay (2020b) concluded that the dense tussock canopy might open up in a later succession phase.
It is interesting to note that individual tussock basal area was unrelated to time since fire. This shows that tussocks of all sizes were lost during succession, rather than smaller (or larger) tussocks suffering higher than average mortality. It seems that tussock plants germinating from seed and surviving through the tussock-building phase have similar probabilities of establishing dominance in the competitive phase as older, larger tussocks weakened by the fire. Thus fires might provide a mechanism for new tussock plants to germinate and establish in an otherwise highly competitive community -a potential answer to the problem of tussock grass regeneration posed many years ago by Grubb (1977). Similar benefits to seedlings of other species have also been reported after páramo fires (Keating 2007, Ramsay 1999, Ramsay & Oxley 1996). Young (2004) suggested fire-induced reproduction for tussocks in East African mountain grasslands. Similar responses by páramo tussock species have been observed by the authors anecdotally, and this would merit further study.
The height of tussocks increased steadily across the range of fire ages in the study, not just in the early years. This corresponds with observations on the fire responses of Argentinian grasses (Peláez et al. 2003). As tussocks mature, their bases rise above the surrounding ground, contributing 10 - 20 cm of additional height. But this does not explain most the height increase, which is dictated by leaf length. Since grass leaves grow constantly from their meristems near the leaf base (Duru & Ducrocq 2000), taller tussocks could be produced by leaves that lived longer as time since fire increased. As noted earlier, previous studies have shown reduced leaf longevity immediately after fire, supporting this possibility. Alternatively, leaf longevity might be similar throughout the years of post-fire recovery, but the leaves might grow more rapidly in later phases of the succession in response to local environmental factors, leading to taller plants. There is evidence from studies of other grasses that leaf length varies greatly according to environmental factors (Barre et al. 2015, Sugiyama & Gotoh 2010). Of course, both explanations could occur simultaneously. Much of the competitive advantage of tussock plants derive directly from this increase in height, so tussock grass growth deserves more attention, particularly with consideration of the potential impact of future climate change on páramo ecosystems.
The increase in tussock height through time is also significant because it produces fuel for the next fire (Laegaard 1992). Cabrera & Duivenvoorden (2020) found vegetation height was a principal predictor of páramo above-ground plant biomass, and negatively related to specific leaf area. Our results demonstrated a rapid build-up of the proportion of dead leaves by the end of the tussock building phase, with a later build-up of biomass subsequently. By 2 y after fire, more than 60% of the leaves within tussocks were dead, increasing to approximately 80% by 10 y -similar levels to those reported for the same species in an Ecuadorian páramo by Suárez and Medina (2001) and in a Colombian páramo by Hofstede et al. (1995), but higher than the 44% of less contiguous Festuca orthophylla tussocks in the Bolivian Altiplano (Fernandez Monteiro et al. 2011). Although the rapid inclusion of dead leaves in the first 2 y after fire contributes potential fuel to the next fire, it is the continued rise in tussock height that builds up much of the combustible material in later years. The presence of significant quantities of fuel not only increases the chances of fire spreading though the vegetation once it has started (Pels & Verweij 1992), it also increases the likelihood of a high-intensity fire, resulting in higher plant mortality and more damage to ecosystem services (Ramsay & Oxley 1996, Zomer & Ramsay 2020a). More frequent fires, with lower fuel loads, might be less damaging, and might even promote productivity (Hofstede et al. 1995) and plant diversity (Bremer et al. 2019, Keating 2007).
As the tussocks grow in height, light reaching the ground decreases rapidly, especially from 2-6 y after fire. By 8 y after fire, median light levels were less than 10% of that above the tussock canopies. This is similar to that reported in other studies: around 3% of the above-canopy light reached the base of the same species of tussock plant in a Colombian páramo seven years after a fire (Hofstede et al. 1995), and values of <10% light transmission at the base of mature Festuca orthophylla tussocks in the Bolivian Altiplano (Fernandez Monteiro et al. 2011).
Nevertheless, in our study, most sites included a few places with a more open canopy, where up to 60% of PAR was able to reach the ground. Thus, there are still some limited opportunities for shade-intolerant species to establish in even late-stage páramo grassland.
All of the measures of tussock development estimated in this study showed high variance. Tussock grasses are inherently variable in size and density, and so sufficient replication is needed to obtain representative statistics (Fernandez Monteiro et al. 2011, Ramsay & Oxley 2001). In addition, fires themselves are inherently variable. Differences in fire regime (particularly fire return frequency and fire intensity) result in spatial patchiness of plant mortality and recovery at both coarse and fine scales. The impacts of fire also depend on usually unknown, local weather conditions during the fire (such as wind speed, wind direction, and the number of dry days before the fire). Therefore, it is perhaps not surprising that the estimates in our study showed such high variance.
Although our results confirmed that páramo tussock grasses recover in predictable ways after burning, none of the trends were consistent enough to provide a reliably precise estimate of time since fire. The combination of spatial variability in the tussocks themselves and the impact of fire on vegetation means that the estimates of time since fire based on tussock grasses cannot be used confidently to determine recent fire history with precision. In some páramos in the northern Andes, giant rosette plants have been proposed as effective biological indicators of time since fire (Garcia-Meneses & Ramsay 2014, Ramsay 2014, Zomer & Ramsay 2018). Unfortunately, these plants are not present in all páramos, and in places where they are absent there are no other obvious candidates for time since fire indicators.
The absence of regular, cloud-free satellite imagery in many páramo areas makes it difficult to monitor fires remotely (Borrelli et al. 2015). Therefore, predicting and managing the impacts of climate change and fire suppression in the páramos on biodiversity and ecosystem services depend on good quality fire records taken locally. These registers should not only record the times fires occur, but also attempt to describe their spatial extents. In particular, the managers of protected areas with fire suppression practices should begin to collect dependable records of fires so that the effectiveness of fire management policies and their impact on biodiversity and ecosystem services can be better monitored. Such records would also facilitate more studies like the one presented here, enhancing our understanding of páramo ecology more generally. Recently introduced payment for ecosystem services programmes that promote fire exclusion in páramos provide another mechanism to generate research opportunities (Bremer et al. 2019).
Fires have been characteristic shapers of páramo grasslands since their appearance after the last glaciation and the coincidental arrival of people. Tussock grasses dominate these grasslands, and their responses to fire play a significant role in determining the responses of other plant and animal species. Interactions between species (such as facilitation and competition) are driven by space availability, microclimate and light regimes, all of which are mostly dictated by tussock grasses. In addition, the principal reason for farmers to set fires is linked to the productivity of these grasses. Understanding how tussock grasses respond to fires of different kinds, and the consequences for other organisms, are key concerns of páramo ecology and management. This topic is particularly relevant today because of better enforcement of local fire reduction policies, and potential changes linked to climate change.












 uBio
uBio