Introduction
Contributions to the conservation of bird species are possible with studies of the reproductive biology which reveal important data that can aid understanding of ecological relationships (Martin 2004) between biotic and abiotic factors. In the Neotropical region ornithological studies have increased considerably in recent years (Xiao et al. 2017), but the basic aspects of the biology of many bird species remain limited. Among them are reproductive aspects, mainly knowledge about nests and eggs, mainly Flycatchers (Heming et al. 2013). The availability of food resources influences the start of a breeding season (Sick 1997) and combination with choice of nest site and time invested in parental care are crucial for the survival of nestlings (Saether 1985, Mezquida & Marone 2001).
Rusty-Margined Flycatcher ranges from Costa Rica and Panama to northern South America (Mobley & Kirwan 2020). This species is usually one of the most common flycatchers in the regions of Brazil in which it occurs (Sick 1997). Four subspecies have been described, two of which occur in Brazil: Myiozetetes cayanensis cayanensis and Myiozetetes c. erythropterus (Piacentini et al. 2015). Myiozetetes c. cayanensis is relatively widely distributed in Brazil in comparison with M. c. erythropterus, which is restricted to southeastern Brazil (Mobley & Kirwan 2020). Few data are available on the reproductive biology of M. cayanensis (Carvalho 1960, Oniki & Willis 1983, Dyrcz 1991, Pascoal et al. 2016), and most of the studies have been conducted in countries other than Brazil (Mobley & Kirwan 2020). In the present study, we obtained detailed data on the reproductive biology and biometrics of M. c. cayanensis from a lowland terra firme forest in southwest Brazilian Amazonia, including its breeding season in Brazil, which provide important insights into the life history patterns of the flycatchers.
Material and methods
We conducted this study at the Zoobotanical Park of the Federal University of Acre (UFAC), in Rio Branco, capital of the Brazilian state of Acre. The Zoobotanical Park (09°57’08.9”S, 67°52’22.5”W) is a forest fragment with an area of ca.100 ha surrounded by an urban matrix. It is considered to be one of the largest urban forest fragments of the Rio Branco (Meneses-Filho et al. 1995). The vegetation is composed of successional forests of varying ages dominated by the cycle of mass mortality and succession of the bamboo Guadua weberbaueri, and dense understory with many vine tangles (Meneses-Filho et al. 1995, Silveira 1999). The region’s climate is humid tropical, with a dry season (May-October) and a rainy season (November-April) (Duarte 2006). The avian assemblage of the ZP is composed by 150 birds species distributed over 36 families, and some species are migratory and others have restricted geographical distribution (Guilherme 2001).
We found the nests during non-systematic observations made between 2012 and January 2020. We monitored all nests found, irrespective of stage, until they became inactive. We measured eggs and nestlings using a millimeter ruler and analogue (2012-2014) or digital callipers accurate to 0.01 mm (2015-2020). We weighed eggs and nestlings using a Pesola® scale with a capacity of 100 g and accurate to the nearest 1 g (2012-2014) or a digital scale accurate to 0.05 g (2015-2020). We collected nests after they became inactive and deposited them in the collection of the UFAC ornithology laboratory. Nests were described according to the standardized scheme proposed by Simon and Pacheco (2005).
We trapped and ringed Rusty-Margined Flycatcher in the years 1999-2002 (1624.8 net / hours), 2004-2006 (1367.3 net / hours) and 2009-2019 (57948.2 net / hours). We used 12.0 × 2.5 m mist-nets with 36 mm mesh and banded individuals with numbered metal rings supplied by CEMAVE (Centro Nacional de Pesquisa e Conservação de Aves Silvestres), under the scope of project 1099, coordinated by EG (senior bird bander, reg. no. 324654). We collected morphometric data (wing, tarsus, bill, head, tail, total length and cloacal temperature) only from 2002 following the protocol outlined by Proctor and Lynch (1993).
