Introduction
Large branchiopods are widely distributed in the worldwide, generally associated with temporary water bodies, which are more abundant in the arid and semi-arid regions of the planet (Perez-Bote et al. 2005, Rogers 2013). These primitive crustaceans are constituted by three orders: Anostraca (fairy shrimps), Notostraca (tadpole shrimps) and Diplostraca (Mabidi et al. 2016), this last one comprise more than 200 species described for the world (Tiwari 1966a, 1966b, Martin 1992, Martin & Davis 2001, Belk et al. 2002, García & Pereira 2003, Brendonck et al. 2008, Rabet et al. 2012, Rogers & Padhye 2014, Rogers & Padhye 2015, Padhye et al. 2015), of which 23 species belong to the family Leptestheriidae (suborder Spinicaudata, spiny clam shrimps) where most of them is represented by the genus Leptestheria. (Brendonck et al. 2008, Babu & Nandan 2010, Rogers et al. 2020).
In Colombia there is not any valid record of Leptestheria species, nevertheless, this genus has been reported in the Magdalena Valley and the Orinoco River without specifying the species (Roessler 1995). The aim of this paper is to report on the first record of Leptestheria venezuelica in the north of Colombia, which expands distributional range of this species, also a brief description of the specimens found is presented.
Material and methods
Study site. The work was carried out in a temporary pond in Maicao sector, department of La Guajira located between 11°23ʹ04.63ʺN; 72°16ʹ31.10ʺW (Fig. 1A) in the north of Colombia, the pond is a product of the temporal rains, the flow of the water disperses through the low áreas of the site, around the pond a sub-xerophytic vegetation dominated by legumes plants, Prosopis juliflora and Acacia farnesiana was observed (Fig. 1B). The rainfalls regime in the area is bimodal, present a high dry period between December and April, in May and June present the minimal rainfalls period, finally, in September start the high rainfalls and extend to November (OrjuelaRojas et al. 2011).
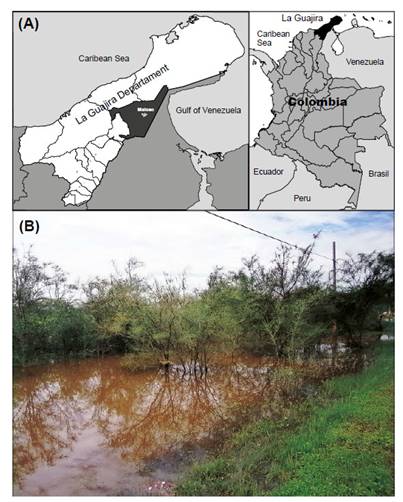
Figure 1 Location of collecting of the Clam Shrimp Leptestheria venezuelica Daday, 1923, new record from Colombia. (A) Location of Maicao, La Guajira, northern of Colombia; (B) photograph of the temporal pond.
Biological collections. Biological samples of Leptestheria venezuelica were collected with a zooplankton net (mesh size = 100 μm) in aleatory points of the pond and preserved in 70% ethanol. Leptestheria venezuelica were processed for taxonomical identification including the examination of the whole specimen and dissection of selected appendages. Dissected appendages were mounted on slides with glycerin and sealed with Canada balsam; then, appendages were photographed using a camera ZEISS model AxioCam ERc 5s under Microscope ZEISS Primo Star and stereo microscope ZEISS Stemi 305 (Carl Zeiss, Oberkochen, Germany). Individuals were measured with the calibrated software ZEN 2 (Blue edition of ZEISS company) in lateral position, from the anterior end of the rostrum to the posterior margin of the telson. The identification of the species recorded herein followed Fryer (1987). Kaji et al. (2014), Shu et al. (2015) and Timms (2016). References were consulted for data on leptestheriids in Daday (1923), Nayar & Nair (1968), Sars (1900), García & Pereira (2003) Simhachalam & Timms (2012), Tiwari (1966a; 1996b) Rogers et al. 2012, Rogers et al. 2020. The examined specimens both dissected (slides) and undissected (vials, ethanolpreserved) samples were deposited at the Museo de Colecciones Biológicas Universidad del Magdalena (CBUMAG) Colombia, where they are available for consultation and further examination.
Results
Material in collection: COLOMBIA, La Guaji-ra, Maicao 18 km near box culvert roadside pool (11°23ʹ04.63ʺN; 72°16ʹ31.10ʺW) collected by J,Serna. Nov. 2018; 6 females (CBUMAG:MAC:02010) and 9 males (CBUMAG:MAC:02011) in the Museo de Colecciones Biológicas Universidad del Magdalena.
