Introduction
The genus Prosopis (Leguminosae: Mimosoideae) comprises approximately 45 tree and shrub species, distributed across arid and semiarid environments of the Americas, Africa and Asia (Burkhart 1976, Catalano et al. 2008), most of which have high economic and ecological importance (e.g. Simpson 1977, Pasiecznik 2001, Catalano et al. 2008). Around 39 South American species have a centre of diversity in Chaco (Argentina, Bolivia, Paraguay around 27 species). Around six to seven species, are found in the arid Pacific western side of Chile and Peru, with a wide diversity of landraces and local putative hybrids, creating taxonomic confusion (Burkhart 1976, Brücher 2012, Whaley et al. 2019).
The coast of Peru is recognised as part of one of the world’s oldest and driest deserts (Hartley et al. 2005), where plants derive moisture from fog and seasonal Andean fluvial influx (e.g. Péfaur 1982, Dillon et al. 1991, Whaley et al.2010b). West of the Andean mountain chain, aridity dominates the length of the Pacific seaboard for around 3200 kilometres, through Peru, north and central Chile. A trend to hyperaridity increases towards the 18°S latitude - the ‘arid divide’. The aridity traverses the Andes to Bolivia and Argentina, to form a diagonal swathe from Pacific Equatorial dry forest to Chaco; throughout this biome, Prosopis is frequently the key arboreal hardwood component of the vegetation. In Peru especially, Prosopis are the dominant species (Ferreyra 1987, Whaley et al. 2010a,b, Beresford-Jones 2011, Whaley et al. 2019) where-as phreatophytes-they are resilient to prolonged aridity whilst also adapted to fog capture (Whaley et al. 2010b). Prosopis trees (frequently called ‘algarrobo’ in south America or ‘huarango’ in southern Peru) are multi- use species, and throughout human history have provided essential resources of forage, food and fuel (Beresford- Jones et al. 2009). All their biomass can be used and many species adapt well to silvopastoral and agroforestry production (e.g. Roig 1993, Felker et al. 1981, Pasiecznik et al. 2001). Bioarchaeological studies suggest that South American Prosopis have played a vital role in the ability of people to settle in arid regions, where these species have occurred for at least 8,000 years (Beresford-Jones et al. 2009, 2011, McRostie 2014, McRostie et al. 2017). Today, most Prosopis-rich forest in Peru, Chile and Chaco, continues to be deforested for fuel and agricultural expansion. In the Ica region of Peru, the few marginal Prosopis forest relicts still existing, are key to biodiversity, ecosystem health and man (Beresford-Jones et al. 2009, 2011, Whaley et al. 2010a,b). However, degradation and climate change-related wildfires are now ongoing (Whaley et al. 2019, p: 34, 84), and to varying extents, attributable to tree mortality herein examined.
The Prosopis species in western Peru -the focus of this paper- are primarily classified as Prosopis pallida (Humb. & Bonpl. ex Willd.) Kunth and Prosopis limensis Benth. This follows separation of the two previously synonymised species, and the recognition of Prosopis juliflora as not occurring naturally in Peru (see Burkhart 1976, Mom et al. 2002, Burghardt et al. 2010, Palacios et al. 2012). Prosopis in Peru are highly variable with human selection, hybridisation and translocation since the dawn of pre-Columbian history (Whaley et al. 2019); so hereafter, we simply use ‘Prosopis’ to encompass all the coastal Peruvian Prosopis species, forms and varieties.
The heterogeneous architecture of Prosopis, together with its prolific provision of nectar and fruits provides multiple niches for insect species in arid conditions (Simpson 1977, Núñez-Sacarías 1993). Although most insects are naturally associated with the tree, due to their prevalence and economic potential Prosopis insects have often been categorised as plagues in Peru (Díaz Celis 1977, 1995, Díaz 1997). A study of insects associated with Peruvian coastal Prosopis species reported 176 species in Ica and Piura regions, from 1991 to 1993 (Núñez-Sacarías 1993), whilst a site-specific study in Piura recorded 129 species (Juárez et al. 2016).
Gall midges in forest and Prosopis trees
Enallodiplosis discordis belongs to the gall midge family (Diptera: Cecidomyiidae), where the true significance and role of many species in forest ecosystems is unknown. As many Cecidomyiidae are small inconspicuous flies they can easily be overlooked (Gagné 1994, Skuhravá 2005), but doubtlessly are significant and widespread components of forest and agricultural ecosystems (see e.g. Cilbircioğlu 2009, Altamirano et al. 2016), with many forest species still undescribed (Jaschhof & Jaschhof 2014). Cecidomyiidae biology is varied but many are gall-formers, hence their common name; some are fungivorous, free-living phytophages or predators. Gall midges may constitute the largest family of flies (Gagné 1994) with more than 6,590 species described (Gagné & Jaschhof 2017).
Little research has been conducted on gall midges in Prosopis, although a few studies have focused on the mesquite gall midge (Asphondylia prosopidis) which produces bud galls in P. glandulosa Torr. in south-west USA (e.g. van Klinken et al. 2009). Asphondylia prosopidis is reported in Israel as responsible for the formation of ovary galls in P. farcta (Gerling & Kugler 1973, Kay 2007), and has been studied for potential biocontrol of Prosopis (Park et al. 2009, Zachariades et al. 2011). In South America a non-specific study of 29 Prosopis galls in central Chaco (Salta, Argentina), suggested 62% of gall formation was induced by Cecidomyiidae, with P. elata, P. nigra and P. ruscifolia hosting three species, with several unidentified (Fernandes et al. 2002). Further work in Argentina investigated the potential of Prosopis biocontrol insect species (McKay 2007), reporting Asphondylia aff. prosopidis as a phytophagous gall midge inducing galls on the peduncle and ovary of P. nigra and other Prosopis species. Seventeen gall-forming taxa were recorded on P. alba in Argentina, of which four to five appeared to be Cecidomyiidae (Carabajal de Belluomini et al. 2009).
Neotropical Prosopis species are known as hosts for other Cecidomyiidae species including: Hemiasphondylia mimosa (in Colombia on P. juliflora); Liebeliola prosopidis (in Argentina on P. strobulifera); Tetradiplosis sexdentatus (in Argentina on P. alpataco and P. campestris); and Rhopalomyia prosopidis (in Argentina on P. alba, P. alpataco, P. campestris and P. flexuosa) (Gagné 1994). However, all these are gall-forming species, and as such, distinct from the non-gall-forming Enallodiplosis discordis (inChile on P. tamarugo and Peru, described here from P. pallida and P. limensis).
Enallodiplosis discordis, was first recorded and described in Prosopis tamarugo in Pampa Tamarugal (Canchones), Atacama Desert of northern Chile (Vargas et al. 1989, Gagné 1994). Here, Prosopis forest was all but eradicated during the nineteenth-century Chilean nitrate boom (Monteon 1979, Estades 1996, Wisniak & Garces 2001). In 1964 the Chilean government established a Prosopis reforestation project of c.100,000 ha in the Pampa Tamarugal (Habit et al. 1981), and -as a managementtool- produced a guidebook to insect plagues of Prosopis (Vargas et al. 1989) in which a cecidomyiid plague of P. tamarugo is identified as ‘Contarinia sp.’ (see Fig. 1). Subsequently, the species proved new to science and was described as Enallodiplosis discordis (Gagné 1994). Enallodiplosis is a monotypic genus and one of 27 genera of Neotropical Cecidomyiidae, showing no close relationship to one another or to the formal tribes (Gagné 1994).

Figure 1: (a) the effect of Enallodiplosis discordis on Prosopis aff. tamarugo, Pampa Tamarugal, Chile, published in Vargas et al. (1989); (b) the larvae E. discordis (‘Contarinia sp’.) (×30 original state), attributed to foliar damage of Prosopis leaflets. The larvae appear orange in colour in full sun and pale cream in shaded conditions.). Reproduced by kind permission [Images © O. Whaley]
Insect emergence as forest plagues
Trade globalisation and climate change have seen forest ecosystems under increasing attack from emergent insect plagues and pathogens causing forest die-back worldwide (Ramsfield et al. 2016). Often insect pests are merelythe most apparent killer, alongside a complex of geneticand chemical interactions from opportunistic microbesincluding bacteria and fungi (see Boone et al. 2013).Cause and effect are difficult to pin down, with insectsresponding to fragmented climate-stressed forest, withintroduced pathogens. Emergent insect plagues in forestsare attributed to: (i) climate change - conducive to insect physiology and reproduction, (ii) introduced pathogensthrough global trade, (iii) fungal hybridisation, (iv) habitat fragmentation and degradation (Liebhold et al. 2012,Mitton & Ferrenberg 2012, Adams et al. 2013, Boone etal. 2013, Tobin et al. 2014, Roy et al. 2014, Branco et al. 2015, Flower & Gonzalez-Meler 2015). For example, introduced Asian beetles Agrilus planipennis (emerald ash borer) and Anoplophora glabripennis (Asian longhornbeetle) are devastating regions of temperate forests where most research is focused - and Dendroctonus ponderosa (mountain pine beetle), responding to milder winters, assist wide destruction of Pinus contorta (lodgepolepine) and Pinus ponderosa (ponderosa pine) (Bale et al. 2002, Harvell et al. 2002). In Australian temperate forest,insect related die-backs are emerging; Eucalyptus viminalis is suffering die-back over an area of around 2,000 km2 of New South Wales, with widespread infestation of an endemic but novel species of eucalyptus weevil Gonipterussp., (Coleoptera: Curculionidae) (Ross & Brack 2015).
High reproductive rates and dispersal capacity mean insects easily target native forests (Menéndez 2007), and are able to cause more damage in water-stressed trees (Jactel et al. 2012, Haavik et al. 2015). Demonstrably globalised movement and deforestation raise the frequency and opportunity for fungal infection and hybridisation (Brasier 2000). Worldwide, studies are showing dieback in ancient trees, partly attributable to insect attack (Lindenmayer et al. 2012). There is a lack of research into insect plagues in Neotropical dry forest.
Prosopis are desert trees referred to as of ‘unsurpassed resilience’; the shattering of this resilience requires consideration of many possible causes, including fungal pathogens, but here we look solely at die-back associated with E. discordis.
The key aims of this paper are to:
- Detail the life cycle, taxonomy, and larval stages of E. discordis,
- Analyse the interaction and effects of E. discordis in Prosopis trees and foliage, with observations of distribution and historical ecology,
- Chart the socio-ecological impact of Prosopis dieback and pod production,
- Assess the relationship of E. discordis with ENSO and climate change,
- Suggest methods for physical and biological control, whilst highlighting areas for further research.
Materials and methods
Enallodiplosis discordis life cycle.- Observations were made almost exclusively in situ (with no rearing incaptivity) in the regions of Ica, Lambayeque and Piura,between 2005 and 2018, using photography and handlens for magnification in the field (larvae and adult size<1 mm). Monitoring observations were repeated monthly(as permitting by funding). Larval diurnal/nocturnal activity was supported by repeat photography. Plastic membranes were used to intercept the fall of mature larvae below infested tree canopies (Fig. 2). The larval pupation and emergence were observed over 24 hours during periods of one week. Larvae were placed in glass tubes to observe pupation within Prosopis leaf-litter (Fig. 2), with adults studied by netting off branches. Illustrations of larval development stages and adults were illustrated from fresh material using a drawing microscope. Additional information and images were gathered from herbarium specimens with use of a scanning electron microscope (SEM) at the Royal Botanic Gardens, Kew. Methodology for SEM specimen preparation was as follows: E. discordis larvae and adults were detached from herbarium Prosopis specimen collections and mounted. Larvae from specimens in ethanol were dried using a Tousimis Autosamdri- 815 Critical Point Dryer (Tousimis, USA). The larvae were mounted on SEM stubs with double-sided sticky tape (Agar Scientific Ltd, Stanstead, UK); exposed surfaces were covered with a layer of platinum using a Quorum Q150R ES sputter coater (Quorum, East Sussex, UK). The mounted specimens were examined using a Hitachi cold field emission S-4700 SEM (Hitachi, High Technologies Corporation, Berkshire, UK) and digital images of larvae and leaf attachment structures were recorded.

Figure 2: (a) trapping Enallodiplosis discordis larvae, falling to pupate during the night under Prosopis tree canopy using plastic sheet; (b) third instar larvae, in the morning having dropped on to sheet (rudimentary cocoon in tube inset); (c) technique of trapping adults during early morning - moving vertically-held sheet covered in vegetable oil over leaf-litter; (d) larvae fallen on concrete surface where most desiccate and die, not having leaf-litter in which to pupate; (e) Prosopis pods (huaranga or algarroba) once gathered in abundance for food and forage (this image 1994). [Images © O. Whaley]
Enallodiplosis discordis larval diagnosis.- SEM images (in Plates 2-3) were taken with a tabletop Hitachi TM3000. Drawings (Plate 1) were made using a Wild Heerbrugg microscope with drawing tube. The larval specimens were mounted in Canada balsam or Hoyer’s medium following the method outlined in Gagné (1994).
Enallodiplosis discordis direct observations.- Field observations of Prosopis condition and E. discordis infestation, were conducted between 2002 and 2015 across the arid coastal distribution of Prosopis (west of the Andes) in Peru. Locations, herbarium specimens and photographic records were made using GPS, Google Earth and -more recently- the Locus Map Pro™ mapping application on an Android phone. The intensity and activity of larvae on Prosopis foliage was observed, with added noting of biocontrol predators. Any other plant species associated with Prosopis, were recorded and examined for presence of E. discordis. These species included sub-canopy shrubs such as Pluchea spp., Lycium americanum, Vallesia glabra; and forest tree species: Acacia macracantha, Capparis spp. and Parkinsonia spp. Crops of adjacent industrial agriculture, including asparagus, avocado, grapes, and tomatoes were also monitored and examined for E. discordis. Historical distribution data over the last 60 years was gathered by the examination of herbarium collections of Prosopis from accessions collected 1950-2016, held at K (UK), MOL (Peru), PRG (Peru), SGO (Chile), UNICA (Peru) and USM (Peru) (herbaria codes after Holmgren et al. 1990). Herbarium specimens were examined for evidence of larval exuviae, desiccated larvae and diagnostic spots on the leaves.
Monitoring of E. discordis infestation was done in Peru (with associated herbarium collections) within 105 relict populations of Prosopis, and repeated over the duration of the study, other observations were made in Chile and Argentina. Herbarium specimens collected during field visits were deposited in herbaria: K, MOL, USM.
