Introduction
The order Scorpiones currently has 2645 species described, grouped into 163 genera and 18 families (Rein 2021). Despite the small number of described species, when compared to spiders, scorpions have a wide geographical distribution, being found on all continents, except for Antarctica (Brownell & Polis 2001, Lourenço 2018). The greatest diversity of scorpions is concentrated in tropical and subtropical regions of the globe, inhabiting different environments, such as deserts, savannas, and forests (Lourenço 2002, Lourenço & Eickstedt 2009, Porto et al. 2010).
According to data from the Notifiable Diseases Information System (SINAN) platform, cases of scorpionism in Brazil reached more than 33000 from 2015 to 2020 (SINAN, 2021). The highest incidence of scorpion stings occurred in the South and North regions, and the highest mortality and morbidity rates were concentrated in the Southeast and in the State of Amazonas, associated with delayed health care. However, the North, Northeast and the Southeast coast had less access to antivenom (Wen et al. 2020). Accidents caused by venomous animals represent a serious public health problem in Brazil, and among the cases notified by SINAN, those caused by scorpions are the most frequent in the state of Ceará (67.2%) (Braga et al. 2020).
Different scorpion species can coexist in the same geographic area, but the toxic potential of scorpion venoms is not uniform, with almost all the most dangerous species belonging to the Buthidae family, including the dreaded genera Androctonus and Buthus (north Africa), Parabuthus (South Africa), Mesobuthus (India), Leiurus (Near and Middle East), Centruroides (North and Central America) and Tityus (South America) (Abroug et al. 2020). Despite the great biodiversity of scorpions in the tropics, ecological studies on these arachnids are still scarce, even in the face of the evident environmental changes that are transforming the Neotropical regions. In Brazil, studies involving the ecology of arachnids are more frequent in the Amazon and Atlantic Forest biomes, both of which interestingly have similar characteristics: closed vegetation with high humidity and rainfall, aspects that are different from other Brazilian biomes such as the Cerrado and Caatinga (Lira et al. 2019).
The Brazilian scorpion fauna is underestimated by factors like lack of specialists and research incentives, in addition to the existence of sampling gaps in several areas (Porto & Brazil 2010). In the Northeast region of Brazil, the numbers related to the scorpion fauna are still inaccurate, considering that the vast majority of studies are focused on public health. There is an association between scorpion species and xeric environments, suggesting that scorpion assemblages do not vary much in conserved fragments from different phytophysiognomies (Carmo et al. 2013). The gradual increase in scorpionism incidence rates may be a result of the expansion of scorpions’ habitats (Bucaretchi et al. 2014).
The State of Ceará is mostly covered by the Caatinga biome, whose scorpion fauna contains at least 33 described species (Porto et al. 2014, Esposito et al. 2017, Lira et al. 2019). However, it is estimated that about 41% of the Caatinga has never been researched, and 80% has been poorly sampled (Tabarelli & Vicente 2004). If we exclude in these data studies with scorpions, the unstudied area of Caatinga rises to 70% (Porto et al. 2014). Insufficient knowledge about the geographic distribution of scorpions in regions of the Caatinga justified this study with the aim of updating the list of scorpion species causing accidents reported in the state of Ceará.
Material and methods
Characterization of the study area. - Ceará has an estimated population for 2019 of 9 132 078 inhabitants, occupying the eighth position among the most populous Brazilian states (IBGE 2019). Located in the north of the Northeast Region of Brazil (5°12′0″ S, 39°18′0″ W), and is limited by the Atlantic Ocean (N and NE), the states of Rio Grande do Norte and Paraíba (E), Pernambuco (S) and Piauí (W). Its total area is 148 894 757 km², corresponding to 9.37% of the total area of the Northeast Region (IBGE 2019). The state has 184 municipalities, with about 75% of the population occupying urban areas (IBGE 2019). The predominant climate, in 98 of the 184 municipalities is warm tropical semi-arid. Surrounded by relatively high relief formations, such as plateaus and cuestas, Ceará is bounded by the Serra da Ibiapaba (W), the Chapada do Apodi (E), the Chapada do Araripe (S) and the Atlantic Ocean (N). The dominant vegetation is the Caatinga (about 46% of the territory), however, there are also present tropical forest, savanna, and coastal vegetation (Borges-Nojosa et al. 2019).