In general, we used a Pesola® scale with a capacity of 100 g (1 g precision) to weigh birds and nestlings prior to 2014, and a digital scale (0.05 g precision) during 2015-2020. We used a millimetre ruler to measure wing, tail and total length, and analogue callipers (0.05 mm precision) until 2014 and digital callipers (0.01 mm precision) in 2015-2020 to measure bill and tarsus. For each biometric, data were taken from each adult and juvenile individual only the first it was trapped. During ringing, we distinguished adults and juveniles based on the presence of juvenile plumage (see Sibley 2010, Johnson et al. 2011). We examined each trapped individual to determine molt in the remiges and rectrices (Sibley 2010) and presence or absence of a brood patch (Redfern 2010), and we included only adults in the analysis. We measured cloacal temperature with a digital thermometer (32.0-42.9 °C, accurate to 0.1 °C) and calculated minimum longevity from the first day an individual was banded to their last recapture (Scholer et al. 2018).
We searched for records of active Myiozetetes cayanensis nests, irrespective of subspecies, including those with nestlings, up to June 5th 2020, on the Brazilian Wikiaves site (Wikiaves 2020). We verified the date, location (Brazilian state and municipality), the author, and the content of each photograph (nests with eggs or nestlings). For the purposes of the study, we considered all the photographs from all localities, except those of a given nest taken on the same or subsequent days. We compiled a list of the number of records per month over the course of the year, including active nests and nests with nestlings, to determine the period of the species’ breeding season in Brazil, in general. Citizen science plays an especially important role in the compilation of data on the natural history of bird species (Tewksbury et al. 2014, Turnhout et al. 2016).
We calculated the incubation period from the date the last egg was laid until the last chick hatched. Post-hatching, we measured the mass of nestlings every two days to minimise our impact on nests. We banded young with a numbered metal ring while still in the nest. We considered the nestling development period based on the hatching of the first nestling and the abandonment of the nest by the second chick. The day of hatching was treated as day 0 (Oniki & Willis 2001). For nests found with nestlings, we estimated age based on the mass of the nestlings, comparing them with data for young monitored from hatching. We applied the equation proposed by Ricklefs (1967) to determine the growth rate of the nestlings:
W(t) = A / (1+e[- K(t - ti)]
where W(t) is the mass of the nestling at age t, A is the asymptote of the growth curve, K is the constant growth rate and ti is the inflection point of the growth curve. We ran the equation in the R software, version 3.5.1 (R Core Team 2018). We used the Mayfield (1961) method to calculate reproductive success rates, and determined apparent success as the ratio between the number of successful nests and the total number found (Jehle et al. 2004).
Results
We found nine Rusty-Margined Flycatcher nests, which all were active (three in January, February, and March 2013, two in August and December 2014, one in January and two in August 2015, and one in January 2015). All nests were constructed in open areas near water. Six nests were built in support plants above water (Fig. 1a, and three nests were built near wasps colonies in lemon tree Citrus sp. (Rutaceae) (Fig. 1b). On average, nests were placed 1.80 m above ground (range 1.43-2.15 m). We found four nests under construction, three with eggs (two of which had been abandoned), one with nestlings, and one was inaccessible. All nests were built of most pliable dry grass, few fine woody steams (thickness 2.01 mm), roots, and dry leaves. Internally, dry grass arranged irregularly (Fig. 1c). In one nest we found string thread. In other nest we found pieces of plastic and crochet string too in its external lining. All nests conformed to the closed / globular / fork types (Fig. 1d), arranged in two to six thin branches from the main branch. We present mean measurements of nests (n = 3) in Table 1.

Figure 1 Rusty-Margined Flycatcher Myiozetetes c. cayanensis nests found in a terra firme forest fragment in southwest Amazonia. (a) Nest built 1.43 m above water; (b) Nest built near wasps colonies; (c) internal view of nest characteristics; and (d) lateral view of an nest sited in fork. All photographs by JL.