Region of Ica, southern Peru (P. limensis) (including marginal areas of adjacent regions, Ayacucho and Huancavelica) 2001-16.
Departments of Piura, La Libertad and Lambayeque in northern Peru (P. pallida) 2006, 2007, 2012, 2013, 2015-17.
Tarapacá region, Elqui Province, northern Chile (P. alba, P. chilensis, P. tamarugo) 2005, 2010, and El Loa Province (P. aff. alba, P. tamarugo) 2016.
Province of Corrientes and Chaco, northern Argentina (P. affinis, P. alba, P. kuntzei, P. nigra) 2010.
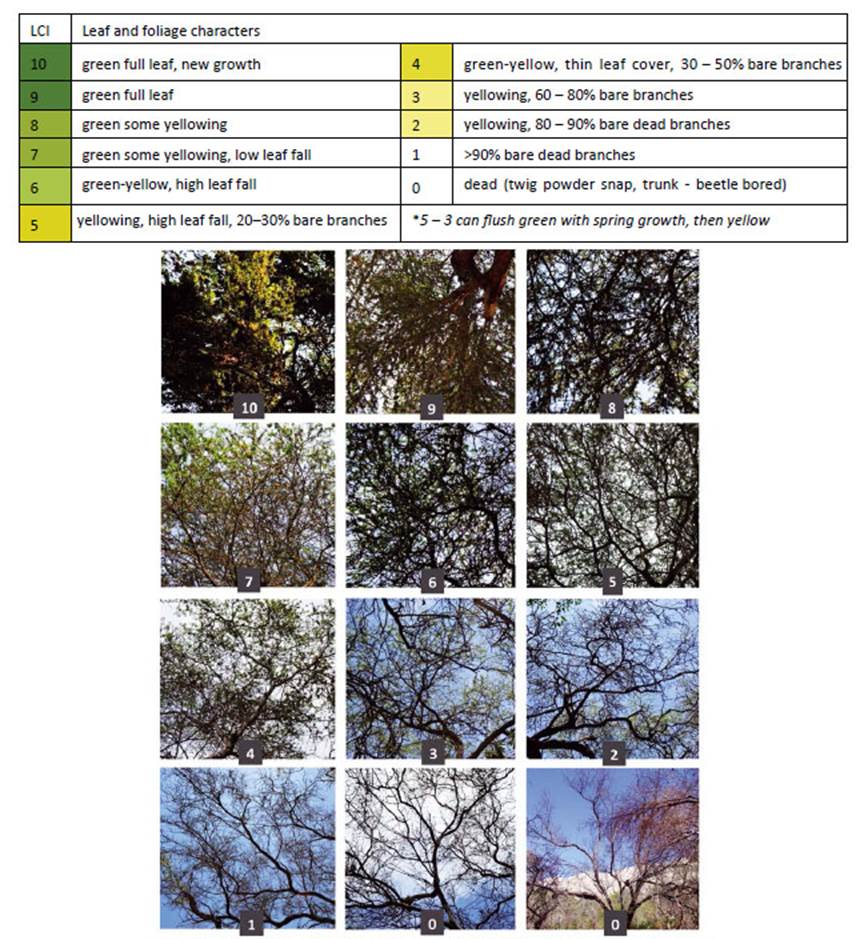
Initially, we assessed the intensity of E. discordis infestation in Peru using a rapid assessment of leaflet counts. Prosopis species in Peru have compound bipinnate leaves composed of 2-4 pairs of pinnae, and 8-15 leaflets per pinna. Ten leaves were selected randomly (on the western- most side of each tree) for presence or absence of larvae (using a hand lens ×10/20). The infestation value was 0-10, (0 = no larvae observed and 10 = present on every leaflet). In Prosopis trees with inaccessible branches, estimations were made from available leaves and leaf-litter. However, as the presence of E. discordis quickly became ubiquitous-with consequential loss of leaflets-it was found that a Leaf Cover Index (LCI) became more useful as an indicator of infection intensity and defoliation (Fig. 16). LCI was conducted with 0-10 classification of leaf coverage (0 = leafless dead tree, 10 = full foliage [normal] coverage). The LCI was tested by students and specialists and-with a few minutes of training-the results produced less than one degree of discrepancy.
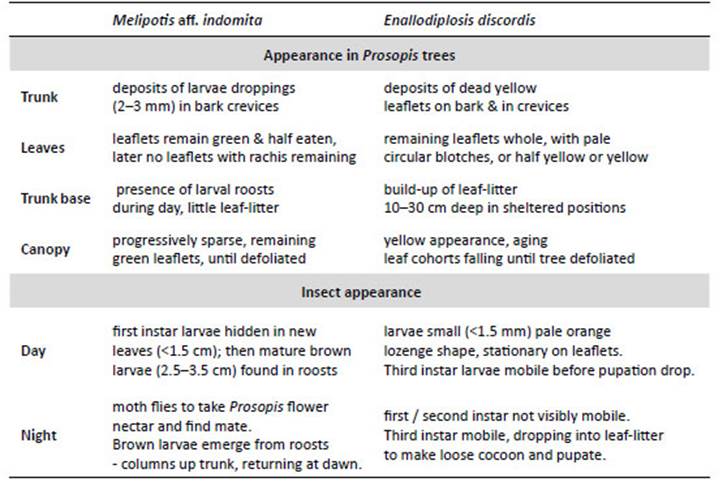
NOTE: Peruvian Prosopis species are not considered deciduous. In the department of Ica, Prosopis do not shed leaves as a phenological response to season; however, in Piura and Lambayeque Prosopis trees can shed a small percentage of their leaves (about 10%) between April and May, following fruit production (February to March). Also, after several seasons of extreme drought, Prosopis will sporadically shed leaves or even self-prune (pers. obs.). Furthermore, as noted by Díaz (1997), we observed that after unseasonal rainfall in Piura and Lambayeque, Prosopis leaves can lose chlorophyll and fall. The canopy develops a pale yellow ‘burnt’ aspect, occasionally referred to locally as ‘tostadera’, where opportunistic mould fungi appear to speed leaf necrosis. Prosopis trees in these conditions were ignored. Only when E. discordis was identified as the cause of leaf shedding was LCI used. Defoliation in Prosopis is also caused by Melipotis aff. indomita-a known pest of Prosopis (see e.g. Deloach & Cuda 1994). We were not able to ignore this contiguous plague species, widely prevalent as a Prosopis defoliator from 2001, and a likely contributing factor to the declining condition of the Prosopis until 2007/08 (see Appendix for further information, Fig. 14).
Drone monitoring.- By 2015-16 the ground-truthed mortality of Prosopis in Lambayeque was widely apparent across the canopy, with Prosopis trees appear ing dark grey, leafless, and skeletal amongst otherwise healthy species. Using a Sensefly eBee™ Classic survey drone and Pix4D photogrammetric software, we generated georeferenced images of mixed forest habitat to combine with ground-truthed GPS point layers (Garmin GLO), with tree species identified. This allowed us to develop a technique to distinguish dead P. pallida from other species (Acacia macracantha, Beautempsia [Capparis] avicenniifolia, Bursera graveolens, Colicodendron [Capparis] scabridum, Cordia lutea, Cynophalla [Capparis] flexuosa, Loxopterygium huasango, Parkinsonia praecox and Vallesia glabra) within an open regenerating forest structure (published in Baena et al. 2017).
Distribution and climatic niche of Prosopis and Enallodiplosis.- To represent known distribution of E. discordis, we produced a climate niche model for both Prosopis and E. discordis (Fig. 13) to gain insight into potential impacts and distribution related to Prosopis, but not as a vigorous exacting model. For Prosopis: 11,605 collected specimens and observation records were downloaded from GBIF (26 May 2014). We filtered these by removing duplicates and species not from South America, resulting in 1576 specimen records. We also removed Prosopis species widely considered to have been introduced to Brazilian cerrado (P. juliflora and P. pallida) (De Souza Nascimento et al. 2014).
Using Google Earth, we filtered only georeferenced specimens and removed mistaken localities (i.e. those in the sea or those wildly disjunctive) leaving 523, to which we added 47 records from our collections. We then used these 570 records to run the Maxent model (Phillips et al. 2006, Phillips & Dudik 2008). For Enallodiplosis: we used our 547 records from Peru and Chile, discounting those from Argentina. We examined the 19 BIOCLIM (Hijmans et al. 2005) variables, which we were planning to use as our prediction variables. From the 19 variables we removed nine, which either exhibited geographic disjuncts (sharp changes in values across the landscape) or were highly similar (Spatially autocorrelated). We used the 10 BIOCLIM predicting variables within the Maxent software-employing the default setting (20% of locations were used to test the model’s performance)-as these have shown to give robust reliable results (Phillips & Dudik 2008, Davis el al. 2012).
Prosopis pod production.- Between 1995 and 2017, the socio-economic impact of the plague was gauged by assessing historical pod production from local authorities and informal interviews with around 60 communities. Information was also collated from Prosopis-dependent communities invited to take part in the ‘Huarango Festival’, initiated in 2005 in the city of Ica to celebrate Prosopis biodiversity and products (Whaley et al. 2010b). We interviewed commercial and non-commercial producers of Prosopis syrup, flour and honey, including commercial artisanal operations such as: La Españolita and La Perla (Piura), Santa Maria de Locuto (Tambo Grande), Algarrobina Heroica Villa, Tallan (Catacaos), Miskyhuarango, Samaca Productos Orgánicos (Ica), as well as local Prosopis beekeepers and honey sellers. A few families that had migrated to urban areas, from rural Prosopis-dependent communities, were also interviewed. The key aim was to determine the seasonal timing and quantity of Prosopis pod harvest, before and following El Niño of 1997/98 and historically in memory and experience. In discussion, we also asked whether pod gatherers could remember Prosopis tree insects, or changes, associated with a collapse of pod production. Additional information was also gathered from the Instituto Nacional de Recursos Naturales (INRENA), park rangers and officers of the Servicio Nacional de Sanidad Agraria del Perú (SENASA), and agroindustry entomologists.
Results and discussion
Enallodiplosis discordis - Diagnosis
Adult.- Head lacking occipital protuberance and eyes separated laterally and at vertex. Male antennae with 12 binodal flagellomeres, each node with one circumfilum (Fig. 6). Female antennae with only seven flagellomeres, each short, pyriform. Wing with R5 reaching posteriad of wing apex, M4-CuA evanescent beyond base. Tarsal claws untoothed, curved beyond midlength; empodia as long as claws. Abdominal terga weakly sclerotised, their only vestiture a posterior row of setae. Male terminalia with gonostyli completely setulose, hypoproct bilobed and slightly longer than cerci, aedeagus slightly shorter than the hypoproct. Ovipositor short, the cerci ovoid and discrete.
NOTE: The full diagnosis of E. discordis larvae, was published in Gagné & Whaley (2020), here we include an edited version for ease of access.
Larvae
The three instars are strikingly different from one another (Fig. 5). Unlike the first instar of most cecidomyiids, E. discordis does not migrate at this stage, but begins to feed directly upon hatching, often settling on the egg case (Fig. 3). The first instar lacks obvious structural morphology, except for the head with its long mandibles, the fore and hind spiracles, and dorsal crest. The second instar is also stationary and often settles directly upon the first instar placement to feed (Figs. 3, 4), although it can also move to another fresh leaflet if necessary. Its head is disposed ventrally, and the dorsum has a series of conspicuous, long dark setae. The third instar is the crawling stage, when, after completing full growth, the larvae drops from the plant and burrows into the Prosopis leaf-litter. The head is also ventrally disposed, but the body is covered with sculpturing and has a pair of ventral pseudopods on each of the first seven abdominal segments.
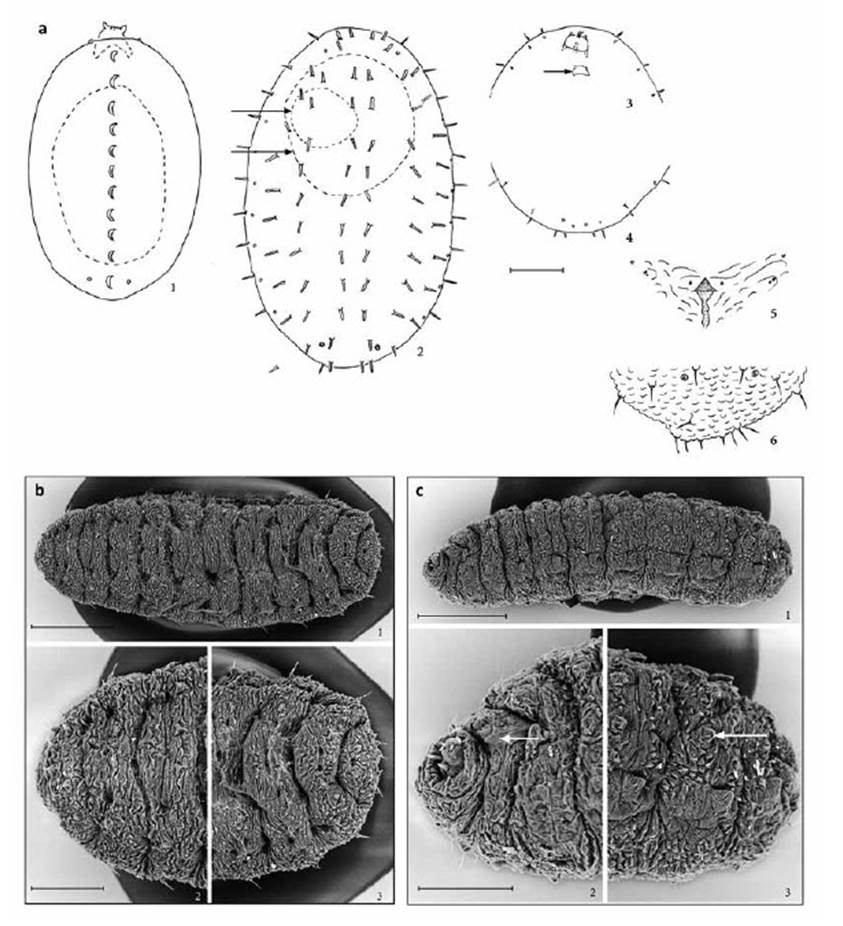
Figure 3 Enallodiplosis discordis, larvae. (a) First instar, dorsal (1), dashes show silhouette of egg case below larva; second instar dorsal (2), dashes show silhouette of egg case (upper arrow) and first instar (lower arrow) below larva; head and thoracic segments, ventral (3), arrow indicates head capsule of first instar; last three abdominal segments, ventral (4); third instar, spatula and associated papillae, ventral (5); eighth and terminal abdominal segments, dorsal (6). Scale lines: (1) 0.05 mm; (2-4) 0.10 mm; (5-6) 0.05 mm. (b) E. discordis third instar, dorsal: entire larva (1); thoracic segments (2); sixth to terminal abdominal segments (3). Scale lines: (1) 250 μm; (2-3) 150 μm. (c) E. discordis third instar, ventrolateral: entire larva (1); head and thoracic segments (2), arrow indicates spatula; seventh to terminal abdominal segments (3), arrow indicates pseudopod. Scale lines: (1) 250 μm; (2-3) 100 μm.