Taxonomy
Suborder Spinicaudata Linder, 1945 Family Leptestheriidae Daday, 1923 Genus Leptestheria Daday, 1913 Leptestheria venezuelica Daday, 1923
Female. Carapace. Female carapace 5.0 - 5.9 length (Fig. 2A; 2C). (n = 65); the umbo is present on anterior margin; arched in the dorsal anterior margin with little granulations, short setaes present in row on the carapace growth lines like that of the male carapace (Fig. 3A). Head: with an angularly shaped rostrum. A rostral apex with wide fornices meet and a well-developed occipital sharp spine anteriorly curved. (Fig. 4A) present a concavity above the eye. First antenna: indistinctly segmented, with 14 - 18 lobes bearing sensory setae in frontal margin. (Fig. 5B). Second antenna: indistinctly segmented, with 13 - 15 segments on upper flagellum and 13 - 14 segments on lower flagellum, each segment with 1 - 5 dorsal spines and 3 - 5 ventral long setae (Fig. 4C). Thoracopods: first thoracopod with setose lobe on anterior side (Fig. 8). Female ninth and tenth thoracopod with epipods swollen and cylindrical in shape (female egg mass supporting appendages) (Fig. 7A and 7B). Telson: with two rows of 24 - 33 sharp spines, being larger and thin those terminal spines and with much finer spines between them. Telson filaments delicate (two), plumose on distal end, arising behind first telsonal spine. terminal part of the telson ends in two claw-shaped cercopods, dorsally closed in females than in males (Fig. 9B).
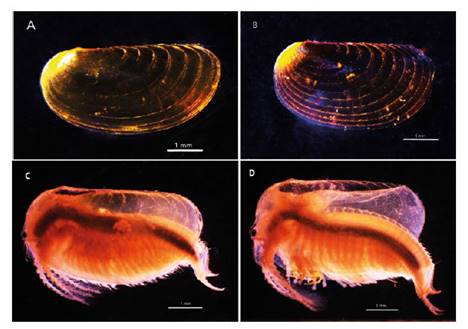
Figure 2 Microphotograph from stereomicroscopy, the left lateral view of the clam shrimps Leptestheria venezuelica showing the carapace female (A), the carapace male (B), habit of the female (C) and the habit of the male (D).
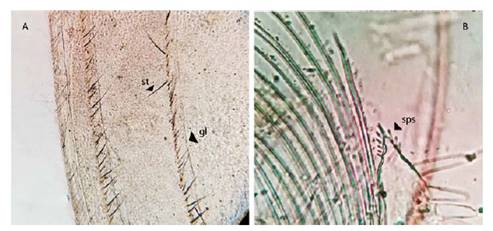
Figure 3 Microphotograph of parts by Leptestheria venezuelica. the grow lines (gl) of the carapace in both sexes (A), the setaes (st) in the lines; the terminal structure of the first endite (sps) in the claspers of the males (B).
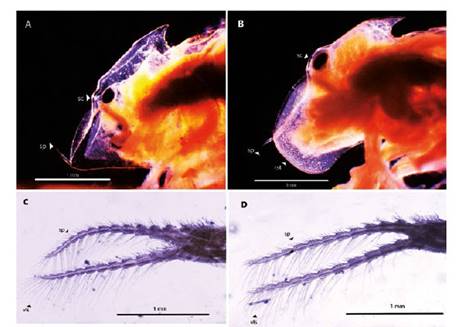
Figure 4 Microphotograph of the parts from Leptestheria venezuelica. Left lateral view of the female head (A) and male head (B) by Leptestheria venezuelica showing the eye (sc), the occipital spine (sp) and the rostrum in Aand B; the second antenna of the female (C) and the second antenna of the male (D) showing the ventral long setaes (vls).
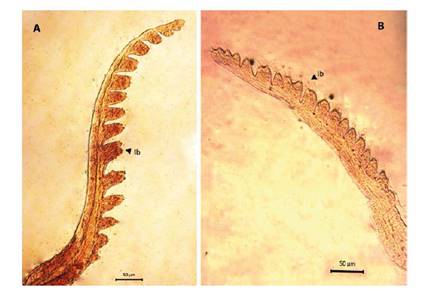
Figure 5 Microphotograph of the first antenna by Leptestheria venezuelica, show the lobes with setaes (lb) in both sexes, the first antenna of the male (A) and the female (B).
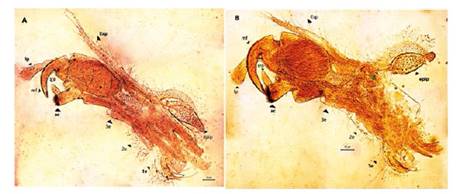
Figure 6 Microphotograph of the right claspers in males of Leptestheria venezuelica. the first clasper (A) and the second (B). lp: long palp; mf: movable finger endopod; srp: short palp; ac: apical club; 1e, 2e and 3e: first, second and third endite respectly; epip: epipodite; Exp: exopodite.
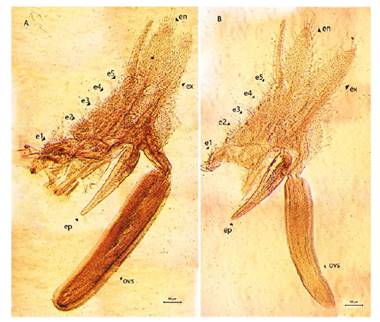
Figure 7 Microphotograph of the right thoracopods in females of Leptestheria venezuelica, the ninth (A) and tenth (B) thoracopods, show the epipodite (ep); the endites (e1-e5); the endopod (en); the exopodite (ex) and the cilintdrical structure for the egg mass supporting (ovs).