Table 1 Characteristics of Rusty-Margined Flycatcher Myiozetetes c. cayanensis nests (n = 3) found between 2012 and 2020, in a terra firme forest fragment in Acre, Brazil, southwest Amazonia.
In four nests the clutch size was two eggs, in two nests was three eggs, and in one nest was four eggs. Eggs were predominantly white with pale and dark brown blotches, generally concentrated at the larger end (Fig. 2a). Mean egg mass was 2.8 ± 0.5 g (range 2.0-3.5 g; n = 13) and size 21.8 ± 1.2 × 15.4 ± 0.5 mm (range 21-25 × 15-16 mm). Of all the nests monitored with eggs, in only one nest three of the four eggs failed to hatch. Incubation period was 15 days. Daily nest survival showed that there is 100% chance that a nest will fledge at a least one juvenile. Daily survival rate during the incubation period was 90%. Assuming an incubation period of 12 days, Mayfield success was 20%, and apparent success was 46% during the incubation period.
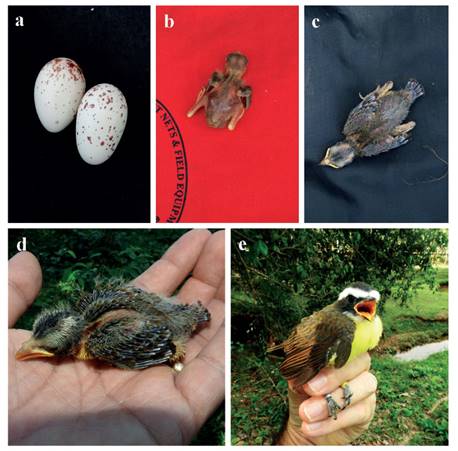
Figure 2 Development of Rusty-Margined Flycatcher Myiozetetes c. cayanensis nestlings in a terra firme forest fragment in southwest Amazonia. (a) detail of the eggs, (b) recently hatched nestling, and (c) five-day-old nestling (Nest 2, Figure 2); (d) 14-day-old and (e) 23-day-old nestling (nest 3, Figure 2). (Photos a, b and c by JL; d and e by EG).
We monitored the development of six nestlings in three nests (three nestlings in nest 4 from September to October 2014, one nestling from nest 5 in January 2014, and two nestlings from nest 9 in Janury 2020). Nestlings hatched with light pink skin, few plumes on the back and head, and eyes closed (Fig. 2b). After five days, the eyes are slightly open, and the feathers of the remiges and rectrices start to develop (Fig. 2c). We monitored three nestlings from hatching.
Mean hatch weight was 2.3 ± 0.58 g (range 2-3 g; n = 3) and nestling weight average 20 g (n = 6) after 14-16 days, and 25.5 g (n = 3) on day 23 (Fig. 2d, e), the oldest age recorded of any nestling (Fig. 3). Two chicks fledged after 22 days, while four chicks fledged after 23 days (Fig. 3). We ringed all chicks in the nest (ring codes F51460, F51463, F51454, F51492, F67598 and F77742). In two nests all nestlings were infected with maggots under skin on the head. No nestling monitored was predated after hatching. The longest nestling period was 23 days and chicks fledged at a mean mass of 24.2 ± 1.3 g (range 22-25.5 g; n = 6).
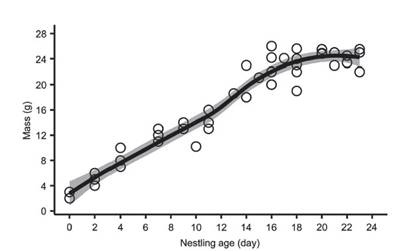
Figure 3 Logistic growth curve for six nestlings of Rusty-Margined Flycatcher Myiozetetes c. cayanensis, monitored in a terra firme forest fragment in southwest Amazonia between 2012 and 2020.