Figure 4 Enallodiplosis larval stages. (a) Newly laid egg, (i) first instar larvae at 1-2 days (ii); (b) First instar larvae at 3-4 days; (c) Second instar larvae at 4-5 days; (d) Mature second instar larvae; (e) Third instar larvae at 7-9 days; (f) Third instar larvae during skin change in fixed position; (g) Mobile third instar larvae preparing to drop to leaf-litter; (h) Third instar larvae covered in sticky leaf exudate allowing view of internal pigmented fat body. [Images © O. Whaley]
First instar (Figs. 3a, 4a): Length 0.21-0.30 mm. Colour - shiny translucent, turning pale yellow. Body ellipsoid, above convex with dorsal longitudinal carina at each segment middle, flat below, segmentation inconspicuous. Head is large with elongated styliform mandibles. Two pairs of spiracles present, one pair anterior on prothorax, the other dorsally on eighth abdominal segment. Papillae not apparent at x600 power resolution; body otherwise featureless.
Second instar (Figs. 3a, 4c, d): Length 0.70-0.82 mm. Colour - body from pale yellow to light orange, contrasting with the prominent dark brown setae. Body ellipsoid, convex above, flat below, segmentation defined by peripneustic spiracular system and papillar pattern. Head oriented toward venter, with elongate pair of styliform mandibles. Spiracles of eighth abdominal segment larger than others. Six dorsal papillae with long thick setae present on all thoracic and abdominal segments, except two on eighth abdominal segment. Two pairs of pleural papillae present on thoracic and first to eighth abdominal segments: the most dorsal of each pair with seta similar to that of dorsal setae; the most ventral pleural seta diminutive, barely longer than width of base of papilla. Eight terminal papillae, the four dorsal-most with setae as long as dorsal setae, the four ventral-most without setae.
Third instar (Figs. 3c, 4f, g, h.): Length 1.1-2.4 mm. Colour - pale yellow to dark orange. Body spindleform, slightly dorsoventrally flattened, segmentation, with pair of pseudopods on venter of first to seventh abdominal segments, integument covered with verrucae. Head oriented ventrally, with elongate pair of styliform mandibles, cephalic apodemes same length as head capsule. Spatula (Fig. 3c) - extruded anterior portion triangular, the posterior shaft weaker, narrow, of variable length. Six dorsal papillae with long setae present on all thoracic and abdominal segments, except two on eighth abdominal segment. Pleural papillae with setae as long as dorsal papillae. Terminal segment with eight papillae: six with setae same length as proceeding dorsal and pleural papillae, two with somewhat shorter setae. Prothorax with pair of sternal papillae; on each side of spatula a pair of lateral papillae, one with short setae, and a ventral papilla with short setae. Ventral and sternal papillae not detectable on body posterior of first abdominal segment. Anus well-defined on venter.
Life cycle observations
The inconspicuous nature and small size of E. discordis-in combination with ground level emergence and copulation confined under the partially obscured canopy of Prosopis trees-has contributed to a lack of research on this insect in South America.
Oviposition.-The female E. discordis lays translucentpale yellow to white eggs, oblong capsule in shape (±50µm), barely visible to the naked eye. Oviposition takes placeon the upper and lower surface of Prosopis leaflets (Fig. 6).
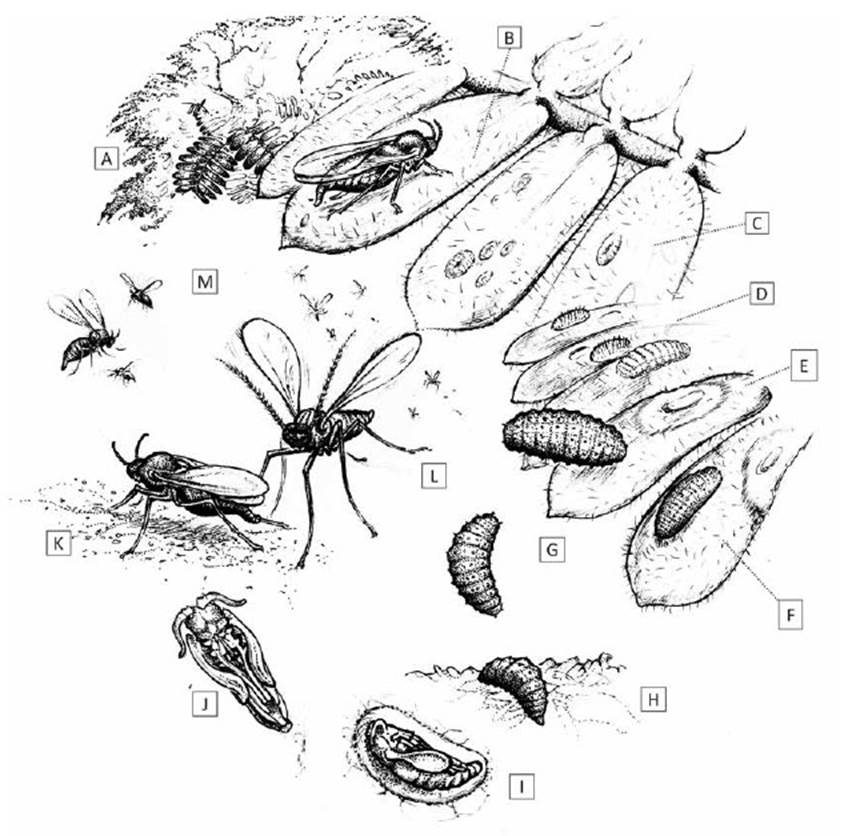
Figure 5 Lifecycle of Enallodiplosis discordis. (a) Prosopis tree host, with pinnate leaves of 8-12 paired leaflets; (b) oviposition on Prosopis leaflet; (c) first instar larvae, often found singly on leaf surface, or up to 10+ larvae per leaflet when few leaves available; (d) second instar larvae with longitudinal rows of short dark setae; (e) leaf necrosis ring formation from larval position; (f) second instar larvae during skin change; (g) third instar larvae moving to leaflet tip and then dropping to ground; (h) on the ground, larvae wriggles slowly into leaf-litter to depth of 15-25 mm; (i) larva pupation in rudimentary cocoon (see Fig. 2); (j) pupa before eclosion, ventral view; (k) female on ground attracting males for mating; (l) males swarm in mating frenzy just above ground, not ascending; (m) females ascend vertically into the tree canopy, walking from leaf to leaf during oviposition. [Drawing © O. Whaley]

Figure 6 Enallodiplosis discordis adult breeding behaviour and pupation. (a) female and male E. discordis; (b) SEM image of male antennae with binodal flagellomeres and nodal circumfilum; (c) pupal case in leaf-litter; (d) pupal case SEM image; (e) female, with dark orange abdomen and short antennae of seven flagellomeres; (f) male showing long antennae, around same length as body; (g) freshly fallen third instar larva starting pupation (biro for scale), inset shows larvae flexing to move down into leaf-litter for pupation; (h) female starting oviposition in foliage around 7.30 am (underside of Prosopis leaflet); (i) males approach female for mating, second mated female (red arrow); (j) imago females waiting in raised positions on leaf-litter to mate before flying upwards. [Images © O. Whaley, (a) © Ana Bravo].
Perhaps in response to climatic conditions -during hotter months, the lower surfaces can be favoured. The female walks between leaves along the leaf rachis and pinna before depositing between 50 and 80 eggs on leaflets. The larval density is 1-3 per leaflet (Fig. 9), suggesting the female uses leaf selection to reduce egg density. This equates to 22-44 larvae per Prosopis pinna; 132- 164 larvae per leaf (in Peruvian Prosopis). Under conditions of severe infestation with competition for space, egg density can achieve 10-20 larvae per leaflet (Fig. 9). Larvae emerge after 1-3 days remaining on the same leaflet. After 2-3 days the larvae (150-200 μm in length) (Fig. 6) display a translucent pale-yellow colour, with an internal orange peripheral fat pigmentation. From the first instar larval position, the host leaf begins to change colour and turn yellow.
Feeding and development.- Development takes place throughout the year, but with increasing intensity as season warms October to December and into April. Larvae of gall midges have piercing-sucking mouthparts and most adult mouthparts are capable of ingesting liquids. Adult mouthparts consist of the dorsal labrum, lateral labella, and a ventral hypoproct lateral to the labella maxillary palpi (Gagné 1994). Enallodiplosis discordis larvae (apparently) derive nutrition from the leaf by perforation of the leaf cuticle into the mesophyll using a pair of styliform mandibles; possibly favouring access through the stomata (see Discussion), completing development in 6-10 days. The first instar skin change is difficult to observe with a hand lens: it rolls up and does not remain stuck to the leaf as in the subsequent skin changes (Fig. 8). Cohorts of different-aged larvae appear together on leaflets. In crowded conditions, the larger third instar larvae crawl over the first and second instar larvae (Fig. 9a,b even at times appearing cannibalistic. During the second instar (Fig. 4c), the eight longitudinal rows of short dark setae become apparent (400-600 μm); larvae may move extremely slowly on the leaflets, if at all, largely at night. During the day in hot weather (±35 °C)-and often during skin changes-they may move to the underside of leaves (Fig. 9). However, the second instar (Fig. 4) larvae generally remain in the same position on the leaf surface feeding. Under most climatic conditions, following skin change, a desiccation spot or ring develops on the leaflet from the larval attachment point (Fig. 9).
After the third instar skin change and larval detachment, the exuviae (0.6-0.8 mm long) are visible adhered to the leaflet (Fig. 8). Although not always the case; removal of the larvae during the third instar skin change, exposes what appears to be the remains of both instar exuviae (as in diagnosis above), larvae having stayed in position during the second instar. The third instar can be observed during the day, arching back and forth, detaching from the exuviae by flexing the tail in the air and finally releasing the head (Fig. 4). During the third instar, the larvae move around very slowly on leaflets for a few hours (see below), progressing to the leaflet tip in the early evening, where they arch themselves back and forth again, until dropping during dusk or at night- presumably to avoid desiccation from ground heat and predation by ants. This larval ‘rain’ is easily observed by placing a plastic membrane under infested trees (Fig. 2). Larvae burrow into the leaf-litter; when prevented from doing so by a hard surface (concrete, for example), they accumulate in great numbers (Fig. 2d).
Notably, larvae can change colour, from cream to progressive shades of orange pigmentation, with increased exposure to sunlight (Fig. 7,9). When sufficiently hydrated, the leaflets (infested with larvae), leak sap, and the mobile third instar struggle through this resulting exudate, and in doing so the larvae become covered in sap. This allows observation of internal orange pigmented fat body as they become transparent (Fig. 4h).