Capture and identification. - All scorpions in this study were collected through the health surveillance service, developed by the Scorpion Control Program (PDCE) in 165 municipalities (89%) of the State of Ceará, after accident notification. All specimens were captured/collected by municipalities’ endemic agents, following the guidelines of the Scorpion Control Manual of the Ministry of Health (Brasil 2009). The collections were carried out from January 2018 to December 2019, in urban, peri-urban, and rural areas, through spontaneous donations (animal captured by the owner of the residence), accidents due to scorpion stings or active searches by health teams. Captured animals were placed in a container containing 70% alcohol and identified by Relrison Dias Ramalho and Denise Maria Candido (Butantan Institute), as described by Vachon (1974). All specimens were deposited in the scientific collection of the Dr. Thomaz Corrêa Aragão Entomology Laboratory of the Vector Control Center of the Ceará State Health Department, in Fortaleza municipality, under the curatorship of biologist Relrison Dias Ramalho.
Distribution analysis. - In our survey we used some ecological indices, such as: relative abundance (RA%), determined as the ratio between the number of individuals of each species divided by the total number of specimens (n = 999). Sampling resulted from cases of envenomation that were notified to health agents, and whose responsible specimen was captured at the time or was the result of an active search at notification sites. Scientific literature and Global Biodiversity Information Facility (2020) data were used only to be compared with the species found. Distribution maps were created using QGIS 2.18.18 with the GRASS 7.4.0 program.
Results
During the study, 999 scorpions were captured and distributed in eleven species, ten from the family Buthidae and one from the family Bothriuridae (Tab. 1). Scorpions of the genus Tityus C. L. Koch, 1836 represented 44.1% of the total animals collected in the study, followed by those of the genus JaguajirEsposito, Yamaguti, Souza, Pinto da Rocha & Prendini, 2017 (RA=37.4%). The most abundant species was Tityus stigmurus (Thorell, 1876) (RA=40.1%), followed by Jaguajir rochae (Borelli, 1910) (RA=37.2%), Bothriurus asper Pocock, 1893 (RA=8.3%) and Bothriurus rochai Mello-Leitão, 1932 (RA=6.7%). Species of scorpions were distributed across all biomes in Ceará, with the greatest diversity found in the Caatinga (Tab. 1).
Table 1 List of scorpion species collected/received by municipal agents of the Health Surveillance Service of the state of Ceará and in others federative units of Brazil, and the biomes they inhabit. AF = Atlantic forest, CA = caatinga, CE = Cerrado. Alagoas (AL); Bahia (BA); Ceará (CE); Distrito Federal (DF); Espírito Santo (ES); Goiás (GO); Maranhão (MA); Mato Grosso (MT); Mato Grosso do Sul (MS); Minas Gerais (MG); Pará (PA); Paraíba (PB); Pernambuco (PE); Piauí (PI); Rio de Janeiro (RJ); Rio Grande do Norte (RN); São Paulo (SP); Sergipe (SE); Tocantins (TO).
