Introduction
Due to its natural conditions the soil house a diversity of microorganisms, which differ from each other through their functional characteristics, such as, the ability to provide plant growth through the production of hormones, and plant growth regulators (Oliveira et al. 2012), biological control of diseases, and pests (Silva et al. 2015, Simi et al. 2018), and phosphate solubilization (Vera et al. 2002). Thus, with the intermediation of these characters, fungi can promote plant growth. Their role in increasing plant growth and yield, disease resistance, biotic and abiotic tolerance provides an environmentally friendly solution to reduce the use of hazardous pesticides and industrial fertilizers (Diagne et al. 2020).
Given environmental adversities, and climate change, studies have been developed to prospect the existence of microorganisms with agricultural applicability. Nascimento et al. (2018) describe those extreme environments such as highly halophilic soils, and with lack of water availability for housed filamentous fungi in association with cactus.
Phosphorus is a limiting element in agriculture, which has generated a great deal of discussion regarding the use of microorganisms that solubilize this element. In this context microorganisms have already been described as phosphate solubilizers in previous studies (Silva et al. 2015, Silva et al. 2018). This element, in addition to acting directly in the mineral nutrition of cultivated plants, participates in their metabolic processes, being, therefore, a fundamental element in plant development.
Phytohormones or plant hormones are understood as chemical substances that in low concentrations promote the growth of plants, influencing their growth, development, and cell differentiation of tissues (Spaepen et al. 2009). They are signaling molecules that regulate many developmental processes in plants (Fitze et al. 2005), are organic compounds, which are naturally produced in some part of the plant, and transported along the plant, which ends up in specific physiological responses. Because their ability to stimulate or inhibit plant growth, these are also called plant growth regulators. Five main groups of phytohormones are recognized: auxins, gibberellins, ethylene, cytokinins, and abscisic acid (Saharan & Nehra 2011). These phytohormones or phytoregulators are also fundamental in the colonization processes of the root system towards symbiotic processes by microorganisms (Silva et al. 2021).
The suppression of diseases by beneficial microorganisms from the rhizosphere can occur through several mechanisms, such as: antagonism related to the production of antifungal antibiotics, competition for space and nutrients with phytopathogens, and other microorganisms harmful to the rhizosphere, and induction of resistance in plants (Moreira & Araújo 2013). Taking into account the biocontrol, there are several mechanisms used by fungi, among which the production of metabolites, and enzymes with antifungal properties, hyperparasitism, and competition for nutrients stand out (Verma et al. 2007). These characters give these organisms a prominent place in research related to the biocontrol of plant diseases caused by fungi.
Given the above, this study aimed to evaluate the ability of rhizospheric fungi isolated from Opuntia cochenillifera to promote growth in plants through phosphate solubilization, production of 3-indole acetic acid, growth in water restriction, and antagonism against phytopathogens.
Material and Methods
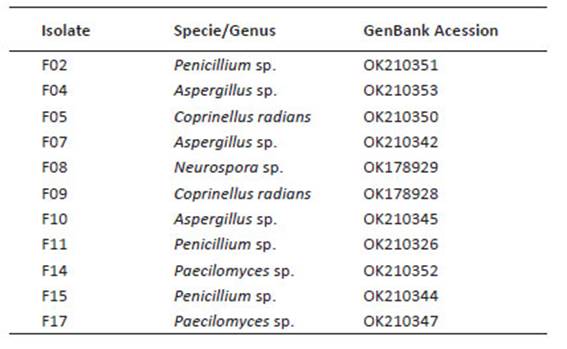
Investigation place, and fungal isolates. The tests were carried out at the Agricultural Microbiology Laboratory of the Agricultural Sciences Center of the Federal University of Alagoas, Molecular Phytopathology Laboratory (CECA-UFAL), and in the Soil Laboratory of the Federal Institute of Education of Alagoas. The rhizospheric fungi were isolated from expontaneous Opuntia cochenillifera from municipality of Ouro Branco, State of Alagoas, Brazil (W: 37°24'45"S, 9°4'47.3"), in sun of 2018, collect 10 g of soil to 15 cm deep. The dilution was carried out to fraction 10-3, 10-4 and 10-5 and inoculated in Petri dishes containing selective culture medium (Tortora et al. 2016). The rhizospheric fungal isolates were identified by sequencing the ITS region of rDNA and are properly deposited and preserved in the Agricultural Microbiology Laboratory of CECA-UFAL and are deposited in GenBank (Table 1). The plant pathogens Colletotrichum sp. and Fusarium sp. are properly deposited and preserved in the Molecular Phytopathology Laboratory of CECA-UFAL.