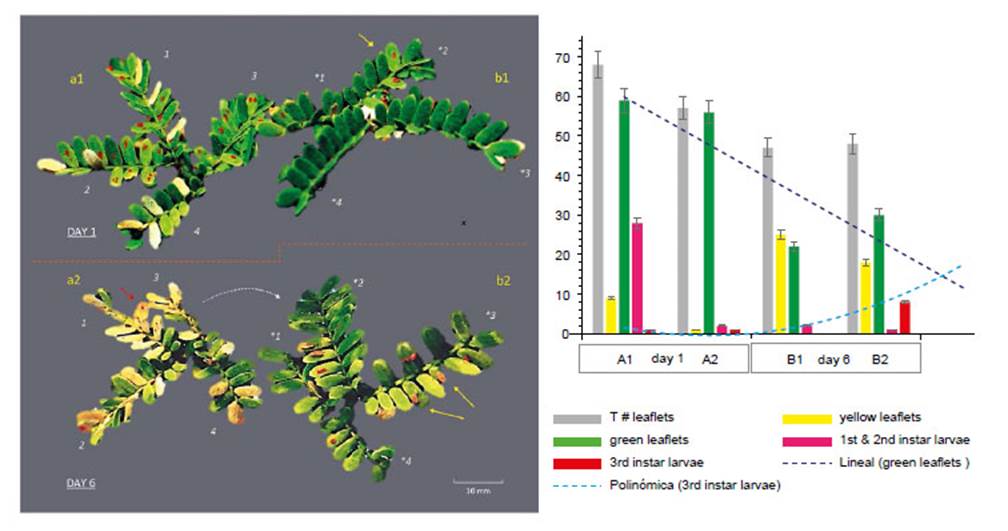
Figure 7 Effect and movement of Enallodiplosis discordis larvae (in situ on two paired Prosopis leaves). DAY 1: (a1) Second instar larvae in typical position, 1-4 per leaflet, some leaflets attacked have begun to lose chlorophyll; (b2) Leaf recently colonised by two larvae in second instar (yellow arrow) with little leaf discoloration. DAY 6: (a2) Most larvae have moved from the desiccating leaflets, some larvae remain and die or are preyed upon (red arrow), necrotic leaflets are falling; (b2) Leaflets are colonised by third instar larvae, that move to leaflet tips (yellow arrows) to drop to the ground to pupate in leaf litter. Some second instar larvae have moved to right leaf (as indicated). *Leaflets’ changed orientation numbers match leaves, red larvae (top) colour enhanced for visualisation. (c) Graph indicating the defoliation of leaflets (in a, b) in relation to E. discordis larval development and feeding movement. [Images © O. Whaley]
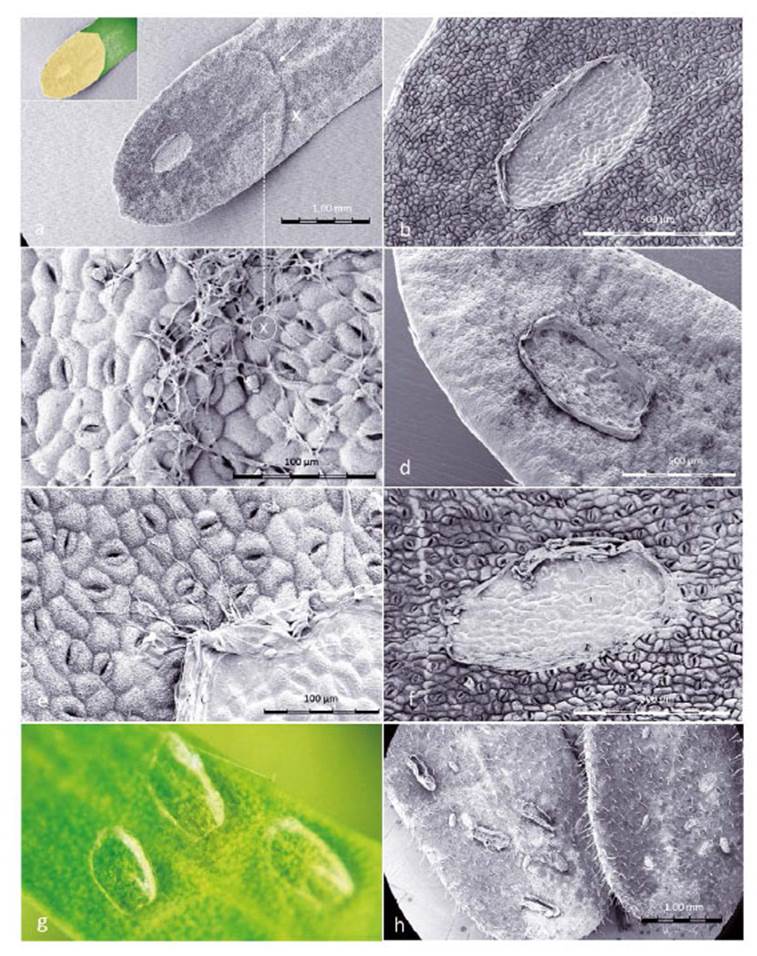
Figure 8 SEM images of Prosopis leaflets at Enallodiplosis discordis larval skin-change sites. (a) Prosopis leaflet (±1.7 mm wide) showing larval skin-change adhesion site with exuviae, necrotic area spreading through half the leaflet. The ridged boundary between desiccation and chlorophyll is visible (white arrow), inset shows leaflet colour; (b) Close-up of site (±600 µm) on the hairless leaflet underside with high density of stomata; (c) Close-up of ridge at point X on image above, with filaments resembling fungal hyphae across surface and entering stomata; (d) Larval exuviae (±600 µm) on upper leaflet surface with evidence of palisade parenchyma cell decay after third instar larvae skin change; (e) Close-up of larval exuviae with resemblance of fungal hyphae apparently growing from exuviae; (f) First instar larval skin change position, with little evidence of leaf perforation but blocking of stomata; (g) Third second instar exuviae sites (photo); (h) Second instar exuviae with suggestion of cannibalism. [Images © O. Whaley]

Figure 9 Enallodiplosis discordis larvae on Prosopis foliage: (a) Larvae of first and second instars, at high density when little foliage remains; (b) ‘Target’ leaf necrosis rings around larval skin change position (white arrow), most prevalent in more humid conditions; (c) Close-up of remaining skin change and leaf necrosis; (d) Characteristic ‘half leaflet desiccation’ following larval colonisation in more arid conditions; (e) Unidentified red galls found uncommonly with E. discordis, north and south Peru [photo Bosque El Cañoncillo, Trujillo]; (f) Typical leaflet necrosis following larval stages, some larvae desiccated or preyed upon; (g) Bunched brachyblast leaves (P. limensis) desiccate spontaneously; (h) Foliage desiccates and flowers fail or are aborted; rarely forming pods, they exudate gum, desiccate and fall [photos taken in Ica, La Libertad, Lambayeque, Piura - images © O. Whaley]. (For normal pod/flower appearance, see Whaley et al. 2019.)
Pupation.- After reaching the ground, arching backwards and forwards, larvae bury themselves in the leaf-litter until reaching 15-25 mm deep (Fig. 6). During a 12-hour period, the larvae make a small rudimentary cocoon (Fig. 6). After 48 hours the pupae (1-1.2 mm) turn dark orange (with black eyes), then complete pupation, turning dark brown after 60-70 hours. The adult hatches after 3-5 days in the leaf-litter. Without leaf-litter as a substrate, pupation rarely takes place and the larvae desiccate and die. After emergence, the adult pulls itself up through the leaf-litter, leaving a transparent white pupal case - clearly visible with a hand lens (Fig. 6).
Emergence and breeding behaviour.- Emergence of adults from the leaf-litter begins around daybreak, between 05:45 and 06:00 and continues to 08:00. Females vary from 1.2-1.6 mm in length, with a red swollen abdomen and distinct short antennae (seven flagellomeres); they crawl across the leaf-litter, until reaching a slightly elevated position above the surrounding leaf-litter, usually on a dead leaf stem or peduncle 2-3 cm above the ground (Fig. 6). Newly hatched females, with wings not fully expanded, make no attempt to fly or crawl to higher positions. By remaining on the leaf-litter, they are less likely to encounter the males in more elevated positions.
The wings of the male dry quickly after emergence to begin swarming, at times in thousands, to peak in a mating frenzy between 07:00 and 07:30. They take narrow, repetitive zig-zag flightpaths in a restricted range 5-30 cm above the ground, covering 10-30 cm in width and a maximum of a few metres in length. Slightly smaller than females, the males (0.9-1.2 mm) appear dark in colour, with large recurved antennae (12 binodal flagellomeres) (Fig. 6a, b). Even in large swarms, they are almost invisible against the ground when viewed from above. This swarming frenzy may be observed at ground level, by looking across the leaf-litter surface (a local ‘huaquero’, who slept under a Prosopis tree, recounted swarming activity annoyance at daybreak every day. Despite years in the Nazca area, he had never seen this before). Little audible sound is heard. Males are attracted to the female pheromones, and possibly by visual signals of the red abdomen. Although not observed, the females probably exsert their ovipositors to broadcast pheromones-as with all other Cercidomyiidae-and then withdraw the ovipositor and cease delivering pheromone.
Males swarm around the females in small, loose-Foliage can be several metres above the ground in the ly alternating groups (5-15 cm wide) competing tocase of pruned trees, or a few centimetres high for un-mate, with copulation taking 2-4 seconds. The femalepruned trees. Males do not attempt to follow the female appears to mate only once but is bombarded by males.upwards but remain swarming at ground level. By 09:20, The female remains in position for 5-20 minutes afterfew females remain on the ground, and some males stay copulation (Fig. 6j) until able to fly with wings fully ex-hovering just above the leaf-litter. The surrounding air panded, after which she flies directly upwards, spiral-temperature at this stage in summer months is 25-30 ling until encountering Prosopis tree foliage. °C; any males still in flight favour the canopy shade rather than patches of sun. By 09:35, nearly all females are found on the foliage, selecting the new light-green leaves and branch tip cohorts for oviposition, rather than darker mature leaves (Fig. 6). When sufficient foliage is available, only one or two eggs are laid per leaflet; conversely, when few leaflets are available (towards the end of E. discordis defoliating cycle), 10 or more eggs are found laid on a single leaflet (Fig. 9). With shortage of available new leaves-after years of infestation-five or more females can be observed per branch (Fig. 6). By 10:00, the number of females on lower branches declines as they disperse up into the canopy, and very few females remain on the ground (1-3 per m2); those remaining tend to be stunted with smaller abdomens. Males are mostly expended and are carried away by ants, activated by the heat. By 10:20, some isolated individuals of late emerging females are observed, although the very few males still flying do not attempt to copulate during short flights.
Numbers of larvae per tree.- To make a rough estimation of population density and reproductive capacity of E. discordis in one location in Ica (5-9 May, 2007), a water-trap tray (23×18 cm; 414 cm2) was placed 3.5 metres from the trunk under a typical mature Prosopis tree (c.40-60 yr., 50 cm diameter at breast height [DBH]) canopy and observed for four consecutive windless nights. A total of 160 E. discordis final instar larvae were trapped (having dropped to pupate).
Given the tree canopy area of around 80 m2, it can be assumed that at least 50000 (77295 given equal foliage density and infection above tray) mature larvae dropped per night from a single tree, equating to at least 1.5 million per month. This suggests that during the peak larval infestation period (4 months per year), 6 million individual E. discordis larvae could reach maturity on an average sized tree. Besides collecting the larvae, 145 adult males and 33 adult females were also trapped, suggesting a male:female sex ratio of around 4:1. The number of larvae reared through to adult emergence, suggests this ratio might vary with temperature -in a warmer environment an inverse ratio was found.
Larval movement and feeding.- Observation of larval movement-(made in Lambayeque and elsewhere) over 6 days noted: (i) larvae are most mobile during the night; (ii) they move from their diurnal positions, from the second instar; (iii) they often migrate to the leaflet underside to feed and moult. Often by the second instar, larvae are compelled to move, as the leaflet has begun to desiccate or drop off-depending on its exposure to sun and humidity. Larvae can, if compelled, move to feed several leaflets away from the original leaflet, even crossing to the opposite pinna. They move during the night and early morning, corresponding with lower temperatures and higher percentage relative humidity (RH) (Fig. 7), stomatal opening periods, also perhaps with higher leaf turgor providing better sap flow. At least in final instars, the size of mandible stylets and the posture of larvae suggest they might access leaf interior nutrients through upper epidermis stomata.
Enallodiplosis discordis larvae apparently respond strongly to temperature and humidity, becoming active at night and during high humidity and low temperatures. This may be a strategy to avoid desiccation, and to feed during times of highest metabolic rate in the Prosopis host. This is clearly unrelated to predator avoidance; in that they are most exposed during the day. Prosopis have evolved to maintain a favourable water potential in hyperarid desert conditions by opening stomata at night, and during high photosynthetic activity in the early morning. This observation supports Sudzuki’s (1985) concept of Prosopis hydraulic lift -a physiology to which E. discordis may have evolved. There does appear to be a relationship with E. discordis feeding times and Prosopis host tree metabolism. Research in P. tamarugo in Pampa Tamarugal, Chile demonstrated that 35-year-old trees at 04:00 had 80% of the stomata open at 84% RH (8 °C), contrasting with 83% of stomata closed at 12:00 midday-a time with the hottest temperature and lowest RH (33% RH / 30 °C) (Sudzuki 1985). Likewise, the Prosopis sub-canopy percentage RH (26 September 2014, Lambayeque) rises steeply at night to >84% RH from around midnight until peaking in the morning around 06:30 at 91% RH. After 09:30, humidity drops rapidly to <60% RH at 11:00 and to <50% RH from 12:00 midday until around 17:45, when it rises quickly to >70% RH by 19:15, an hour after sundown (our project Tinytag™ dataloggers).
Mechanism for feeding.- The sedentary posture of E. discordis larva, means the head and mouthparts are concealed beneath the body, effectively hiding observationof feeding. By careful removal of larvae from leaflets it isclear they derive sustenance directly from the leaflets,leaving a slight depression and perforation in the leaf epidermis-probably due to the uptake of sap and/or alteration of the mesophilic cell structure. In the first instar especially, removal of larvae exposes a pale-yellow exudatethat may include digestive enzymes. Interestingly, underthe SEM or light microscope there is no consistent evidence of perforation of the leaf cuticle for feeding during skin changes. However, as the leaf usually desiccates fromthis point (Fig. 8,9), the feeding appears to take place inthe same location. Research demonstrates Cecidomyiidaelarvae, have evolved salivary proteins that, when injected into the leaf, induce systemic molecular-level changesin host plants to produce tissue growth. (Johnson et al. 2015). We found no easily discernible structural changesto leaf tissue that might suggest that E. discordis is usingother chemical messaging secretions to facilitate feeding.The surface morphology of Prosopis leaves around skinchange and feeding sites, shows a ridge formation aroundthe site observable under the SEM (Fig. 8, 9f).
Intriguingly, an unidentified red pubescent gall-like nodule sometimes occurs concurrently with E. discordis infestation (Fig. 9e) and is conceivably related. These nodules appear as the same colour and size as the female and might have evolved to deter other females from oviposition in areas of high infection. Cecidomyiidae gall-formers have been shown to be able to induce galls several centimetres distant their location (Sopow et al. 2003).
In summary, movement of E. discordis larvae is very restricted and rarely more than c.15 mm from the leaflet on which the egg was deposited and is then confined to the Prosopis leaf pinna. They remain largely stationary during the day, though move slowly when necessary-around and between leaflets-at night (Fig. 7), until the final third instar after skin change when they drop to pupate.
Damage to Prosopis foliage
Both on the upper and lower surfaces of Prosopis leaflets, E. discordis larvae appear as tiny compressed capsules (≤1.2 mm), orange to yellow or cream in colour. After larval movement from a feeding or skin-change position on a leaflet (exuviae often remain), a symptomatic pale blotch or ring appears (Fig. 8), suggesting the injection of enzymes that dissipate. Over the next 1-3 days the leaflet turns pale cream, progressively along the branch from the new leaf cohorts that are infected. Cohorts of Prosopis leaflets then quickly become yellow (or cream) and necrotic, falling after 3-10 days (Fig. 10). The dying leaflets give the tree a ‘burnt’ appearance (Fig. 10). Leaves desiccate after infection, depending on the climatic conditions (related to foliage moisture content, species, etc.). In the most humid conditions, leaves rapidly turn yellow (Fig. 10g), however in the driest conditions we observed-such as in San Pedro de Atacama, Chile-P. alba leaves remain with a ring around E. discordis larval perforation, turning yellow slowly, over weeks not days. When the leaves desiccate rapidly, first instar larvae on the same leaves become ‘stranded’ and can desiccate and die (Fig. 9).