| Taxonomic group | Federative Unit of Brazil | Habitat | Reference |
|---|---|---|---|
| BUTHIDAE | |||
| Tityus stigmurus | AL, BA, CE, Fernando de Noronha, MG, PB, PE, PI, RN, SE, SP | CA, CE, AF | Bertani et al. (2018); Brazil & Porto (2010); Freitas & Vasconcelos (2008); Furtado et al. (2020). |
| Tityus serrulatus | BA, CE, DF, ES, GO, MG, MS, PR, PE, RJ, RN, SC, SE, SP, TO | CA, CE, AF | Brazil & Porto (2010); De Souza et al. (2009); Lira et al. (2019b); Lira et al. (2013). |
| Tityus martinpaechi | BA, CE, PB | CE, AF | Brazil & Porto (2010); Porto et al. (2014); Foerster et al. (2019). |
| Tityus confluens | CE*, GO, MS, MT, PA, PI, PR, SP, TO | CE, AF | Bertani et al. (2005); Carvalho et al. (2017); Porto et al. (2014); Lourenço & Aparecida-da-Silva (2006); Lourenço & Aparecida-da-Silva (2007). |
| Tityus maranhensis | CE*, MA, PI, TO | CE, AF | Lourenço, Jesus-Júnior & Limeira-de-Oliveira (2006); Porto et al. (2014); Kury et al. (2016). |
| Jaguajir rochae | AL, BA, CE, MG, PB, PE, PI, RN, SE, SP | CA, CE | Benício et al. (2019); Bertani et al. (2018); Esposito et al. (2017); Lira et al. (2018). |
| Jaguajir agamemnon | BA, CE, GO, MA, MG, MT, PE, PI, SE, TO | CA, CE | Benício et al. (2019); Esposito et al. (2017); Lira et al. (2020); Furtado (2015). |
| Physoctonus debilis | BA, CE, PA, PE, PI, RN | CA, CE | Brazil & Porto (2010); Porto et al. (2014); Esposito et al. (2017); Lourenço (2007b); Lourenço (2017). |
| Ananteris franckei | BA, CE, PE, PB | CA, AF | Foerster et al. (2019); Giupponi et al. (2009); Azevedo et al. (2016); Porto et al. (2010). |
| BOTHRIURIDAE | |||
| Bothriurus asper | AL, BA, CE, DF, MA, PB, PE, PI, RN, SE | CA, CE, AF | Brazil & Porto (2010); Porto et al. (2014); Lira et al. (2018); Lira et al. (2020); Mota (2006); Porto et al. (2010); De Souza et al. (2020). |
| Bothriurus rochai | AL, BA, CE, MA, PB, PE, PI, RN, SE | CA, CE, AF | Brazil & Porto (2010); Porto et al. (2014); Lira et al. (2020); Porto et al. (2010); De Souza et al. (2020). |
* First record for the state of Ceará, Brazil.
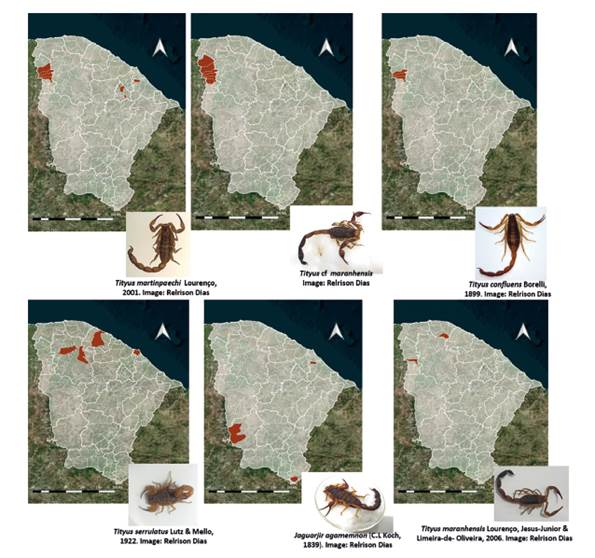
Tityus stigmurus, J. rochae and B. rochai, were the most widespread scorpion species (Fig. 1), while Jaguajir agamemnon (C.L. Koch, 1839), Tityus maranhensisLourenço, Jesus-Júnior & Limeira-de-Oliveira, 2006 and Tityus cf. maranhensis were limited to a few municipalities in the state of Ceará (Fig. 2).
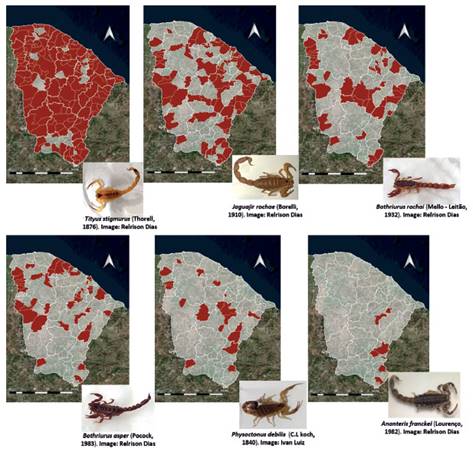
Figure 1 Distribution of the most common scorpion species in the municipalities of the state of Ceará, Brazil.
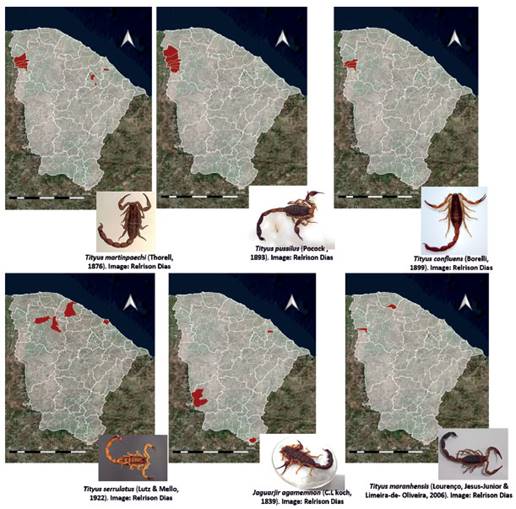
Figure 2 Distribution of scorpion species with the lowest occurrence in the municipalities of the state of Ceará, Brazil.