Table 1 Identification and accession number of rhizospheric fungi associated to cacti Opuntia cochenillifera.
Phosphate solubilization assay. The fungal isolates were previously grown in Potato Dextrose Agar (PDA) medium in Petri dishes for five days. Subsequently a mycelium disk of approximately 1 cm was removed from the plate and inoculated in Erlenmeyers flasks containing 100 mL of NBRIP culture medium (Nautiyal 1999), and incubated for 15 days in an orbital shaker under continuous rotation of 120 rpm at room temperature. Every five days, 1 mL of supernatant from the culture medium was removed from each Erlenmeyer, which was placed in microtubes and stored (≈6 °C) for further analysis. At the same time, the pH of the samples was measured at the same collection intervals (5, 10, and 15 days).
After the collection, 1000 µL of each sample was centrifuged at 10000 rpm for five minutes. Then 145 µL of the sample supernatant was transferred to new microtubes, adding 570 µL of distilled water and 285 µL of ammonium vanadate-molybdate (5% molybdate, 0.25 ammonium vanadate (v/v)). The samples were reserved for 10 minutes, and the absorbance was read under optical density at 420 nm in a spectrophotometer (model SP-2000, Biospectro) (Malavolta et al. 1997, Silva 1999). The concentration of P in µg.mL-1 was determined using the equation y = (0.3041X2 + 0.2566X + 0.0213) x100, where X corresponds to the obtained absorbance values, and is interpreted values: low solubilization (< 50 µg.mL-1), medium solubilization (50 - 100 µg.mL-1), high solubilization (101 - 500 µg.mL-1), and high solubilization (>501 µg.mL-1).
The experimental design was in completely randomized blocks with four replications in a split-plot scheme in time, where each treatment corresponded to a fungal isolate. The control treatment corresponded to Erlenmeyer containing only the culture medium without fungus. The data obtained were subjected to analysis of variance (F) using the Sisvar software (Ferreira 2014), and the means were compared using the Tukey test (p ≤ 0.05), and regression analysis (R²).
Quantitative production of 3-indole acetic acid (IAA). The rhizospheric fungal strains were previously grown in PDA culture medium for five days. Then 5 mm diameter discs containing hyphae, and spores were transferred to Erlenmeyers with liquid DYGS culture medium (glucose 2.0 g, peptone 1.5 g, yeast extract 2.0 g, KH2PO4 7H2O 0.5 g and MgSO4 7H2O 0.5 g) (Rodriguez Neto et al. 1986) supplemented with 100 μg.mL-1 of L-tryptophan.
Cultures were kept in constant orbital agitation (100 rpm) at 26 °C for five days. IAA production was evaluated using the colorimetric method described by Gordon and Weber (1951). A 1 mL aliquot of each supernatant was transferred to test tubes, to which 2 mL of Salkowski reagent was added. The tubes were kept in the dark for 30 min (Hartmann et al. 1983). The pink color was indicative of the presence of phytohormone being quantified by reading in a spectrophotometer (model SP-2000, Biospectro) with optical density of 530 nm of absorbance. The IAA concentration in the culture medium (y) was determined by comparison with a standard curve, using commercial IAA, through the equation y = 34.507X2+43.802X+0.843, where X correspond to the obtained absorbance values. Isolates were classified according to Hartmann et al. (1983) who establish the following parameters for IAA production: low production (<1μg.mL-1); medium production (1-10μg.mL-1); high production (11-50μg.mL-1), and superior production (>51μg.mL-1).
The experimental design was completely randomized with five replications. The data obtained were submitted to analysis of variance (ANOVA) using the Rstudio software (2020) based on the R language (R Team Core, 2013), and submitted to the Tukey test (p ≤ 0, 05).