Figure 10 Prosopis tree and forest die-back. (a) Characteristic dense upper canopy foliage in healthy tree, with Enallodiplosis discordis yellowing in lower branches (P. limensis, Poroma, Ica, July 2007); (b) Yellowing of canopy after 8+ years of repeated defoliation, re-sprouting with immediate E. discordis infection (P. pallida, Tucume, Lambayeque, Sept 2015); (c) Foliage re-sprout in single branch remaining alive, following 2015-16 El Niño rains (P. pallida, Salas, Lambayeque, Feb 2016); (d) Fallen leaflets accumulating in sheltered conditions, with antlion (Myrmeleontidae) traps (P. limensis, Usaca, Ica, July 2007); (e) Dead trees following 4-5 years defoliation (P. limensis, Cahuachi, Nasca, Mar 2006); (f) Dead trees after 7-8 years defoliation (P. pallida, Bosque Pomac, Lambayeque, Nov 2018); (g) NIR image from drone, grey dead Prosopis and red/orange Capparis spp. and Vallesia glabra (La Pena, Lambayeque, Nov 2013); (h) Following El Niño rains, dead Prosopis contrast with other re-sprouting species including Capparis spp., Cordia lutea, V. glabra (La Pena, Salas, Lambayeque, Feb 2016). [Images © O. Whaley, (g) © J. Moat]
The physical appearance of Prosopis tree foliage during E. discordis plague outbreaks, appears to be identical to those observed in Chilean Prosopis (Vargas et al. 1989) (Fig.1). The Vargas et al. (1989) guide provides detailed observation, saying: ‘Leaflets show circular blotches of a pale colour that correspond to the positions where the larvae have fixed themselves to suck the sap’ (Foliolos presentan manchas circulares de color blanquecino que corresponden de sities donde se fija la larva para chupar la savia’).
Phenology.- Enallodiplosis discordis was recorded throughout the year (throughout monitoring decade) as a prolific plague in Prosopis. During the spring (October-December) in southern Peru, as temperatures increased to a range of 12-32 °C and with the new leaf cohorts, numbers of larvae increased significantly (Fig.11). They continued to multiply during the late summer months (December-April; c.15-37 °C) and thereafter declined with the onset of the austral winter (May-September; c.6-31 °C). Whilst annual peak temperatures are not hugely different in Lambayeque and Ica, Lambayeque experiences much higher humidity and rarely drops below 13 °C even in winter (according to project dataloggers). Accordingly, the seasonal temperature minimum seen in southern Peru, is moderated in northern Peru, where the plague appears to sustain more intensity with somewhat reduced seasonality. The occurrence and intensity of E. discordis as a defoliant, has been variable over the last few years, perhaps due to unseasonal climate shifts and the effects of El Niño Southern Oscillation (ENSO) 2015/16. It is notable that Enallodiplosis appears to respond positively to heat, and that El Nino of 1997/98 raised land temperatures at c.5 °C over seasonal averages through much of the cycle (Takahashi 2004). More climate related research is clearly needed.
Tree resilience and mortality.- During winter months, Prosopis trees in the Ica region, are partially reliant on fog moisture that is trapped by foliage and drips on to soil beneath the trees, seeing seasonal development of superficial root networks to capture this moisture (Whaley et al. 2010b, 2019). As a result, defoliation (from E. discordis) results in a positive feedback cycle, where less foliage = less fog drip, and the diminishing moisture input is compounded by sub-canopy ground heating; the ground becomes less shaded, exposed and desiccated, leading to tree mortality. Conversely, any large Prosopis trees that grow near water sources, can ‘outgrow’ the defoliation through repetitive leaf renewal -the water and the high insolation rates sustain productivity. However, they produce few flowers and the majority are aborted, with most pods failing to develop (Fig. 9h). The lack of precipitation -and the continued defoliation (in wind-sheltered conditions)-produces deep leaf-litter layering (Fig. 10d). However, the majority of Prosopis have little access to near-surface ground water, and after several years of defoliation die back, or succumb with a failure of hydraulic conductivity and carbon starvation (see Sevanto et al. 2014). Tree mortality may be accelerated by basidiomycete and other fungal pathogens. For example the polypore fungus Phellinus rimosus, was found on dead branch stumps (Usaca, Rio Poroma, near Nazca, Ica region). But with a global distribution, here its association is most likely to be as an opportunistic saprophytic decomposer (pers. comm. Brian Spooner, RBGK).
A continual process of defoliation by E. discordis (and Melipotis see below), gradually debilitates the tree and- although immensely resilient-the Prosopis tree becomes susceptible to drought stress and fungal attack. Branches die back progressively (see Fig. 10), even self-pruning, until the trunk itself desiccates. The tell-tale signs confirming tree mortality are: (i) branches being completely leafless, (ii) beetle holes (Cerambycidae) appearing on all sides of the main trunk, (iii) the splitting and delaminating of bark on the main trunk, (iv) all round tree, the outer twigs snapping test, produces a puff of wood dust (Fig. 10).
It is important to distinguish between the stark visual effect of Prosopis leaf shedding-due to short precipitation events outlined-and that of E. discordis-mediated leaf necrosis. Unseasonal rainfall events (likely climate change) can cause yellowing of whole tree canopies, whereas, E. discordis infestation yellowing is progressive, dispersed, and with cohorts of leaves turning yellow from the older to the newer leaves (Fig. 9,10).
Leaf necrosis.-The process of leaf necrosis is more than likely to include microbial agents, with features such as the formation of a pale ring around the larval attachment and skin-change points, suggesting fungal (or filamentous bacteria) infection. SEM images provide some evidence, with apparent filaments resembling fungal hyphae, visible over the leaf surface and entering the stomata (Fig. 8c). Research is needed to conclude if any fungal pathogen is intimately linked to E. discordis, or whether-at a foliar level-larvae simply provide an entry pathway for opportunistic microbes.
Distribution and Infestation of Enallodiplosis discordis
By 2006-2007, the presence of E. discordis in Prosopis trees (including nursery seedlings) in coastalPeru was found to be nearly universal-occurring in almost every tree sampled over the distribution (Fig. 11).This included all relict habitats in the department of Ica(Fig. 11) that were frequently visited where Prosopis is largely reduced to isolated relict clumps (1-50 trees) inriparian margins, or communal orchards (huarangales)5-150 trees (key relicts are: Copara, Coyungo, Huarangal, Ingenio, Jumana, Ocucaje, Palpa, Pampa de Villacuri, Poroma, Pozo Santo, Rio Seco, San Pedro, Samaca,Tingué, Ullujaya, Usaca, Las Trancas). Also, all accessible relicts of Prosopis forest (including reserves) in LaLibertad, Lambayeque, Piura and Tumbes. Enallodiplosis discordis larvae were also found occurring on ornamental trees in Lima. In total, 813 observations weremade in 105 relict forest patches in regions of southern and northern Peru. A further 10 observations were made in Chile and 15 in Argentina.
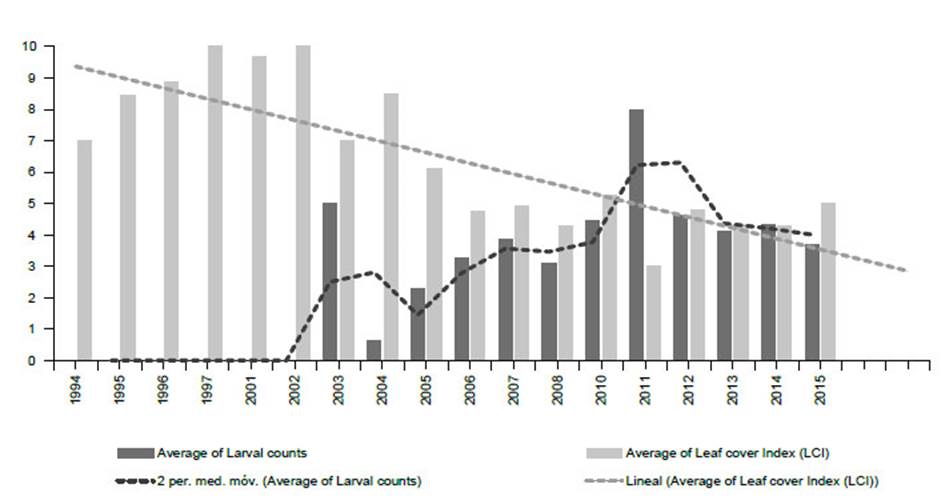
Figure 11 Monitoring Prosopis tree health and E. discordis larvae using 813 georeferenced observations from 1994-2015 (northern and southern Peru combined), represented here with yearly average values. Tree health is indicated by leaf cover (Leaf Cover (canopy) Index (LCI) see Fig. 16) and intensity of plague by larvae counts (presence/absence of larvae with leaf monitoring). This provided a consistent picture of declining Prosopis health with increasing larval presence. Since 2015, repeatedly monitored trees were found dead or moribund as linear trend suggests.
From 2001 to 2005 Prosopis tree health declined rapidly in southern Peru (LCI decline from 10 in 2000 to <5 in 2006) (see Fig. 11) with localised reduction of infestation after 2014, whilst increasing in northern Peru. Our herbarium Prosopis specimens indicate that Enallodiplosis began to take hold as a plague in the Nazca region of Ica in 2001 (see below), and by 2003/4, Enallodiplosiswas found in every tree monitored in the region of Ica, southern Peru. From 2005, Enallodiplosis was discovered in every Prosopis observed in northern Peru (Lambayeque, La Libertad and Piura). Our current recorded distribution of E. discordis is shown with red dots (Fig. 13). Using the Geospatial Conservation Assessment Tool, GeoCAT (Bachman et al. 2011) we make a rough estimation of the distribution area of E. discordis with Prosopis defoliation over of a minimum of 4,384 km2 (275 points area of occupancy with 4 km cell width).
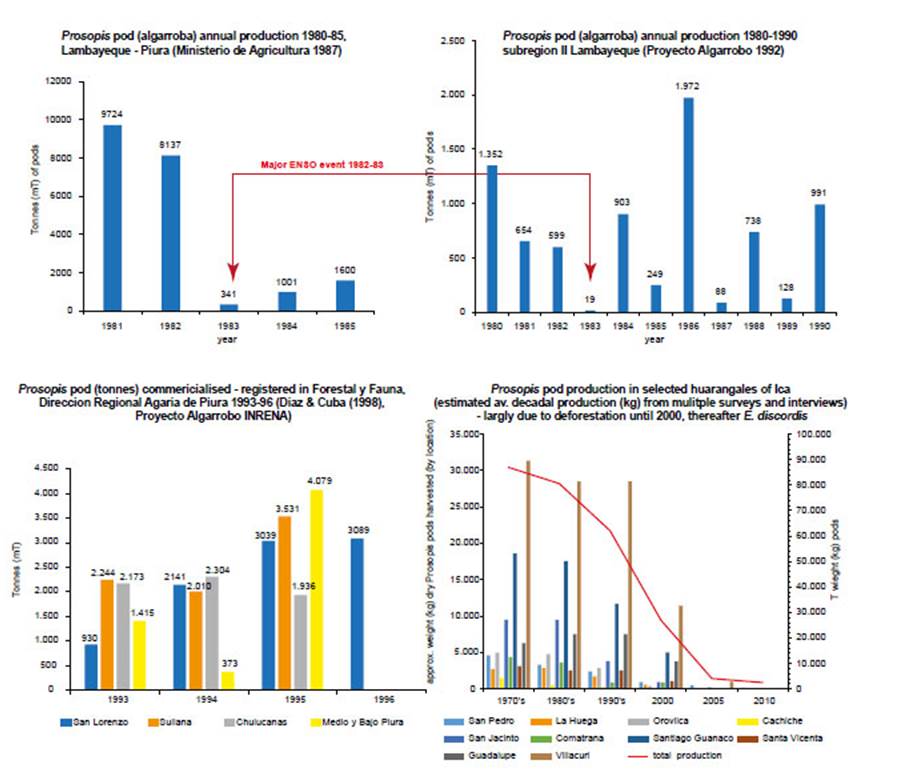
Figure 12 Prosopis pod production (P. pallida = algarroba, P. limensis = huaranga). (a) Prosopis pod (algarroba) annual production (mT) 1980-85, Lambayeque and Piura (Ministerio de Agricultura 1987); (b) Prosopis pod (algarroba) annual production (mT) 1980-90, subregion II Lambayeque (Proyecto Algarrobo 1992), red arrows indicate the 1992-83 El Niño event; (c) Prosopis pod (algarroba) commercialised and registered in ‘Forestal y Fauna de la Dirección Regional Agraria de Piura’ (mT) 1993-96 (Diaz & Cuba (1998) Proyecto Algarrobo INRENA); (d) Prosopis pod (huaranga) production (kg) in selected woodlands of Ica Region derived from estimated approximate average decadal production (kg) from surveys and interviews 1970s-2010.
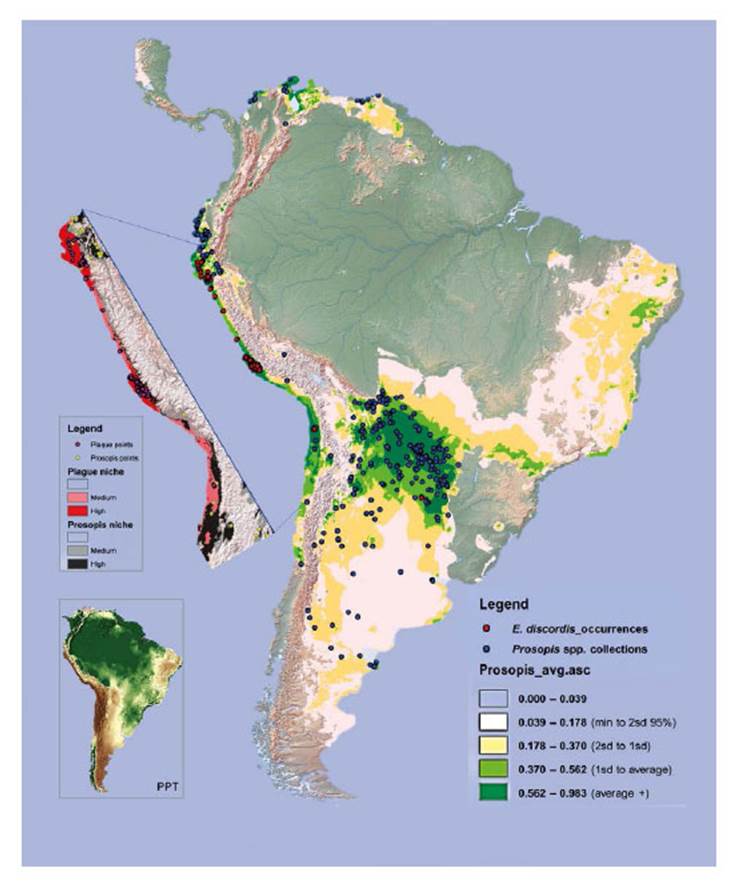
Figure 13 Niche models for Enallodiplosis discordis and Prosopis tree genera (c. 39 species) in South America. The Prosopis niche with high to medium predictions are dark to light green, and that of E discordis shaded red (cut out). Records of E. discordis occurrences (mostly as plague although not Argentina or Chile), are indicated by red dots and Prosopis voucher collection localities blue dots. Pink areas represent less robust predictions for the E. discordis niche areas and are generally not optimum niche areas for natural Prosopis distribution. Notably within these pink areas several occurrences of E. discordis were found on ornamental Prosopis trees. The inserted image (right), is shown to highlight aridity, with light to dark brown being lowest rainfall and green highest.