For the first time, this study registered for the state of Ceará the species Tityus confluens Borelli 1899 in the municipalities of Ubiapina, São Benedito and Ubajara, and Tityus maranhensis in the municipalities of São Benedito, Guaramiranga and Morrinhos (Chapada da Ibiapaba, Sobral macro-region) (Fig. 2). Our study extends the distribution of these species to the state of Ceará, which today had no records.
This study registered a total of 12 species of scorpions for the state of Ceará (T. stigmurus, Tityus martinpeachi Thorell, 1876, Tityus confluens, Tityus serrulatus Lutz & Melo, 1822; T. maranhensis, Jaguajir Agamemnon, J. rochai, Physoctonus debilis (C.L.Koch, 1840, Anantheris franckei Lourenço, 1982, Bothriurus asper and Bothriurus rochai) however, one of them shows characteristics of both T. maranhensis and T. pusillus, and is identified as T. cf. maranhensis. Although other scorpion species have already been described for Ceará (Esposito et al. 2017), for example, the buthid Troglorhopalurus lacrau (Lourenço & Pinto-da-Rocha 1997) and the chactids Hadrurochactas brejo (Lourenço 1988) and Hadrurochactas araripe (Lourenço 2010) (endemic to the Caatinga biome) (Azevedo et al. 2016), during the period of our study, no specimens were found and no accidents involving these species were reported.
Discussion
Scorpions from Ceará. - The number of species found in this study corroborates data from the survey by Porto et al. (2014), which shows that the richness of the Caatinga scorpion fauna is similar in the states of Ceará, Piauí, Pernambuco and Paraíba, ranging from 7 to 10 species. Among the species of scorpions of the family Buthidae, the most abundant in Ceará were T. stigmurus and J. rochae, species also widely distributed in other northeastern states (Carmo et al. 2013, Lira et al. 2019, Porto et al. 2010, Lira & Albuquerque 2014, Araújo et al. 2010). Although most areas of the Caatinga biome do not have records of any species of scorpion, about a third of the Caatinga species can be found in the state of Ceará (Porto et al. 2014, Azevedo et al. 2016).
Tityus stigmurus, commonly known as northeastern scorpion, was the most widespread and abundant species of medical importance in Ceará, probably due to its adaptation in an environment with significant human population density. This scorpion mainly inhabits urban environments, but it also can be found in natural ones (Furtado et al. 2020, De Souza et al. 2009). This is a generalist species that inhabits regions of the Atlantic Forest, Caatinga and Cerrado (Bertani et al. 2018, Lira et al. 2019, Furtado et al. 2020). In natural environments, it is found inhabiting regions of modeled crystalline and hinterland relief covered by the phytoecological unit called “Caatinga do cristalino”, vegetation found in about 70% of Ceará (Moro et al. 2015, Fernandes & Queiroz 2018).
According to Lira et al. (2019a), J. rochae, J. agamemnon and P. debilis are classified as specialists in open-forests, inhabiting regions of Caatinga and Cerrado, but which may show a reduction in their distribution, considering future climate change scenarios. This means that climate changes, especially those related to the increase in temperature, would expand arid and semi-arid areas such as those in the Caatinga biome, possibly changing the distribution pattern of species, especially those with a high degree of specialization in the habitat (Huang et al. 2016, Lira et al. 2019, Foord et al. 2015). In a study by Lira et al. (2019), in the state of Pernambuco, it was observed that the most common scorpion species in the Caatinga were B. rochai, J. rochae, T. stigmurus and P. debillis, consistent with our results for several of the species found in the state of Ceará.
Typical of the Caatinga and Cerrado formations, Physoctonus debillis is found exclusively in some locations of the Northeast region of Brazil and has long been the subject of taxonomic confusion (Esposito et al. 2017, Lourenço 2007, Lorenço 2017). The rare genus Physoctonus Mello-Leitão, 1934 has three described species, P. debilis and P. striatus, inhabiting the northeastern Caatinga region, while P. amazonicus is found in transitional areas of savannas and forests in the southeastern Amazon (Lourenço 2017), supporting the hypothesis that an earlier link between the Caatinga and the Amazon savanna and Guyana enclaves could have influenced the speciation process during the rainy and dry periods (Lourenço 2001).