In vitro antagonism against Fusarium sp. and Colletotrichum sp. For the antagonism test against Fusarium sp., and Colletotrichum sp., the rhizospheric fungal isolates, and the target phytopathogens were previously grown in PDA culture medium for five days at room temperature to obtain young cultures. The paired culture methodology was adopted, which consists of the deposition of the phytopathogen and the antagonist on the same Petri dish at equidistant opposite poles, providing a direct confrontation between the rhizospheric fungi, and the phytopathogen.
The control treatment consisted of inoculating only the phytopathogen in one pole of the plate. The plates were incubated for seven days at room temperature. After this period the evaluation was carried out, which consisted of measuring the radial mycelial growth of the pathogen (cm). The data were applied in the formula: IMG% = [(⊘plate x CMP)/⊘plate]x100 (Silva et al. 2019) where: IMG% - Percentage inhibition of mycelial growth; ⊘ plate - diameter of the plate used in the experiment; CMP - pathogen mycelial growth.
The experimental design was completely randomized with five replications. The data obtained were submitted to analysis of variance (ANOVA) using the RStudio software (2020) based on the R language (R Team Core 2013) and submitted to the Tukey test (p ≤ 0.05).
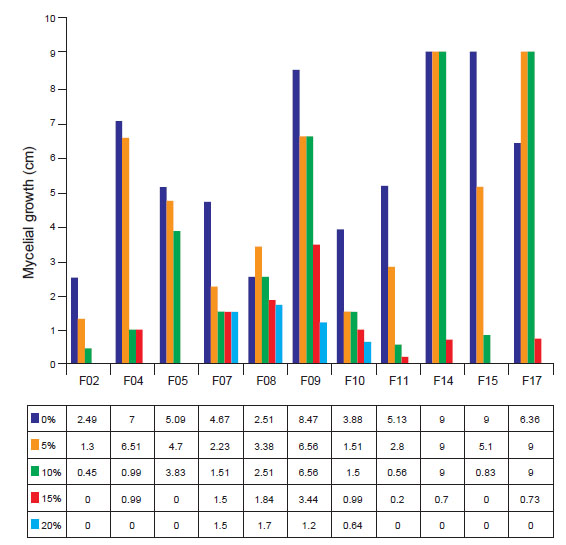
Growth under water activity. To evaluate the growth of rhizospheric fungal isolates in water restriction conditions, PDA culture medium was placed in 90mm Petri dishes. To provide the different concentrations of water stress, 5, 10, 15, and 20% of Mannitol (v/v) was incorporated into the culture medium. The control consisted of the culture medium without Mannitol. The plates were incubated at room temperature, and evaluations performed at seven days after incubation.
The experimental design was completely randomized with five replications. The data obtained were submitted to analysis of variance (ANOVA) using the RStudio software (2020) based on the R language (R Team Core 2020) and submitted to regression analysis (R²).
Results
Phosphate solubilization. Through analysis of variance, differences were detected between the isolates (p ≤ 0.05), as well as response to regression (R² = 100). Thus isolates F04, and F05 were the most efficient in the P solubilization (Fig. 1), with better results than others. However, no interactions between incubation time, and phosphate solubilization were detected, considering that this characteristic is prominent in each of the isolates, which have different behaviours.
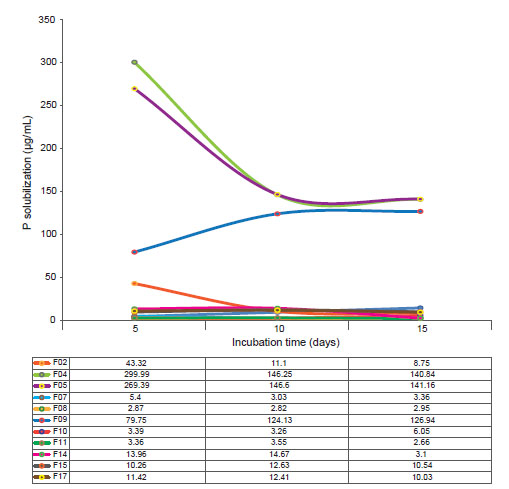
Figure 1 Phosphate solubilization for the rhizospheric fungi associated to cacti Opuntia cochenillifera. Table shows values of P solubilization per incubation time.