The constant growth rate (K) of the nestlings was 0.18 (range 0.02-0.14; SE = 0.03) with a growth asymptote of 22.8 g (26.99-29.71 g; SE = 6.9; Fig. 3). Daily survival for nestling period was 100%. Mayfield success during the nestling period was 100%, assuming chicks remained in the nest for 23 days. Apparent success was 100% in the nestling period.
We trapped adults (n = 67) and young individuals (n= 38, including re-captures) in all months of the year except in August. During all years monitored, most of young individuals were captured between January and March and in October (n = 24; Fig. 4a). We found active nests in January-March, August, and December (Fig. 4a), and we recorded individuals with brood patch between August and April (n = 23; Fig. 4a) and in June (n = 1) while we recorded majority of moulting individuals from September to May (n = 62; Fig. 4a).
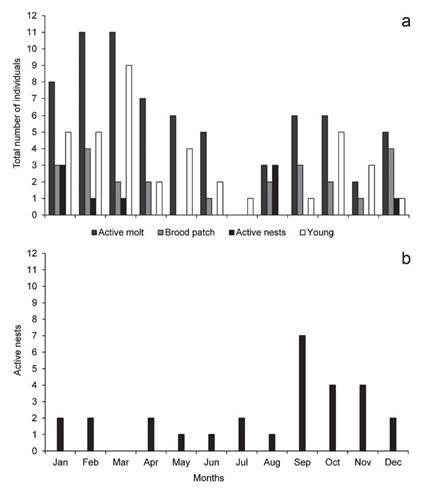
Figure 4 (a) Rusty-Margined Flycatcher Myiozetetes c. cayanensis trapped during the study period in a fragment of terra firme forest in southwest Amazonia in 1999-2002, 2004-06 and 2009-19, and active nests during 2012 to 2020. The bars show the numbers of ringed juveniles, molting, the presence of vascularized brood patches and active nests. Re-traps are not shown; (b) Monthly distribution of 28 active nests of Rusty-Margined Flycatcher Myiozetetes c. cayanensis in 11 Brazilian states between 2008 and 2019. Source: Wikiaves (www.wikiaves.com.br).
We obtained 28 citizen science records of active nests of Rusty-Margined Flycatcher (two of which contained nestlings) between 2008 and 2019 from 11 of the 26 Brazilian states, as well as the Federal District. Photographs of active nests were found in all months of the year except for March (Fig. 4b), although most records (53.5%; n = 15) were obtained between September and November (Fig. 4b). Most records (n = 13) were from Brazil’s North region, with eight from the Midwest and seven from the Southeast region.
We recorded morphological information of 93 M. cayanensis individuals trapped between 2002 and 2019. Measurements of individuals were: weight 27.7 ± 4.4 g (range 20-52.4, n = 93); wing 87.7 ± 4.9 mm (range 78-107, n = 93); tarsus 18.7 ± 2.9 mm (range 11.5-26.6 mm, n = 92); bill 14.6 ± 1.6 mm (range 10-17 mm, n = 56); head size 34.6 ± 2.5 mm (range 27.9-37 mm, n = 11); tail 70.8 ± 7.4 mm (range 45-98 mm, n = 93); total length 174.3 ± 8.1 mm (range 149-191 mm, n = 79) and cloacal temperature 40.9 ± 1.5 °C (range 39.6-42.9 ºC, n = 4).
We observed longest live time from 119 captures and ringed and posterior recaptures of 22 individuals between 1999 and 2019. Of the ringed individuals, 90.9% were recaptured more than once (n = 20). Longest minimum longevity was recorded for individual F16643 banded as an adult on 12 February 2014 and last re-trapped on 8 December 2016 (two years, nine months and 26 days, or 1,034 days after banding). Three other longestlived individuals were F16621, banded on 12 February 2014 and re-trapped on 21 June 2016 (two years, four months and 26 days, or 878 days after banding), F26529 banded on 26 March 2014 and re-trapped on 16 August 2016 (two years, four months and 21 days, or 873 days after banding) and F16621 banded on 12 February 2014 and re-trapped on 21 June 2016 (two years, four months and 9 days, or 861 days after banding). We re-trapped the individuals E61472, F26592, F36397, and F51497 all after one year (378-572 days) and the other 12 individuals at intervals of <1 year (2-154 days).