Species such as Tityus martinpaechiLourenço, 2001, T. serrulatus Lutz & Mello, 1922, T. maranhensis and T. confluens were found in a more restricted region of Ceará, the Chapada da Ibiapada, inhabiting municipalities with Caatinga vegetation, humid and dry forest (Moro et al. 2015). Chapada da Ibiapada is one of the last remnants of the Atlantic Forest in Ceará, formed by humid mountains, and low surfaces covered by Caatinga which has periods of prolonged drought (De Moura-Fé 2017, Bétard et al. 2007). Ananteris franckei Lourenço, 1982 was described based on specimens from Exu (Pernambuco) and latter recorded by Lourenço et al. (2013) in the Crato region, in the Ceará, inhabiting the leaf litter layers of dry forest (Lira et al. 2018).
Tityus martinpaechi is morphologically similar to T. stigmurus, occurring in the Caatinga and Atlantic Forest of the states of Bahia, Ceará and Paraíba (Souza et al. 2009, Porto et al. 2010). Its morphological similarity with T. stigmurus may have influenced the lack of taxonomic records and accidents in the state of Bahia (Porto et al. 2010). Tityus confluens and T. maranhensis were registered for the first time in the Caatinga by Porto et al. (2014). T. confluens is the taxon that has generated a lot of systematic and biogeographic discussion, but our findings extend the distribution of T. confluens to Caatinga vegetation, reinforcing the hypothesis of a Chaco-Cerrado-Caatinga corridor also called the ‘open forest diagonal’ (Werneck et al. 2012) and supports the theory of the existence of distribution corridors proposed by Lourenço (2015) for South American scorpions.
The specimens collected in the municipalities of Ibiapina, São Benedito, Tianguá, Ubajara, Viçosa do Ceará and Guaramiranga, showed similar characteristics to T. maranhensis and T. pusillus. For this reason, we consider the identification as T. cf. maranhensis. The review of the subgenus Archaeotityus, performed by Moreno-González et al. (2019), showed a disjunct distribution of T. pusillus (Alagoas, Bahia, Paraiba, Pernambuco, Rio Grande do Norte and Sergipe) and T. maranhensis (Maranhão). It is possible that all specimens we found are actually T. maranhensis, however other phylogeographic studies will be needed for corroboration. The Archaeotityus subgenus, the oldest species group within the Tityus genus, seems to share with scorpions of this genus homologous toxins active in voltage-dependent sodium channels (Borges et al. 2012). Thus, although T. (Archaeotityus) maranhensis still has a restricted distribution in Ceará, its expansion could result in its inclusion in the list of species of medical importance in Brazil.
Among the species of the family Bothriuridae, only B. asper and B. rochai was registered in Ceará. According to Araújo et al. (2010), the reproductive activities of these two species are related to the increase in food resources during the rainy season of the Caatinga. The reproductive period of scorpions reaches its maximum when there is an abundance of food, associated with the rain and temperature regime (Araújo et al. 2010). The seasonality that occurs in the Caatinga seems to be the predominant factor influencing the behavior of these species in search of sexual partners and food, considering that B. rochai is classified as a specialist in open forest, whereas B. asper is a generalist species (Lira et al. 2019b, De Souza et al. 2020). Thus, excluding the species that presented taxonomic, systematic and/or biogeographic inconsistency, we consider that the state of Ceará has 11 species of scorpions.
Ecological Comments
Factors such as disordered urbanization and population growth tend to increase the possibility of human-scorpion contact. However, climatic factors and habitat type are the most important drivers of the distribution of these animals (Rafinejad et al. 2020). Urbanized habitats can produce different dynamic patterns in a population when compared to natural habitats (Mansouri et al. 2021). This is because the urban environment provides greater availability of food and water, in addition to the absence of natural predators, which facilitates adaptation and allows scorpions to reproduce more than once a year (Szilagyi-Zecchin et al. 2012).
