Introduction
The Straight-billed Woodcreeper Dendroplex picus ranges from Panama to northern South America (Marantz et al. 2020). Thirteen subspecies of D. picus are recognized (Marantz et al. 2020). The eastern extreme of the Brazilian state of Acre and adjacent areas of the lowlands of southeastern Peru and northeastern Bolivia represent the limit of the distribution of D. p. peruvianus in southwestern Amazon (Schulenberg et al. 2010, Guilherme 2016, Marantz et al. 2020). The Straight-billed Woodcreeper is typically found, in the state of Acre, in upland forests, along edges and in open areas, including anthropic clearings with palms, and urban areas. Records of reproduction in the Woodcreeper come mostly from Majewska and Oteyza (2013) in Venezuela, supplemented with some details from French Guiana (Ingels & Giraud-Audine 2013) and northern Brazil (Oniki & Willis 1983). Here we provide new records of breeding and nestling growth, the first in a subspecies of the Straight-billed Woodcreeper from the southwestern Brazilian Amazon.
Material and methods
We studied the Woodcreeper in the Zoobotanical Park (ZP, 09°57’08.9”S, 67°52’22.5”W) of the Federal University of Acre, which includes a forest fragment (~100 ha) mostly surrounded by urban areas of the city of Rio Branco (Guilherme 2001, Souza et al. 2020). We measured (analogue callipers, 0.05 mm precision) and weighed (Pesola® scale, 1 g precision) eggs and nestlings every two days. The incubation period was the interval from the date the last egg was laid until the last egg hatched, based on nest 1. The nestling period began at the first hatching and ended with fledging of the last nestling (day 0, see Oniki & Willis 2001). We estimated growth following Ricklefs (1967) using the equation:
W(t) = A / (1+e[- K(t - ti)]
Where W(t) is the mass of the nestling at age t, A is the asymptote of the growth curve, K is the constant growth rate and ti is the inflection point of the growth curve. We run the equation in the R software version 3.5.1 (R Core Team 2018).
We captured woodcreepers from 1999-2019 (in 60940.3 net/hours) using 36 mm mesh nets (12 × 2.5 m) and banded all birds with numbered bands (CEMAVE, SNA no. 32465-4). All birds captured were measured only once at the time of first capture. During ringing, we distinguished adults and juveniles based on the presence of juvenile plumage (see Sibley 2010, Johnson et al. 2011). We examined each trapped individual to determine moult in the remiges and rectrices (Sibley 2010) and presence or absence of a brood patch (Redfern 2010), and we included only adults in the analysis. We calculated minimum longevity from the first day an individual was banded to their last recapture (Scholer et al. 2018).
Results
We found nests in open areas near the forest edge (December 2012, October 2013). Nest 1 was built in a broken trunk of a dead Bactris gasipaes palm (Fig. 1a). Nest 2 was built in the cavity of the trunk of a Samanea saman (Fabaceae) (Fig. 1b, c).

Figure 1 Active Dendroplex picus peruvianus nests in a forest fragment in southwestern Amazonia. (a) Detail of nest 1 (red arrow). (b) Nest 2 in trunk. (c) Detail of entrance of nest 2.
Nest openings were 1.1 and 1.46 m above the ground. The chamber of nests contained pieces of tree bark (Fig. 2a). Measurements of nest 1 and nest 2 were: entrance diameter of trunk was 14.5 and 12 cm and incubation chamber until entrance was 57 and 39, respectively. Both nests had a clutch size of two eggs. In one nest, eggs were laid daily. Eggs were predominantly white (Fig. 2a). Mean egg (n = 4) mass was 5.8 ± 0.5 g (5-6 g) and size 24.5 × 19.00 ± 0.06-0 mm (24-19 × 25-19 mm). We observed two adults on nest 1 during incubation period. The incubation period was 16 days.
We monitored the development of two nestlings only in nest 1 beginning two days after hatching. Nestlings hatch with light pink skin, yellow gape and slightly dark bill tip. Hatchlings had black plumes on the head, alar, humeral, and caudal tracts, and closed eyes (Fig. 2b). After four days, the eyes are slightly open, and the feathers of the remiges and rectrices start to develop (Fig. 2c). Nestling mass reached a mean 20-25 g after only 3-4 days, 38 g on day 8 (Fig. 2d) and 48-42 g on day 12, the heaviest recorded of any nestling (Fig. 2e, 3a). Chicks fledged after day 15 (Fig. 2f). We banded the chicks in the nest (codes H-91425 and H-91420). The longest nestling period was 15 days (Fig. 3a) and chicks fledged at a mean mass of 40.5 ± 2.1 g (39-42 g). The constant growth rate (K) of the nestlings was 0.31 ± 0.07 (0.26-0.42) with a growth asymptote of 46.3 ± 14.2 g (42.7-52.3 g).
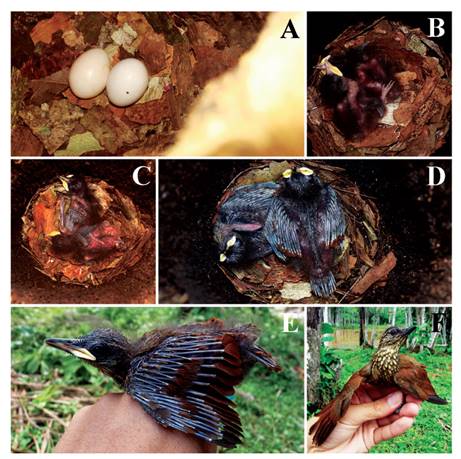
Figure 2 Development of Dendroplex picus peruvianus nestlings in a forest fragment in southwestern Amazonia in 2012 and 2013. (a) Detail of eggs and incubation chamber. (b) 2nd day of monitoring. (c) 4th day. (d) 8th day. (e) 10th day. (f) 15th day.