In this aspect, isolate F04 had the highest P concentrations with 121 µg.mL-1 from the fifth day of incubation, followed by the isolate F05 with a concentration of 100 µg.mL-1. Phosphatase activity, responsible for the solubilization of P, decreased for isolate F04 on the tenth day of incubation. For isolate F05, the phosphate solubilization stabilizes from the tenth day. The isolate F09 has a solubilization peak on the 15th day of incubation, and it can be considered that the phosphatase activity of this fungal isolate is constant and that it needs more time to obtain higher concentrations of P solubility. Isolates F04, and F05 showed the higher phosphate solubilization capacity.
For the pH, most of the fungal isolates studied showed stability in the acidification of the medium, especially the isolates F07 and F08 (Fig. 2), although they presented a moderate rate of solubilization. In addition, isolates F04 and F05 showed the greatest variations in terms of the drop in the pH of the medium throughout the experimental period (R 2 = 100), where this negatively followed the solubilization of P, that is, the highest solubilization rates were accompanied by a lower pH.
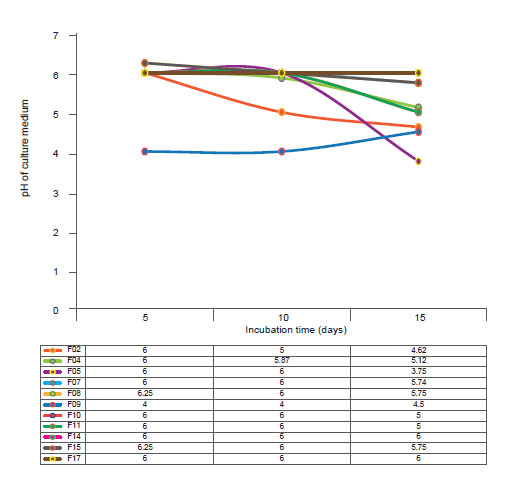
Figure 2 pH of the rhizospheric fungi associated to cacti Opuntia cochenillifera. Table shows values of pH culture media per incubation time.
Statistical analysis (p ≤ 0.01) showed that the highest IAA productions, characterized by colorimetric changes in the medium, were observed for isolates F02 and F07, which are classified according to Hartman et al. (1984) as high IAA producers (Fig. 3). Also, according to the same authors, the other isolates, although with lower IAA values, are classified as high producers of this phytohormone.
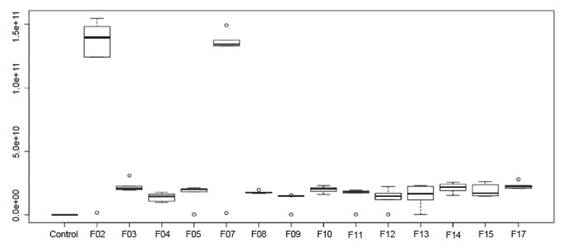
Figure 3 Production Indole 3-acetic acid by rhizospheric fungi associated to cacti Opuntia cochenillifera.
Through statistical analysis (p ≤ 0.05) it was possible to detect that the fungal isolates used presents inhibitory potential against the mycelial growth of Fusarium sp., and Colletotrichum sp. Most isolates showed direct inhibition through competition for space and nutrients in the culture medium, as well as inhibition by volatile compounds, which is explained by the presence of inhibition of mycelial growth of the phytopathogen even with the slow growth of the antagonist (Fig. 4).
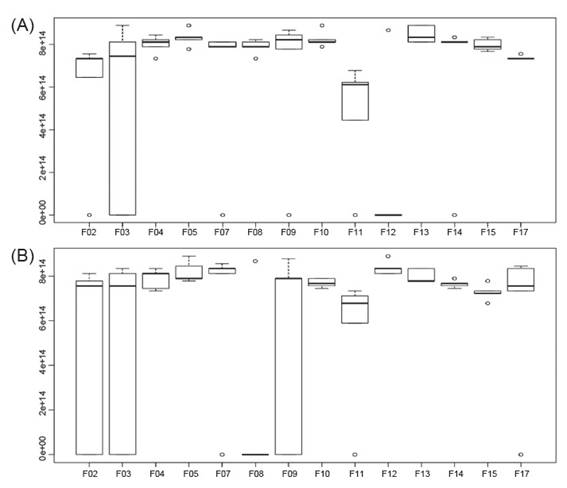
Figure 4 Inhibition of mycelial growth of Fusarium sp. (A) and Colletotrichum sp. (B) by rhizospheric fungi associated to cacti Opuntia cochenillifera.