We first recorded E. discordis as a Prosopis infestation in 2001, identifying the species in 2003 and reporting as a serious plague in coastal Peru (Whaley et al. 2010a, b). From 2001 until present, in both southern and northern Peru, Enallodiplosis continues to be widespread. Prior to 2001, it was overlooked or misidentified in Peru, but evidence suggest it never occurring as a plague. Nevertheless, in Pampa Tamarugal, Chile, E. discordis (‘Contarinia sp.’[sic]) (Fig. 1) was reported as specific to Prosopis species, attacking P. tamarugo more than the other four Prosopis species present (P. alba, P. chilensis, P. strombulifera and the rare P. burkartii) (Vargas et al. 1989). The species was reported as a plague, described as becoming ‘prevalent in the trees during the beginning of spring, and in certain years developing into severe infestations and producing defoliation of Prosopis'.
Baseline research in Peru.- Although E. discordis was recorded in Chile in 1966 (2nd March 1966; C. Klein in Gagné 1994), it appears never to have been recorded in Peru prior to our research. However, this species could well have gone unnoticed in the ‘background’ biodiversity of Prosopis for reasons outlined. There are few baseline entomological studies of Prosopis in Peru, to provide conclusive evidence. Núñez-Sacarías (1993) provides us with a study of insects associated with Prosopis and records an unidentified Cecidomyiidae species in Piura (CICIU 11-130-93; the specimen no longer exists) with no mention of species details. In 2003, Bravo Calderón made a detailed evaluation of insects in algarrobo (P. pallida) foliage in Lambayeque, northern Peru. The Bravo Calderón team evaluated Prosopis forest areas (familiar to the present study) in: Motupe (Tongorrape, El Cardo, Yocape, La Capilla), Olmos (Cascajal, El Puente, San Cristóbal Chico y Grande) and Salas (Tempon, Dos Cerritos); 35 ‘plague or biocontrol’ species were collected from July to December 1997. Significantly, they report only an unidentified micro moth ‘pegador de hojas Olethreutidae family (Tortricidae)’, as being a principle cause of widespread defoliation, present in 100% of the trees and affecting 75-100% of the foliage (Bravo Calderón 2003). This study is also significant, being prior to the 1997/98 El Niño. Despite detailed investigation, they do not report Cecidomyiidae, or anything to suggest the prevalence of E. discordis as a misidentified plague. Conversely, from 2005 we recorded E. discordis as the principle and ubiquitous insect plague species of Prosopis foliage, in the same areas of Olmos, Motupe and Salas. However, it is again possible that E. discordis-at any stage of life cycle and in extremely low numbers-could perhaps have been overlooked in such a study.
Further historical evidence.- To further gauge the novelty and distribution of E. discordis as a plague, we examined historical and contemporary herbarium collections of Prosopis. Specimens record symptomatic evidence of the species through leaf spotting, desiccation, exuviae and dried first instar larvae. We revised Peruvian and Chilean Prosopis species herbarium specimens deposited from 1946 to 2015 and held at herbaria: K, MOL, PRG, SGO, USM. We found no evidence of E. discordis (as a plague species) prior to 1998. We discovered evidence to confirm its existence as a plague in May 2001 (Rio Poroma, Nazca) and June 2001 (Rio Ica) in the Ica region (Whaley & Beresford-Jones coll. 2201, 2601, 4901, 026, 062 (K!). In northern Peru, possible E. discordis (appearing as low-density small orange larvae) was found from 1985 P. pallida collection from Ferreñafe, Lambayeque (27 July 1985; Percy Zevallos 47 [K! herbarium]). However, a single 2003 specimen was found with symptomatic evidence of E. discordis from Chulucanas, Piura (8 October 2003; Rosa Jimenez Valdivia 21). In Chile, the only specimen with evidence of E. discordis was collected in 1980 from Tarapacá province, Camiña (14 November 1980; Lailhacar 59 [SGO! herbarium]) in non-plague density.
Local interviews.- Rural communities in both north and south Peru, offered insightful anecdotal information about changes in appearance of Prosopis foliage. In Piura, following short showers (as outlined) a rapid yellowing of Prosopis foliage can occur, and be referred by communities to as ‘tostadera’, and as ‘relámpago’ in Motupe (Lambayeque). These were the only foliage effects reported by Prosopis-dependent rural communities. The ongoing yellowing of Prosopis canopies, has resulted in widespread worry and confusion in communities with livestock and algarrobina producers, as the trees increasingly fail to produce pods. Significantly, all Prosopis- dependent communities, were not able to recognise E. discordis as an insect or a defoliating plague, despite its widespread occurrence, and had no local name for it. The characteristic leaf spotting and yellowing, with consequential defoliation and collapse in pod production, were not recognised in living memory, and not attributable to a cause apart from a change in historically experienced climate. However, moth plagues were recognised and associated with drought and ENSO conditions; these were ‘pegador de hojas’ (Tortricidae) and ‘gusano defoliador’ (Melipotis spp.) (Fig. 14).

Figure 14 Melipotis moths and Prosopis woodland activity: (a) Melipotis larvae in diurnal hidden roost under plank next to trunk of Prosopis host (1 Sol coin scale, 2006); (b) Melipotis larvae droppings in trunk cleft, with desiccated larvae after defoliation; (c) Adult moth Melipotis aff. indomita, Lambayeque; (d, e) Melipotis aff. indomita, Ica region; (f) Melipotis moth foraging for Prosopis flower nectar at night, Ica; (g) Livestock herders traditionally fatten goats and sheep on Prosopis pod throughout Peru (Topara, Chincha); (h) Huarango (P. limensis) surviving in the domestic settings (Santiago, Ica); (i) Harvesting Prosopis pods for food and forage (1994); (j) Following Prosopis decline in pod productivity charcoal burning became widespread. [Images © O. Whaley, (g) © Claudia Luthi]
In summary review, herbarium specimens and interviews provide evidence that: (i) E. discordis, as a species, was perhaps present in Peru in 1985, but at very low density, certainly not as a plague and easily ignored; (ii) that the species is historically unrecognised as a Prosopis plague in Peru; and (iii) that by 2001 E. discordis had become a plague in Ica, and by 2003, could have initiated as a plague in Piura, that by 2006, had developed throughout Piura and Lambayeque.
Importantly, E. discordis has only been recognised as a Prosopis plague after the ‘extremely strong’ 1997/98 ENSO, and between 1999 and 2001 in Ica it initiated as a plague of Prosopis (Fig. 11).
Enallodiplosis discordis in Chile and Argentina.- In Pampa del Tamarugal (Chile) in April 2010, E. discordis was recorded at extremely low density (<1 leaf in 10) on P. tamarugo foliage. Discussions with park rangers of the Corporación Nacional Forestal (CONAF), revealed that E. discordis had had little impact in recent years and was not considered as an ongoing plague. In 2011, during fieldwork in the Argentinian provinces of Buenos Aires, Corrientes and Entre Ríos, species of P. affinis, P. alba and P. kuntzei were carefully inspected for E. discordis infestations. Probable E. discordis larvae were identified in situ, and only found in a single site in the Chaco region, and at extremely low density on P. affinis and P. alba.. This concurs with the species presence in the country, alongside the identification by Gagné (1994) of probable E. discordis specimens of Brèthes, from Buenos Aires, Argentina. Likewise, the species was recorded in 2012 at San Pedro de Atacama, Chile in P. aff. alba (Whaley unpublished).
The impact on Prosopis pod production
Historical use of pod production.- The most evident indicator of Prosopis forest and tree health, is their pod (fruit) and honey production-stressed trees fail to produce pods, with flower abortion and poor pod development. The prolific annual pod production of P. pallida and P. limensis (see Whaley et al. 2019) in Peru, has been (and remains) fundamental to both the subsistence and commercial economy of dry forest communities in the regions of Ica, Piura, Lambayeque, as well as small parts of La Libertad (as other arid areas worldwide). On the coast of Peru, the nutritious Prosopis pod has underpinned the development of pre-Columbian cultures over at least 10,000 years, whilst the trees have sustained agriculture (Beresford-Jones et al. 2011, Whaley et al. 2010, 2019). In southern Peru, pods are known as ‘huaranga’, and in the north as ‘algarroba’. Despite the fact that rural communities have remained dependent on this an nual resource, there is little published data to quantify Prosopis pod production in Peru; no figures are apparent since 1999, due to interrelated disruption of climatic, cultural and political change. On the north coast, biannual production (one large, one small) is used for forage, but also for human consumption, processed into a range of products including flours, syrups, confectionary and coffee substitutes. In times of abundance, the pods are used as a premium quality animal fodder for fattening or conditioning horses and livestock in the departments of Ica, La Libertad, Lambayeque and Piura. In Ica, forexample, a crop was purchased for Lima police horses (pers. comm. Felix Quinteros) and in Piura used to fatten fighting bulls, even exported to southern Ecuador for the same purpose. The pod crop is stored during the winter months in dedicated clay-sealed storage rooms or ‘algarroberas’ (Díaz Celis 1977, 1995), having been gathered by smallholders, or by itinerant families harvesting on behalf of owners, or from state land (following expropriation of hacienda land during the Reforma Agraria of 1969). State land -nominally at least- was once regulated by the state through the Instituto Nacional de Recursos Naturales (INRENA; now decentralised as Ministerio de Agricultura) following a fee-based concession and reporting mechanism. Although this was often flouted with informal markets, we spoke regularly to several families in the Ica region who paid for concessions and reported pod harvests by the sack. Throughout the pod season, prior to 1999, corrals of pigs, sheep or goats were nearly always found under any Prosopis trees and forests near dwellings (pers. obs.).
Quantifying pod production prior to 1997/98 ENSO.- Prosopis pod production in the Ica region is small compared to that of Piura and Lambayeque, due to the desert confined riparian oases and deforestation of forest relicts before and since the post-colonial period (Díaz Celis 1995, Whaley et al. 2010, Beresford-Jones et al. 2011). Pod prices are dependent on the harvest quantity and the quality. From 1994 to 1997 a standard sack of pods (18-20 kg) was sold for 5-20 soles (PEN) depending on the point of sale, season and year (Fig. 2). In productive years pods could be sold by the ‘quintal’ (100 pounds or 45 kg)-a more common unit in northern Peru. Collated from extensive forest surveys and interviews, we estimate that during the decade up to 1998, the total fluctuating productivity of Prosopis pods in Icawas between 259-492 tonnes per year during a good year, and only around 50 tonnes or less during a poor year. The harvest has clearly declined sharply due to rap-id deforestation for charcoal and agriculture (Whaley etal. 2010). We estimate that around 40% of the total production was lost or consumed in situ by foraging domestic and wild animals and invertebrates; suggesting that around 156-296 tonnes could have been transported asforage and/or traded in Ica in the decades prior to 1998.
This contrasts quantitively with the comparatively large Prosopis forest of northern Peru, where for example, inPiura (areas of San Lorenzo, Sullana, Chulucanas, Medioand Bajo Piura) the pod harvests of 1993 to 1995 had anannual commercial production of 6,800-12,600 tonnes ofpods (Díaz Celis 1995). Some large Prosopis trees in Piura can produce up to 300 kg of pods per year (Grados & Cruz1996) and an average figure of 46 kg of pods per year is also used. A managed plot of algarrobo (Prosopis pallida) could produce 16500 kg/ha/yr in Piura (Díaz Celis 1995).
The actual pod production is far larger-by several orders of magnitude-than the commercialised reported figure, as pods remain unharvested in remote areas, traded in informal unregistered ways, or consumed in situ as wild or domesticated animal forage. A pod production estimate was made for Subregion II Lambayeque; using an average of 46 kg of pods per mature tree, multiplying by the number of trees at estimated average densities from three Prosopis ecosystems, to reach surprisingly large figure of 204,138 tonnes (Proyecto Algarrobo 1992).
In the Ica region the pod harvest collapsed rapidly from 2001, to culminate in 2005, in complete pod production failure south of the region (Santa Cruz, Palpa and Nazca), with no Prosopis pod products available. In the Ica valley, previously highly productive Prosopis trees (such as those in marginal dunes of Pampa Villacuri, San Jacinto and San Pedro) (see Fig. 11, Fig. 12d) failed to produce a harvest. Only a few isolated individuals produced any pods at all (such as in Guanaco, Ocucaje, Santiago de Ica). Flowering was aborted, with remaining flowers devoid of nectar. In 2006, local beekeepers reported no flow of ‘huarango’ (Prosopis) honey, and local market stalls could not obtain any after generations of supply (pers. obs.). By 2009, pods were found only in a few urbanised forests of Ica such as the Angostura. It became possible to accurately record the phenology and quantification of this collapse in pod production through a network of producers during the annual Huarango Festival, established in 2005 (Whaley et al. 2010b) and now in its eleventh year.
By 2006/07 in northern Peru the Prosopis pod collapse was evident, even in areas such as Tambogrande, Piura -a stronghold of forest pod production. Santa María de Locuto, one of the largest consumers of Prosopis pods for commercial purposes, in order to continue production was compelled to purchase pods from as far away as Chulucanas -over 20 km distant- due to lack of production from its surrounding forest (pers. comm. company director).