Discussion
Myiozetetes cayanensis is resident and common in the Zoobotanical Park and campus of the Federal University of Acre in Rio Branco (Guilherme 2001), where the specie is found typically at the forest edge and in open areas, most often near bodies of water. The eastern extreme of the Brazilian state of Acre and adjacent areas of the lowlands of southeastern Peru and northeastern Bolivia represent the limit of the distribution of M. cayanensis in southwestern Amazonia (Schulenberg et al. 2010, Guilherme 2016, Mobley & Kirwan 2020).
In fact, this is one of the few areas in the southwestern extreme of the Amazon basin where M. cayanensis occurs in sympatry with its congener, Social Flycatcher Myiozetetes similis (Guilherme 2016). This occurrence in sympatry should shape the reproductive behaviour of both species with respect to the construction and selection of the nest site, and we suggest a study to verify this hypothesis. The most detailed data on the reproductive patterns of M. cayanensis were obtained during studies in Panama (Skutch 1960, Ricklefs 1976, 1980, Dyrcz 2002), Guiana and Suriname (Haverschmidt 1961, 1971), and northern Brazil (Oniki & Willis 1983). In our study area, Rusty-Margined Flycatcher prefers to nest in open areas and uses plants located in sites with much human activity, like was also observed elsewhere in Amazonia (Pascoal et al. 2016, Oniki & Willis 1983) and northern Venezuela (Verea 2018). The species constructs its nest low above ground or water level (<1 m) and appears to prefer to nest near wasps colonies as reported in Panama (Dyrcz 2002). This undoubtedly reveals a defence mechanism against predators, because when nests were not built near wasps, they were built above water in our study area.
Nest shape was similar to those Myiozetetes c. hellmayri and M. c. cayanensis found in Panama (Dyrcz 2002) and Suriname (Haverschmidt 1971), respectively, and those found in Brazilian Amazonia (Oniki & Willis 1983). This nest type is typical of many Tyrannidae, such as species of the genus Pitangus and Camptostoma (Sick 1997). Use of anthropogenic nest material to construct nests is not uncommon in M. cayanensis. In a Cerrado-Amazonia transition, in north Tocantins, Pascoal et al. (2016) reported that M. cayanensis used synthetic wool and plastic in their nests (Wikiaves data: WA1853400). As it is a species highly adapted to urban environments, the availability of suitable plant material may sometimes be insufficient for nest construction, which leads to the birds using material discarded by humans (Borges & Marini 2010, Marini et al. 2012, Suárez-Rodriguez et al. 2017, Batisteli et al. 2020, Lima & Guilherme 2020).
Clutch size and egg characters are similar to descriptions from Suriname, Panama (Hellebrekers 1942, Skutch 1960, 1985), Guiana (Haverschmidt 1961), Peru (Dyrcz 1991) and Ecuador (Solano-Ugalde et al. 2007) and those described in Brazil (Carvalho 1960). We recorded an incubation time similar to that reported in Suriname (16 days) (Haverschmidt 1971) and Pará, Brazil (14 days) (Carvalho 1960) using the same method of this last author. Incubation time was also equal to that Social Flycatcher in Panama (Skutch 1960). Therefore, we can expect an incubation period estimated from the egg-laying to the hatching of the last egg between 14 and 16 days. Nestling morphology and mass at hatching were similar to the data reported in Peruvian Amazon (Dyrcz 1991), where the nestlings remained in the nest for 21 days. This author reported that chicks fledged with a mass of 25.5 g, similar to our findings. Regarding the growth rate of nestlings, ours are the first calculations for M. c. cayanensis. Our rate (0.18) was lower to the values calculated for four individuals of M. c. hellmayri and other flycatchers in Panama (0.37-0.41), but the growth asymptote was similar (Ricklefs 1976). Growth rate was also lower than that calculated for others Passeriformes at the same study site (Guilherme & Lima 2019, Lima & Guilherme 2020).