Almost all fungal isolates studied showed more than 80% inhibitory capacity for mycelial growth of Fusarium sp. in the test performed. These results may be related to the antagonist mycelial growth rate, as well as to the capacity of production of secondary inhibitory metabolites by the phytopathogen.
Regarding the tests carried out with Colletotrichum sp., only the isolate F08 did not show antagonistic capacity against these phytopathogen. All other isolates showed inhibitory potential for mycelial growth of the studied phytopathogen, with a rate ranging from 75 to 82% of mycelial growth inhibition. In the same way as against Fusarium sp. isolates F04 and F05 showed overlapping and mycoparasitism. The isolate F07 showed a unique characteristic compared to the other strains, where it was observed that it has a high spore dispersion capacity, which contributed to its high performance in the inhibition of the mycelial growth of Colletotrichum sp.
As for the mycelial kinetics of the fungal isolates submitted to water stress, it was possible to observe that the majority presented tolerant behavior up to the concentration of 10% of mannitol (p ≤ 0.05). The isolates F07, F08, F09, and F10 were the most tolerant in the study, where they showed mycelial growth at all concentrations studied.
Regression analysis showed that the mycelial growth of all strains fit the cubic model (R²=99.60; y = 0.001939x + 0.0004939x² + 4.41x³ + 0). This fact is due to the decrease in mycelial kinetics due to the increase in the concentration of mannitol in the medium (Fig. 5).
Discussion
Thus, all fungi of this study showed phosphate solubilization capacity in the NBRIP culture medium, ranging from 10 to 141 µg.mL-1 (≈). Similar results have already been described in the literature, as demonstrated by Oliveira et al. (2012) when studying the solubilization of phosphate by different strains of Trichoderma sp., where the authors reports that such fungi presented mycelial growth and variable P concentration values using the same culture medium.
About the amount of solubilized P as a function of the incubation time, Montaldo (2016) reports that this characteristic is one of the most important regarding the screening of microorganisms to produce inoculants, as it provides constancy in the solubilization of this element. Furthermore, the ability to solubilize phosphates is related to the production of organic acids, as reported by Zaidi et al. (2009) and Zhang et al. (2018).
According to Oliveira et al. (2012) the solubilization of P continues to increase even with the decrease in the pH of the culture medium, which occurs due to the release of soluble acids, which are responsible for the solubilization of P. Thus, it is suggested that the acidification of the medium does not is a rule regarding the solubilization of phosphate, which makes it a desirable characteristic regarding the soil-plant-environment-fungus relationships.
Bakri (2019) in studies with Aspergillus niger for phosphate solubilization highlights that the P solubilization process, through the source of this nutrient in the medium, provides the release of organic acids. These, in turn, grant the fungus the ability to adapt to environments that have abiotic stress, for example, soils with high acidification. This characteristic makes the study of these microorganisms and their interactions with soil and plant even more important.
According to Rawat et al. (2020) the selection of a potent phosphate solubilizing microorganism with multifunctional characteristics to promote plant growth is extremely important for the development of commercial biofertilizers with longer shelf life. Thus, the data presented here are of biotechnological relevance as a subsidy for the development of bioproducts for application in crops.
According to Spaepen et al. (2007) there are numerous environmental and chemical factors that can model the rate of IAA production/biosynthesis by microorganisms, including pH, carbon source, and aeration. Furthermore, it is important to prospect for new promising organisms for the development of inoculant formulations, especially with characteristics that allow them to act in the physiological processes of plants, promoting their growth.
Munasinghe et al. (2017) reports the IAA biosynthesis of the endophytic fungus Colletotrichum siamense, they detected the antioxidant property of IAA. Still, infer that this hormone is considered the main auxin of plants, having as a precursor the amino acid tryptophan controlling many important physiological processes, including cell enlargement and division, tissue differentiation and responses to light. The production of hormones by fungal endophytes could play an essential role in the growth and development of the host plant (Turbat et al 2020).
Fu et al. (2015) state that the IAA acts in the exchange of signals between species distantly positioned in the tree of life, thus, according to the authors, this phenomenon suggests the existence of a widely developed structure in eukaryotes and prokaryotes, allowing production, transfer, and signal perception. Thus, it is possible to affirm the existence of co-evolution between microbial species producing IAA and plant species, with both parties benefiting.