Pod Production post-1997/98 ENSO.- Both the Ministry of Agriculture and communities dependent on Prosopis forest in Piura, reported pivotal shifts and collapses in pod productivity around the two ‘very strong’ El Niño events of 1982/83 (with published figures) and 1997/98 (Fig. 12) (Díaz Celis 1995). A normal annual rainfall in this arid desert region is 20-200 mm, whereas both ‘very strong’ El Niño events registered exceptional rainfall with over 3,000 mm in Piura and Lambayeque (El Niño 1982/83 saw rainfall as follows: Chulucanus >4000 mm, Piura 2147 mm; and El Niño 1997/98 Chulucanus 3735 mm, Piura 1802 mm (see Takahashi 2004), and January to April 1998, areas around the towns of Olmos, Piura and Tambogrande were reported to have received over 4,000 mm of rain (Ordinola 2001).
Interviews with the most elderly have suggested that between 1925 and 1982, the region experienced rather consistently dry conditions (borne out by sea surface temperature El Niño indicator Zone 1-2) and that after El Niño of 1982/83 much of the ‘deforested plains were converted to scrub or regenerated into forest’. This concurs with multiple analyses that class the ENSO cycles of 1925/26, 1982/83 and 1997/98 as ‘very strong’, and the most extreme events over the last century (e.g. Quinn et al. 1987, Takahashi 2004, Cai et al. 2014). Heat spikes, during El Niño of 1982/83 and 1997/98 saw the temperature in Piura and Lambayeque peaking at 5°C over the average, with La Niña following the 1997/98 El Niño producing prolonged drought conditions. Likewise, in Ica the 1997/98 El Niño was followed by a period of drought until 2005 (Servicio Nacional de Meteorología e Hidrología del Peru [SENAMHI]).
Interviews and Ministry of Agriculture data (in Díaz Ce-lis 1995) reveal that between 1984 and 1997 in both Icaand northern Peru-although fluctuating with normal productivity cycles-the production of pods and the rural livelihoods that depended on them, were sustained (Fig. 12c).Therefore, evidence suggests that ‘very strong’ El Niño canproduce a temporary collapse in Prosopis pods, as in El Niñoof 1982/83 (see Fig. 12a, b). The arrival of heavy rainfalland flooding coincide with pod ripening and the fallen podsdecay rapidly. However, after El Niño 1982/83 pod production recovers, and is boosted in subsequent years with highsoil humidity and released fertility (Fig. 12).
This 1982/83 scenario, contrasts completely with asteady decline and collapse in pod production in all areasof Prosopis distribution on the coast of Peru from 2001, following the 1997/98 El Niño (Fig. 12d). This decline is concurrent with the two powerful defoliating agents: firstly, the Melipotis aff. indomita (Lepidoptera: Noctuoidea) plaguethat declined; and secondly, E. discordis that sustained.
Although Prosopis is a phreatophyte, it is probable thata plague trigger is related to unfavourable water balanceresulting in increased sugar content of foliage (see Kulman1971 in Ueckert 1974). Also, climate change induced stressis a factor with little baseline research in desert Peru.
It should be mentioned that the possibility of drought conditions being to blame for defoliation, can be dismissed, in that other tree species in Prosopis ecosystems continued to thrive, including Acacia and Capparis species, Cordia lutea, Pluchea chingoyo and the sub-canopy Vallesia glabra (see Baena et al. 2017).
Fundamental Change following 1997/98 El Niño.- To understand the novel appearance of E. discordis (and Melipotis), we note fundamental change to the Prosopis,environment following the unprecedented El Niño of 1997/98 (Rein 2007, Cai et al. 2014) including groundwater. Water-tables were raised over a space of a few days by 10-30 metres both in Piura and Ica. Furthermore, artisan wells used for several generations in Piura and Lambayeque were ruined, changing from drinkable to saline, or mineralised referred to ‘metallic tasting’, leading to land use and livestock change. Biologically in Northern Peru we observed extensive ‘blanket’ growth of Cucurbitaceae climbers; Luffa operculata (also noted by Ferreyra (1993) after 1982/83 El Niño ) and Momordica charantia (introduced), as well as and the orange-flowered Pseudogynoxys cordifolia (Compositae), all acting as lianas smothering Prosopis forest. This effect is perhaps compounded by atmospheric CO2 fertilisation (Laurance et al. 2004). Eventually many Prosopis trees were smothered contributing to their mortality (see Fig. 10).
As detailed, Prosopis-reliant communities reported a collapse in pod production for the first time in living memory (Fig. 12), specifically in Lambayeque there was a steady decline from 2003, with 80-90% Prosopis tree mortality by 2016 near Motupe, Pómac and Salas (see Baena et al. 2017) (Fig. 12g). In Prosopis forests around Olmos, rural communities reported a single large harvest in 2000 and thereafter, a rapid decline until 2004-05 when pod production collapsed by over 95%, with early reports of unidentified plagues (pers. comm. rural district around Mano de León).
Changes to Prosopis forest livestock management.- Recent decades in Peru have seen radical socio-economic change for rural coastal communities. Access to community forest land has been increasingly blocked by industrial farming and urbanisation. Itinerant herding of goats and sheep through dry forest is in decline as a way of life, with reductions of herds seasonally through Prosopis dry forest (see Perevolotsky 1990, Popescu 2013, MINAG-DGIA). Although figures for livestock forage (or pannage) in Prosopis forest (and ‘algarrobales’) are unavailable, indicative figures include Piura producing around 25% of ‘caprino’ (goats) in Peru in 2006 (MINAG-DGIA).
In contrast to many arid areas, where goats can be highly detrimental to vegetation, evidence suggests that goats (and desert sheep) can maintain and regenerate Prosopis forest in Peru (Piura), in that they facilitate germination and seed dispersal (Perevolotsky 1990, 1991). Significantly during the dry season, they forage Prosopisdry leaf-litter. Perevolotsky (1990) found that goat herds in Piura, increased in size during the severe drought of 1982 (prior to El Niño), indicating the value of dry forest leaf-litter as quality forage.
Given that E. discordis has an obligate relationship with Prosopis leaf-litter to complete its lifecycle (see Fig. 5), the role of forest herbivores may be critical to its regulation. In Peru dry forest, goats and sheep occupy an ecological niche previously filled by the once widespread white-tailed deer (Odocoileus virginianus). The Cronista Padre Bernabé Cobo (1582-1657) reports the forests of northern Peru and Ica as running with huge herds of deer. As well as having a role in forest disturbance previously performed by megafauna (such as the South American ground sloth (Scelidodon chiliensis) found in northern coastal Peru at least until 9000 bp (see Hubbe et al. 2007). The role of goats and sheep in regulation of E. discordis, as a plague, should not be ignored (see supplementary notes below). The on-going situation presents a feedback: where collapse of Prosopis pod forage sees a reduction in forest-ranged livestock, resulting in less consumption of Prosopis leaf-litter and allowing E. discordis to proliferate uninterrupted.
Conclusion
We described E. discordis plague in Prosopis in southern Peru from 2001, and by 2005 in northern Peru. Thespecies continues to be active and ubiquitous today in allthese regions as a widespread defoliant. Our evidence suggests it is a contributor to widespread die-back of Prosopis. We were able to gauge the extent of the Prosopis die-back,used an Sensefly eBee Classic ™ UAV drone for broad-scaleanalysis (including NIR and Red edge imaging), making 37flights in key Prosopis relicts and reserves supported byground-truthed data and plot observation with high resolution remote sensing images (Baena et al. 2017); in many areas we confirmed 80-90% Prosopis mortality (Fig. 10).
Range expansion or introduction.- Our additional observations in Chile and Argentina, suggest that E. discordis is widely distributed and associated with several Prosopisspecies. Notably, in Pampa Tamarugal, Chile-the region ofearliest identification as a plague-E. discordis ‘became prevalent during the beginning of spring and in certain yearsdeveloped into severe infestations producing defoliationof Prosopis’ (Vargas et al. 1989) (Fig. 1). We can hypothesize that El Niño 1997/98 in Peru, triggered extended andsemi-permanent changes conducive to E. discordis plagueconditions (that exist intermittently in Chile), with insufficient climatic and ecological regulation to stem.
There is also an outside possibility of assisted migration from Chile, through a transport mechanism-easily achieved via the stream of large vehicles moving along the Pan-American highway-providing a connection through Tacna ‘aridity barrier’. DNA population studies should be able to the resolve this, and other dispersal and provenance questions.
Niche model for Enallodiplosis discordis.- For further exploratory insight, we ran a niche model, using a known distribution of both E. discordis (Fig. 13) and Prosopis species in South America. Interestingly, the niche response (prediction values) for the E. discordis plague localities are low (>0.6 on a scale of 0-1), suggesting that the model is not predicting well.
This may be due to narrow distribution of observations confined to Prosopis relicts, or to desert landscape discontinuities and conditions, which our model does not include. It could also suggest that the plague is not greatly controlled by climate variables. This is particularly true for the climatically distinct outlying Argentina localities which are predicted with extremely low values (below 0.01). This is not really expected-as the Prosopis niche model is very robust-and is therefore suggestive that either the E. discordis as a plague (or as normal biodiversity) has not yet occupied its full niche, or, that climate is not the only (or strongest) factor determining distribution within the Prosopis host distribution.
The precipitation variables contributed most to the models. We can postulate that the 1997/98 ENSO conditions, with exceptional rainfall, spikes in heat, humidity and Prosopis productivity, could be a trigger; rapidly expanding the niche without biological regulation (see below).
It is conceivable that this could see rapid adaptive selection of E. discordis into a more virulent strain to reach these niches. This is a simplistic niche analysis but reveals patterns that warrant further investigation. Understanding the transport vectors for assisted distribution also needs research. The plague should be carefully monitored and evaluated throughout South America - especially in arid areas of Chaco and Argentina.
ENSO trigger and debilitated habitat resilience.-
The triggering of E. discordis as a plague, following ENSO 1997/98 with compounding climate change, appears as a ‘perfect storm’ scenario, where Prosopis, already under drought stress, sustained unimpeded attack in degraded forest, without the herbivore regulation of leaf-litter consumption (see below). Subsequent droughts prevented development of nectar-rich habitat (such as Waltheria ovata) to sustain biocontrol agents, that were impeded by increasing agricultural intensification and insecticide usage (that rose exponentially after 1999 (Fig. 14)).
Research is needed to determine the role of opportunistic fungal saprobes accelerating leaf necrosis, following perforation by E. discordis (see Fig. 8), and exploiting favourable climatic conditions. Tree fungal pathogen outbreaks have increased widely since 2000 and are already a recognised effect of climate change and increased global movement (see Brasier 2000, Fisher et al. 2012).
Tipping point?.- Given the keystone role of Prosopis trees in the ecosystem, the die-back is also indicative of threshold breaching with tipping points (see Holmgren et al. 2006, Reyer et al. 2015). In Ica, Lambayeque and Piura, many areas are seeing Prosopis dropping out of the forest system completely; ancient trees are dying and dead forests converted to charcoal (see Baena et al. 2017, Whaley et al. 2019, p. 34), with consequential expansion of farmland. Where forest remains, Prosopis mortality releases nutrients, allowing other forest species (such as Acacia macracantha, Capparis avicenniifolia, Capparis scabrida (Colicodendron scabridum), Cordia lutea, Pluchea spp. Vallesia glabra and the introduced invasive Tamarix aphylla) to thrive (with sufficient groundwater). The hypothesis of arid systems having alternative vegetation states triggered by single events-including a very strong ENSO (Holmgren & Scheffer 2001)-is compelling. And here, the "mighty midge" is also propelled by man-made climatic change and degradation. With profound changes in the world’s oldest desert forests, and a cultural and ecological extinction taking place, it is time to act.
Supplementary notes
Here we provide practical guidance for careful management: (1) Plague development and loss of biocontrol; (2) Melipotis as a plague of Prosopis; (3) Distinguishing Melipotis and Enallodiplosis discordis defoliation; (4) Physical and biological control of Melipotis; (5) Biological control of Enallodiplosis discordis; (6) The role of livestock in regulation of Enallodiplosis discordis; (7) Note on Enallodiplosis discordis as a potential control agent for Prosopis.
1. Plague development and loss of biocontrol.
Following the very strong 1997/98 El Niño, and weak 2000/01 La Niña, interviews with small farmers in the Ica region (2005-08), provided insight. From 2001, they experienced a series of intense insect plagues, including red spider mite (Acari: Tetranychidae) in Lima beans (Phaseolus lunatus) and ‘culi’ (Anthonomus spp.) in cotton (Gossypium spp.). Insect pests developed to such intensity, that many small farmers felt compelled to take out loans to pay for insecticide. At the same time, we observed the first outbreaks of Enallodiplosis discordis (recorded in herbarium specimen vouchers in 2001), and concurrently, widespread outbreaks of Melipotis aff. indomita (Lepidoptera: Noctuoidea) causing defoliation of Prosopis. Biological control agents also proliferated as expected. The key predator wasp of Melipotis larvae- Polistes peruvianus (Hymenoptera: Vespidae)-developed into huge colonies of nests on cliffs and lower horizontal branches of Prosopis (alongside the agricultural fields of riparian valleys) (Fig. 15). However, as both smallholder and industrial agriculture insecticide use increased (in adjacent fields), by 2005, most P. peruvianus wasp populations monitored had been wiped out, leaving only the most isolated colonies. We assume that being hidden during the day, M. aff. indomita larvae were less subject to insecticide, enabling their plague-status proliferation, compounded by a lack of the key biological control agent.