Contrary to observed in Panama, no nestlings infected with maggots in our study area were predated (Dyrcz 2002), but predation of Rusty-Margined Flycatcher nests has been observed in other localities. For example, in the Pantanal wetland 50% of the nests were predated, with a success rate of 25% (Pinho & Marini 2014) and in Suriname, a nest with eggs was predated by the Yellow-headed Caracara (Milvago chimachima) (Haverschmidt 1971). In our study we do not detected predation on nests, indicating that locally the species’ nests do not appear to be heavily predated. Certainly, the construction of nests in sites with difficult access, such as above water level or near wasp colonies, can contribute to the reproductive success of Rusty-margined Flycatcher.
The sum of the evidence from long-term studies and occasional records on reproduction in Rusty-Margined Flycatcher indicates that this species nests preferentially at the onset of the rainy season (Skutch 1960, Ricklefs 1980, Sick 1997, Dyrcz 1991, 2002, Pinho & Marini 2014, Pascoal et al. 2016), with active nests being observed only occasionally in other months of the year (Haverschmidt 1971, Oniki & Willis 1983). In our study area, brood patches and active M. cayanensis nests begin to appear in the late dry season, that is, September, continuing throughout the rainy season (August-April) (Duarte 2006). Photographic records obtained through citizen science show that this pattern is typical of the rest of Brazil. That is, that the species begins to reproduce in transition from the dry to the rainy season, extending on through the rainiest months of the year (Gan et al. 2004). At the beginning of the rainy season, Rusty-Margined Flycatcher benefits from an abundant supply of insects (Kishimoto-Yamada & Itioka 2015) to feed its young. The chicks ingest large amounts of beetle imagos and caterpillars (Dyrcz & Flinks 2003). In the western Amazon, as well as in tropical forests, insects of the orders Coleoptera and Lepidoptera may both occur in greater abundance during the transition between the dry and rainy seasons than at other times of the year (Frith & Frith 1985, Penny et al. 1978, Rodrigues 1992, Checa et al. 2009). Overlap molt was similar to data from Bran-coloured Flycatcher Myiophobus fasciatus and Euler’s Flycatcher Latrotriccus euleri in south Brazil (Repenning & Fontana 2011) and to that observed for Western Kingbirds Tyrannus verticalis in Oklahoma, USA (Jahn et al. 2017).
Variation in mean body mass for the species is consistent with one individual weighed in Amazonia Oriental (Silva et al. 1990). A comparison of the morphometric measurements (wing, tarsus and total length) between Social Flycatcher and Rusty-Margined Flycatcher in our study area did not reveal a significant difference capable of differentiating the two species by these parameters, however the average weight of both species was significantly different (Santos & Guilherme 2017). The size of the head, bill, tail length and cloacal temperatures data had not previously been reported for Rusty-Margined Flycatcher. Regarding the longevity data, ours are the first calculations for this species. Our minimum longevity records were lower with the those available for other flycatchers, e.g. Slate-headed Tody Todirostrum sylvia (six years old) and Olive-striped Flycatcher Mionectes olivaceus (nine years old) in Venezuela (Lentino et al. 2003), and Slate-capped Flycatcher Leptopogon superciliaris (seven years old) in Peruvian Amazon (Scholer et al. 2018). Flycatchers in north America had been reported a variation in minimum longevity between 5-11 years old (Kennard 1975, Klimkiewicz & Futcher 1989).












 uBio
uBio 