Turaeva et al. (2020) state that the production of indole acetic acid by A. niger presents variations in its concentrations over the days and also as a function of the carbon source, which is related to its metabolic processes, since most of the biochemical processes Microbials are related to their secondary metabolism.
Patel et al. (2021) investigating the association between Penicillium sp. in association with bean plants report that the fungus has growth-promoting capacities, such as IAA production, which also indicates to be related to the production of siderophores.
Lubna et al. (2018) point out that the endophytic fungus A. niger shows growth-promoting capacities under in vitro conditions, such as the production of IAA. The authors state that the interaction of this fungus with plants stimulates the production of endogenous plant hormones, as well as the activation of secondary metabolites. Thus, a beneficial interaction with the ability to induce systemic resistance is attributed.
Is important to emphasize the importance of knowledge about the beneficial microorganisms that inhabit the rhizosphere of cultivated or not cultivated plants, especially those located in more extreme and degraded environments, as is the case of the fungal isolates studied here. This knowledge fosters subsidies for biotechnological processes for agricultural development.
In this sense, Yuniati and Rollando (2018), studying an endophytic strain of Fusarium, detected the presence of volatile organic compounds capable of inhibiting the growth of phytopathogenic bacteria. Furthermore, it is important to mention that this phytopathogenic fungus genus has a strong pigmentation in the culture medium, which is indicative of the production of volatile and non-volatile compounds, which can influence the mycelial growth of the antagonist candidate.
Silva et al. (2017) report that the antagonist mycelial growth rate is one of the main characteristics to be desired when it comes to the selection of promising strains in the antagonism of phytopathogens. Medeiros et al. (2020) studying the in vitro antagonism of Fusarium oxysporum by Trichoderma sp., report the existence of parasitism, which is the ability of a filamentous fungus to penetrate the hyphae and mycelium of the phytopathogen, a behavior also observed in the present study, especially the isolates F04 and F05, as well as mycelial overlap.
It should be observed that most studies related to antibiosis of phytopathogens by the use of antagonist fungi is directed to the genus Trichoderma. In this sense, it is important to apply other species, as demonstrated by Brandão (2017), who detected the antagonistic capacity of the fungus Paecilomyces lilacinus in the biological control of Bipolaris orizae. Wicklow and Wilson (1990), studying the survival of Aspergillus flavus sclerotia in the soil, observed the fungal species that colonized those structures, which makes it indicative of the mycoparasite ability.
The antagonistic activity exerted by the same fungus (isolate) on more than one phytopathogen of a given culture and, preferably, on phytopathogens from different cultures is a very desirable characteristic for potential antagonists, especially in terms of the commercialization of these microorganisms as biological products (Suárez-Estrella et al. 2013), because of the need for biotechnological development of bioformulations for biological control of pests and diseases.
The data contribute to the selection of strains that, by tolerating water stress, and to plant development by adapting to the most extreme environments in terms of water availability. Thus, it is to be expected that the strains maintain in vitro the same characteristics observed in the environment from which they were isolated, that is, an area undergoing a desertification process, absent from cultural treatments, and with an exponential increase in salt index. Moreira et al. (2020) state that in saline soils, inoculation with microorganisms tends to mitigate the effects of NaCl gradients.
Water stress is considered a limiting factor in the cellular structure and metabolism of the plant, especially in its initial phase (Lüttge 2007; Yang et al. 2021), where its metabolic processes are in constant activity and sensitive to the environment. Thus, the reduction in water content causes changes in physiological and biochemical processes, such as changes in cell turbidity, decrease in cell division, decrease in protein synthesis, stomata closure, changes in photosynthesis rates, translocation of nutrients, absorption of ions, increased accumulation of reactive oxygen species (Jaleel et al. 2009; Santos et al. 2022). Thus, the inoculation of plants with microbial species tolerant to this type of stress provides better development and productivity (Laranjeira et al. 20201).
The fungal isolates of this study can solubilize phosphate with low interference of the pH of the medium, produce auxins, develop under water restriction, and inhibit the growth of phytopathogens.












 uBio
uBio