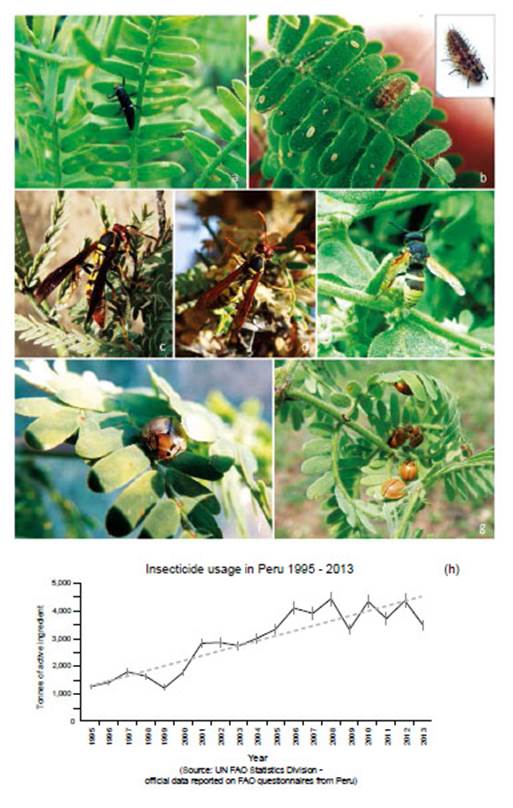
Figure 15 Native insects observed as potential biological control agents of E. discordis. (a, b) Coccinellid ladybird larvae (cf. Cycloneda sp. & Chilocorus sp.); (c, d) The once ubiquitous coastal wasp Polistes peruvianus (Vespidae), also a voracious predator of Melipotis larvae; (e) The potter wasp Pachodynerus peruensis (Vespidae) seen consuming E. discordis larvae; (f, g) Paraneda pallidula (Coccinellidae) typical of Peruvian Prosopis species; (h) Insecticide consumption in Peru following the 1998-99 ENSO event (FAO) with unquantified impact on native biocontrol insect populations. [Images © O. Whaley]
1. Melipotis as a plague of Prosopis trees
We recorded the moth M. aff. indomita causing widespread defoliation of Prosopis in the Ica region over the period 2001-07, during which time its dominance declined and was replaced by the new plague, E. discordis. Melipotis moth species are well-documented defoliating plagues of Prosopis through arid areas of North and South America (Brown 1945, Ward et al. 1977, Deloach 1994): in Louisiana, M. indomita (Walker 1858) as a plague of P. juliflora var. glandulosa (Cockerell in Brown 1945); of Prosopis pallida (Kiawe) in Hawaii 1969 (Oda and Mau 1974); in Texas, M. indomita defoliation causing 58% mortality in P. glandulosa, with foliage reduced by 95% (Ueckert 1974). In South America, Melipotis species were recorded as a Prosopis plague in a wide range of countries, notably: M. ochrodes in Puerto Rico 1936, Chile 1987 and Argentina (Córdoba) 1999 (Mazzuferi 2000); on P. flexuosa and P. chilensis in Argentina (Cates & Rhoades 1977 in Simpson 1977); M. trujillensis and M. walkeri severely attacked P. chilensis (Klein & Campos 1978) in Chile. In Iquique region, Chile, Melipotis species are well-documented as a plague of several Prosopis species reducing productivity. They are "nocturnal and remain grouped together inside tree trunk cracks, underneath salt crusts and in leaf-litter during the day" (Vargas et al. 1989).
In Peru Melipotis moth species can be referred to as ‘gusano defoliadores’ (defoliating grubs) (Fig. 14), and normally remain regulated in low numbers as part of healthy Prosopis forest biodiversity. Melipotis as a plague of Prosopis has been recorded in Piura and Lambayeque by Díaz Celis (1977, 1995), Núñez-Sacarías (1993), Díaz Celis (1997) and Bravo Calderon (2003). Although no widespread defoliation, was observed during fieldwork in Ica and Piura regions (1994-2001), by November 2003, we recorded Melipotis infestations in Prosopis in the Ica (and Nazca), that became widespread by 2005 and declined by 2008; perhaps due to resource competition with E. discordis defoliation. Evidence suggests that Melipotis plagues are triggered by unfavourable water balance resulting in elevated foliar sugar concentrations (Kulman 1971 in Ueckert 1974), this would concur with drought conditions of the period.
2. Distinguishing Melipotis and Enallodiplosis discordis defoliation
Melipotis larvae are found in Prosopis as a normal healthy association (providing forage for woodpeckers, and wasps for example). However, Melipotis infestation as a plague in Prosopis, is easily confirmed by the presence of diurnal larval roosting sites at the base of trunks (see Table 1 and Fig. 14), and by observation of larvae climbing trunks at dusk. Diurnal roosts contain from several hundred to several thousand larvae (in proportion to canopy size). Melipotis larvae are photophobic and crawl rapidly to dark recesses when exposed. Melipotis larvae (in P. limensis in Ica) had preferential selection of young leaves in the outermost leaf cohorts. Once the newest leaves have been consumed, the rest of the foliage is devoured in one to two weeks (Fig. 14). The diagnostic pattern of Melipotis defoliation can easily be distinguished from E. discordis (Table 1), the key features of Melipotisbeing: (i) leaves reduced to naked leaf rachis with few or no leaflets; (ii) branch hollows filled with droppings of active larvae (active larvae have green droppings, thereafter dark brown); (iii) presence of larval roosts in crevices at the base of trunks during the day. Up to 200 larvae per 10 cm2 can be found under any adjacent stones, branches, old clothes, plastic rubbish, or anything dry and dark near the base of the trunk (Fig. 14).
3. Physical and biological control of Melipotis
Several methods of physical control of Melipotis were tried successfully in cultivated Prosopis groves or ‘huarangales’ (see Fig. 14). The most practical method is to physically remove larvae from diurnal roosts, and feed them to hens, which eat them voraciously. Roost traps were created by placing or tying material-such as sack cloth-around the trunk or placing small wooden boards at the base of the trunk. Both serve to attract the entire Melipotis larvae tree population during the day. Over a few nights all larvae can be removed, thus arresting tree defoliation and mortality. Biological control wasps were observed, most commonly Polistes peruvianus (Hymenoptera: Vespidae), as well as large unidentified parasitoid wasps. The endemic black-necked woodpecker (Colaptes atricollis) was also observed excavating root roosts and eating larvae (Pecho et al. 2010).
4. Biological control of Enallodiplosis discordis
Despite hundreds of hours of field observation, and the high insect diversity of Prosopis, only a few biocontrol species were recorded feeding on E. discordis larvae. These were: Chrysoperla spp. (Neuroptera: Chrysopidae) ‘lacewing’ larvae; several species of Coccinellidae (ladybird) larvae (Fig. 15a,b) with adults including Hippodamia convergens and Paraneda pallidula (Fig. 15f,g), and most recently, in Lambayeque Cycloneda sanguinea. The ladybird P. pallidula is the species most associated with Prosopis in Peru, especially in areas without heavy use of insecticide. Ladybird larvae and adults appeared to be more predatory at night, however, in low numbers they are not voracious enough to regulate the vast populations of E. discordis larvae. In Ica and Lambayeque, larvae of Ceraeochrysa sp. and Chrysoperla aff. externa (Chrysopidae) are also effective. These species were observed to consume a succession of E. discordis larvae, grasping with mouthparts and sucking out internal fluids, leaving wrinkled orange remains. Chrysopidae species do have potential as biocontrol agents of E. discordis larvae-as they do for other plagues in the neotropics (Albuquerque et al. 2001). Some native species might be appropriate for mass breeding (Albuquerque et al. 1994).
The recording of E. discordis being predated by rapidly moving wasp species, was more challenging. However, both Polistes aff. peruvianus (Fig. 15c, d) and Pachodynerus peruensis (Fig. 15e), were observed in Chiclayo feeding on E. discordis larvae. Several unidentified small parasitoid wasps were also found searching infected Prosopis leaflets and may also attack E. discordis.
In laboratory conditions in Lambayeque, several otherspecies showed biological potential, including: the insidious flower bug (Orius insidiosus) and a Reduviidae bug.
Two introduced species-already recognised as biological control agents-also show potential to control E. discordis at the pupation stage on the ground; these are: the Clavicipitaceae fungus Isaria fumosorosea (Paecilomyces fumosoroseus) and the nematode worm genus Heterorhabditis.
Lastly, we observed small insectivorous birds feeding on E. discordis larvae, including the house wren (Troglodytes aedon) and the slender-billed finch (Xenospingus concolor). Unpublished research carried out in 2008 on an organic farm in the lower Ica valley, suggests that biocontrol of E. discordis plague is possible in high diversity habitats. Here, where Prosopis-associated plants are cultivated for honey production, we recorded a higher concentration of the biocontrol species in understory plants including: Baccharis salicifolia, Encelia canescens, Grabowskia boerhaaviifolia, Lycium americanum, Phyla canescens, Pluchea chingoyo, Trixis cacalioides and Waltheria ovata. These plant assemblages produce nectar-rich flowers throughout the year (Whaley et al. 2010a, 2019). The lack of any evidence of widespread biological regulation of E. discordis plague to date in Peru, is likely to be a result of degradation including non-targeted destruction of biocontrol species and habitat.
5. The role of livestock in regulation of Enallodiplosis discordis
In protected areas, livestock can threaten natural regeneration, and so exclusion is attempted, as in ‘Santuario Histórico Bosque de Pómac’ (a sole protected lowland Prosopis-dominated forest in Lambayeque, home to endemic species (see Angulo & Sánchez 2016)). The santuario is presently in a serious ecological upheaval, with widespread Prosopis die-back and proliferation of post-rainfall climbers (Luffa operculata and non-native Momordica charantia) (pers. obs. and drone-mounted spectral analysis under permit). Recently, serious conflicts and forest fires have ensued (see Radio Programas del Peru [RPP] https://rpp.pe/noticias/bosque-de-pomac); Pómac: Boletín de Santuario Historico de Pomac N°6, mayo-junio 2018), and are indicative of forest die-back. Given the regulatory mechanism of livestock in Prosopis forest (see above), the relationship between domestic livestock and E. discordis plague regulation here requires urgent research.
During Prosopis forest monitoring it emerged that mature trees with livestock corralled below, maintained foliage, whilst similar aged trees outside corrals, lost foliage and underwent die-back. In the corral, goats and sheep consumed the Prosopis leaf-litter, whilst compacting and soaking the ground with urine.
This saw interruption of the E. discordis life cycle by killing larvae at the leaf-litter pupation stage, permitting leaves to persist not subjected to defoliation cycles. Thus, Prosopis trees in traditional stock corrals were able to retain canopy foliage and produce limited pods, compared with non-corralled trees or those with corralled cattle that did not.
Although overgrazing with goats can accelerate desertification, in Piura research revealed goats in certain densities and forest states, can also be important Prosopis seed dispersers in dry forest (Perevolotsky 1990, 1991, López 2017) especially following El Niño rains. Goats (and sheep) to limited extents, replace displaced herbivores (see Holmgren et al. 2006), in this case, the native seed dispersers such as white-tailed deer (Odocoileus virginianus) and desert fox (Lycalopex sechurae and L. culpaeus) (Huey 1969, Meza 2004) - deer being hunted out and desert fox widely persecuted. This being the case, livestock (at low density) may perform a vital role to help sustain forest health by consuming surface Prosopis leaf-litter, they regulate of E. discordis in the ecosystem - as in the corral.
Today the number of goats and sheep in Peru is apparently in significant decline (by nearly 59% from 1961 to 2012), whilst numbers of cattle are on the increase (rising by 39% from 1961 to 2012) (INEI 2012). Rural communities cite many factors contributing to the decline of forest-run goats and sheep, not least being the loss of Prosopis pod forage as outlined. Other factors include: market decline, community land tenure problems with associated violence and insecurity, agroindustrial expansion blocking itinerant herder access, land abandonment due to drought, urban development, and migration.
To give an indication of this relationship, we conductedseveral small trials by physically removing leaf-litter under Prosopis (mature trees c.90 cm GBH) infested with E. discordis, in Ica and Lambayeque. The sub-canopy leaf-litter (5-10 cm deep, weighing around 20-25 kg) was sweptinto a pile, sprayed with water and composted under plastic to kill pupae. Subsequent leaf falls were cleared 1-2 times per month for three months, with around 1-4 kg ofleaf-litter per clearance. After two weeks, tree canopiesbegan to recover some foliage. Although producing very few pods (consumed by Pacific parrotlets, Forpus coelestis), after two years, trees treated in this fashion, had retained foliage whilst untreated trees died back.
Clearly, the interruption of the lifecycle throughphysical removal of E. discordis pupae in the leaf-litter,does not prevent reinfection from adults travelling infrom other trees, but it does appear to maintain lower levels of E. discordis reinfection from below the tree,enabling the tree to outgrow infestation. Leaf-litter removal is realistically only achievable with Prosopis trees near smallholdings. Further trials of physical interventions are required to allow Prosopis, at least near dwellings, to produce seed and survive. Likewise, it might bean appropriate strategy to save some veteran (patrimonial) Prosopis trees. However, caution must be taken as the leaf-litter will play a role in conserving moisture and insulating the roots from overheating. Here limited irrigation might compensate for this, but again further research is required.
In summary:
- Prosopis trees are less susceptible to E. discordis in the following conditions:
- Growing inside livestock corrals where leaf-litter is consumed. Likewise, trees growing in cattle corrals, where leaf-litter is eaten more out of desperation, but where urination impedes or kills the larva on the ground.
- Cultivated in the domestic or urban setting of garages, villages or next to dwellings, where the ground surface is hard, compacted or regularly swept.
- On sand dune slopes at valley margins or oases - the sand slips away removing and burying pupae. Trees here may also benefit from fog capture.
Notes on Enallodiplosis discordis as control agent for Prosopis
Several Neotropical Prosopis species have been introduced worldwide, especially P. juliflora and P. pallida (often misidentified). In Hawaii, northern India, Ethiopia and Australia especially, introductions cause varying degrees of impact; being highly invasive and detrimental to biodiversity, whilst often providing useful livelihood resources (e.g. Dahl 1971, Sosebee 1973, DeLoach 1985, Cordo 1987, Moran 1993, McKay 2007, Gallaher & Merlin 2010). In the future, E. discordis could be considered as a possible agent with which to moderate non-native Prosopis invasion. We would strongly advise against this for several reasons, but especially, as this insect has already been found on at least six Prosopis tree species, and is unlikely to be selective, making old world Prosopis vulnerable. Furthermore, in a new world order of climate change, pandemics and poverty; good management of introduced Prosopis, could allow the production of valuable local resources, and over the long term, may even assist ecosystems, particularly in irreversibly degraded arid environments needing renewable resources (see Garg 1999, Felker & Moss 1996, Logan 2007, Shackleton et al. 2014). Lastly, Prosopis species from Peru introduced to other countries (see Konda Reddy 1978), may one day be required to restore forest and culture back to Peru.
Prosopis survival and future Research
Some Prosopis individuals (and groups or hybrid swarms) appear to have genetic traits conferring a degree of resistance to E. discordis plague. Across the Pacific coastal Prosopis distribution in Peru, further UAV and ground survey research is needed to: (i) identify genetically resistant clusters / habitat, (ii) identify seed sources, (iii) recognize niche requirements of biological control agents, (iv) locate areas for Prosopis soil seed bank restoration prior to flood events, and (v) research the Achilles heel of E. discordis-its dependence on leaf-litter for pupation.












 uBio
uBio