We captured birds during all months and most young birds were caught October to March, with one in June (74 adults, 11 juveniles, Fig. 3b). Birds had brood patches from September to March (n = 9; Fig. 3b) and birds were molting flight feathers during January to July (n = 33; Fig. 3b). Between 2002 and 2019 we banded 54 individuals: weight 42 ± 7.4 g (25-64.3; n = 55); wing 100.7 ± 11.1 mm (75-157, n = 55); tarsus 21.2 ± 3.4 mm (15-29 mm, n = 55); bill 28 ± 5.5 mm (18-40 mm, n = 36); head size 49.8 ± 1.6 mm (47.4-51.6 mm, n = 9); tail 86.3 ± 8.5 mm (65-109 mm, n = 51); total length 220.3 ± 9.4 mm (198-240 mm, n = 38) and cloacal temperature 41 ± 1.2 °C (39.4-42.6 °C, n = 6).
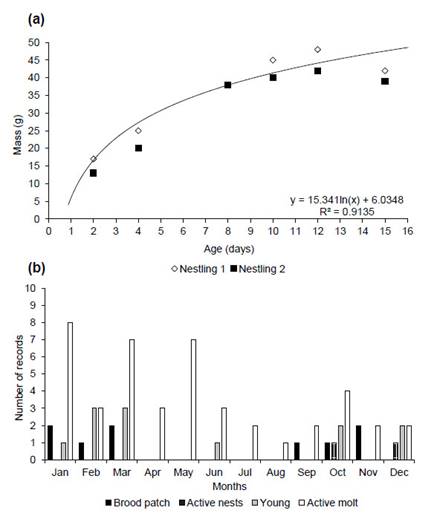
Figure 3 (a) Development and body mass of two nestlings monitored in a forest fragment in southwestern Amazonia in 2012. The logarithmic equation is based on the most developed chick. (b) Dendroplex picus peruvianus trapped in 1999-2002, 2004-2006 and 2009-2019, and active nests 2012 and 2013. Re-traps are not shown.
We made a total of 85 captures between 1999 and 2019, 18 of which involved re-traps, five of which (27.7%) more than once. We recorded a longest minimum longevity for individual G-34976 banded as an adult on January 2010 and last re-trapped on January 2018 (eight years after banding). The second longest-lived individual was G-91705 (four years). Two other longest-lived individuals were G-105899 (three years), G-39925 (three years). We re-trapped the individuals G-14455, G-91756, G-91872 all after two years and G91900, G-14489, G-35000, G-105891 after one year. The other seven individuals at intervals of <1 year.
Discussion
In ZP, D. picus breed in open areas with palms plantations with human activity, like other woodcreepers in Costa Rica (Skutch 1996) and southeastern Brazil (Oniki and Willis 2001). Contrary to south eastern Brazil (Santos et al. 2019) where D. picus is more sensible, the forested surrounding the nesting site in the ZP offers a secure environment for the species survival. In this study, all nesting characteristics were quite similar to those described for a different subspecies in Suriname and Venezuela (Hellebrekers 1942, Majewska & Oteyza 2013). Regarding the growth rate of nestlings, ours are only the second calculations for D. picus. Nestling growth rate in this study was also similar to the only other estimates for the species, in Venezuela (D. p. picus, Majewska & Oteyza 2013). Growth rate was also lower than those calculated for others Passeriformes at the same study site (Guilherme & Lima 2019, Lima & Guilherme 2020).
Dendroplex picus tends to breed preferentially at the onset of, and during, the rainy season (Haverschmidt & Mees 1994, Oniki & Willis 2001, Sanaiotti & Cintra 2001, Majewska & Oteyza 2013, Ingels & Giraud-Audine 2013). In ZP, its nests in transition from the dry to the rainy season (September), extending on through the rainiest months of the year, continuing throughout the rainy season (until March) in Acre (Duarte 2006). At the beginning of the rainy season, D. picus benefits from an abundant supply of insects (Rodrigues 1992) to feed its young, mainly Coleoptera and Lepidoptera larvas like reported to Dendrocincla turdina and Dendrocolaptes platyrostris in Argentina (Cockle & Bodrati 2009, Bodrati et al. 2018). In the western Amazon, insects may occur in greater abundance during the transition between the dry and rainy seasons than at other times of the year, as well as in tropical forests (Penny et al. 1978, Frith & Frith 1985). Overlap moulting and breeding was observed for Glyphorhynchus spirurus in Amazonian Ecuador (Darrah & Smith 2017) and for others dendrocolaptids in central Amazonia (Johnson et al. 2012) mainly in the dry season.
Overall mean body mass for the species is consistent with other studies (Oniki 1978, Silva et al. 1990, Marantz et al. 2020). Wing, bill, tail, and tarsus were all similar to those reported in southwestern Peruvian, Brazilian and Bolivian Amazonia (Hellmayr 1910, Zimmer 1934, Pinto & Camargo 1954). The size of the head, cloacal temperatures and longevity data had not previously been reported for D. picus. Regarding the cloacal temperature data, ours are similar to seven woodcreepers species in northern Brazil (Oniki 1974). The variation in our minimum longevity records is consistent with those available for Dendrocincla fuliginosa and Sittasomus griseicapillus (7-10.5 years old) in Trinidad and Venezuela (Snow & Lill 1974, Lentino et al. 2003).












 uBio
uBio 