Among the environmental factors that can influence the diversity and abundance of scorpions are temperature, precipitation, topography, soil, hydrology, and food availability (Ouici et al. 2020). Most arthropods are highly sensitive to changes in temperature and precipitation and are good indicators of bioclimatic and environmental changes (Lira et al. 2019a). This association between environmental variations and abiotic characteristics promotes considerable changes in habits and microenvironments preferred by arachnids (Carvalho & Oliveira 2016). These venomous arthropods have greater diversity and richness in tropical and subtropical regions of the world.
Most of Ceará has semiarid climate and is being considered as one of the driest states in Brazil, with high average air temperatures during most of the year, and concentrated precipitation in the first semester (Zanella 2005). The coastal region and the mountains of Ceará receive a much greater amount of rain than the hinterland relief depression, presenting different landscape features from those found in the dry interior of the state (Moro et al. 2015). The distribution of scorpions due to the diversity of environments that characterize the contrasting landscapes of Ceará may be influenced by the altitude and maritime nature resulting in the presence of humid and sub-humid areas that move from the coast towards the interior of the state (Silva & Cavalcante 2004).
Characterized as the largest tropical dry forest region in South America (Da Silva et al 2017), the Caatinga is a biome restricted to the Brazilian territory and composes a mosaic of thorny shrubs and seasonal dry tropical forests that form an open forest vegetation (Lira & Albuquerque 2018, Pennington et al. 2009). The highest occurrence of scorpionism is strongly linked to factors of social vulnerability related to low purchasing power, low education and lack of infrastructure (Lira & De Souza 2014, De Almeida et al. 2020). The human population that lives in the Caatinga works mainly in agricultural activities without personal protection equipment, which could also explain the medical relevance of scorpionism in Northeastern Brazil (Carmo et al. 2013).
A study by Foerster et al. (2020) showed that low dispersion animals such as scorpions, exhibit patterns of richness and abundance of species depending on the level of habitat complexity, suggesting that the vegetation structure would be determinant for maintenance of scorpion assemblages in the landscapes of neglected environments such as within the Caatinga. Carmo et al. (2013) showed an association between species of scorpions and xeric environments, suggesting that the assemblages of scorpions do not vary much in conserved fragments of different phytophysiognomies. Landscape characteristics are an important factor in maintaining scorpion assemblages in Caatinga environments. Thus, habitat degradation can negatively affect species distribution. Changes in landscape configuration influence scorpion composition and abundance more than species richness, and although the Caatinga scorpion assemblage is more resistant to landscape changes, it is affected by the presence of cultivated areas (Lira et al. 2021a, Lira et al. 2021b). This indicates that there is greater species turnover in anthropically disturbed environments (Lira et al. 2019c).
Those who think that Ceará is totally covered by the Caatinga are mistaken, considering the diversity of landscapes and vegetation that occur there (Moro et al. 2015). The Cerrado biome (Brazilian savanna) is another area that, associated with the Atlantic Forest (closed forest) and the Caatinga, makes up the ecosystems inhabited by scorpions in Ceará (Lira et al. 2019a); also, the Cerrado is the biome with the greatest chance of fire occurrence in this state (Moro et al 2015). Furthermore, the unsustainable exploitation of native wood, the reduction of original areas by the emergence of urban areas and the expansion of pastures and plantations are the result of anthropogenic pressure that can modify the distribution profile of scorpions (Ribeiro-Neto et al. 2016).
The ecological characteristics of semiarid environments influence the distribution of scorpion species in Ceará, and consequently a greater chance of human-scorpion encounters, resulting in greater chances of risk of accidents. Thus, our results may stimulate the production of studies aimed at scorpions from the Caatinga and other northeastern biomes that are not very well sampled, bringing new information on the biology of the species, toxic effects of venoms and the behaviour of these animals in response to environmental and anthropogenic changes.
Errata
An error in Figure 2 of the work: Braga JRM, Ramalho RD, Sousa JCC de, Almeida IL de. 2022. Scorpions from Ceará State, Brazil: Distribution and ecological comments. Revista Peruana de Biología. 29(1):e21205-e21205. https://doi.org/10.15381/rpb.v29i1.21205.
Due to an editorial mistake, the latest authors version of figure 2 was not considered. The correction refers to the image of Tityus serrulatus Lutz & Mello, 1922. Image: Relrison Dias.
The article files that indicate in the dates of the editorial process: “Correction [Fig 2]: 05/10/2022”, already include the change.












 uBio
uBio