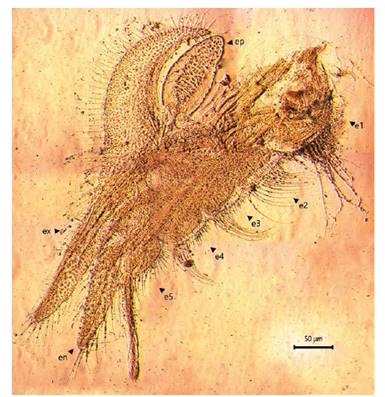
Figure 8 Microphotograph of the right first thoracopod by the female Leptestheria venezuelica, show the endite; the epipodites (ep); the endites (e1-e5); the endopod (en) and the exopodite (ex).
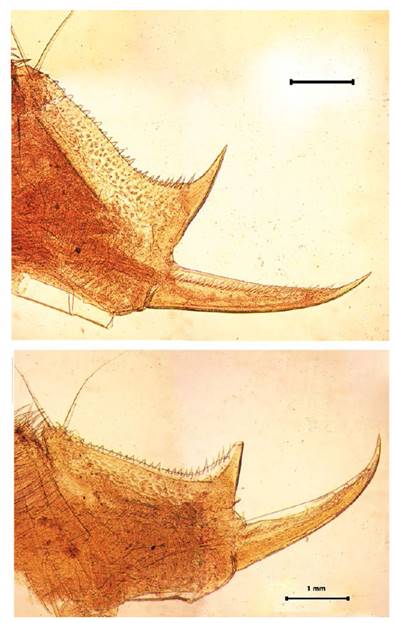
Figure 9 Microphotograph of the telson of the male (A) and the telson of the female (B) by Leptestheria venezuelica, show the row of spines (sp); the cercopod (cr) and the plumose setaes (st).
Males. Carapace: Male carapace 5.0 - 6.2 lenght, 2.4 - 3.0 height (Fig. 2B, 2D), (n = 45); slightly larger and carapace more rectangular than females. Area between growth lines minutely granulated, ventral margin of carapace with minute spines and setae (Fig. 3A). Head: The rostrum of the male is much broader and blunter, roundly spatuliform in lateral view. with well-developed ventrally arched occipital spine (Fig. 4B). The sharp spine situated a short distance behind the point where the fornices meet, usually straight and directed than the spine of the females. Dorsal margin of the head with shallow concavity above the eye. First Antenna: Male first antenna indistinctly segmented, with 14 - 16 lobes bearing sensory setae in frontal margin (Fig. 5A). Second Antenna: indistinctly segmented, with 13 - 14 segments on upper flagellum and 13 - 14 segments on lower flagellum, each segment bearing 3 - 6 dorsal spines. All segments with 3 - 4 ventral long setae (Fig. 4D). Thoracopods: 22 - 23 pairs with the two pair of claspers in males, the first and second thoracopod form a clasper, with strong indentation at base of immovable finger. Movable finger strongly curved with apex acute, the first (Fig. 6A) thicker than the second thoracopod (Fig. 6B); the first endite of the clasper present a tip with diminute spines, characteristic of the genre (Fig. 3B). The second and third endite (e2 and e3) have a filtering function in the anterior thopopods, present variable setaes; Distal end bearing noticeably short blunt spines on ventral margin; Endite 5 immovable finger stout, with strong thick acute spines on clasping border. Endite 4 stout, with simple terminal setae; palp (edite 5) stout, with two segments, bearing setae only on distal segment, arcuate, of two palpomeres, lightly. (endopod) broad basally, tapering and hooked distally; apex with many small scales; large palp, palpomere length subequal in both claspers; distal palpomere (endite 5 outgrowth) slightly elongated, apex with fine setae; small palp (endite 6 outgrowth) cylindrical, nearly twice as long as broad, directed anteriorly or slightly posteriorly, with apex covered with fine setae; palm (endite 6) broadly rectangular, projecting slightly obtusely, gripping area covered with small roughly conical, blunt tipped spines, increasing in size posteriorly.
Total length of palp slightly exceeding half length of movable finger. Endite 1 strongly curved, with acute tip bearing two serrate terminal spines. Endites 2 and 3 lobulate, with pedunculate setae.
Telson: Male telson with two rows of 28 - 32 sharp spines much finer spines between them, being larger and thin those terminal spines. Telson filaments delicate, plumose on distal end, arising behind first telsonal spine (Fig. 9A).
Discussion
The species herein recorded in addition to the records about leptestheriids by Roesser (1995) in Colombia enrich the knowledge of the leptestheriid-fauna of the contry and promote the search of important information about the diversity of taxa. Leptestheria venezuelica described for first time by Daday (1923) has been recorded in Aruba, Venezuela and recently in Chile (Belk et al. 2002, García & Pereira 2003, Rogers et al. 2020), demonstrates the gradual widening of distribution of the species without lose their endemism for South America.












 uBio
uBio 


