Introduction
The genus Capsicum belongs to the Solanaceae family and includes chili peppers and peppers; within this botanical family, Capsicum, stands out among the 90 genera of commercially important vegetables worldwide (Tripodi & Kumar 2019). This genus is native to tropical and subtropical America in an extensive region from Mexico to the southern part of the Andes, in which archaeological evidence suggests its use from the year 6000 BC (Tripodi & Kumar 2019). Capsicum comprises 42 species, including Capsicum annuum L., C. baccatum L., C. frutescens L., C. pubescens (Ruiz and Pavon) and C. chinense Jacq., (Qin et al. 2014, Vallejo-Gutiérrez et al. 2019); the first three are the most cultivated in the world (Anjos et al. 2018). Chili peppers and peppers have a wide variety of shapes, sizes, and colours of fruits, in which the hot ones are used as spices, and the sweet ones as vegetables, in addition to having decorative, medicinal, and cosmetologically purposes (Gálvez et al. 2021).
In Ecuador, the use of genus Capsicum dates from the Valdivia culture (3800 - 1500 BC). Eleven species are reported including the five already mentioned, as well as Capsicum dimorphum (Miers) Kuntze, C. hookerianum (Miers) Kuntze, C. lycianthoides Bitter, and C. rhomboideum (Dunal) Kuntze (Yánez et al. 2015) and C. galapagoense Hunz and C. chesmanii endemics to Galapagos Islands (Lucatti et al. 2013). More than 70 traditional varieties are cultivated and have been collected according to the Instituto Nacional de Investigaciones Agropecuarias (INIAP) (Yánez et al. 2015, Monteros-Altamirano et al. 2018).
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) causes losses in vegetables in tropical and subtropical areas of the world (Latournerie-Moreno et al. 2015, Li et al. 2021), due to the direct damage caused by the sap sucking involving chlorosis, leaf deformation and plant weakening, as well as indirect damage resulting from the presence of a fungus (Capnodium spp.) that produces sooty mould and interferes with the photosynthetic process (Ortega et al. 2019). However, the transmission of viral diseases is the most important damage caused by B. tabaci (Lorenzo et al. 2016, Guo et al. 2020).
The commonly used control measures against insect pests in horticultural crops are based on the use of organo-synthetic pesticides; however, these products are mostly toxic to the environment and to non-target species and favour the development of resistant populations (Nombela & Muñiz 2010). An effective, economical, and environmentally compatible method for the control of insect pests is host plant resistance (Ballina-Gomez et al. 2013).
Painter (1951) defined resistance as the genotypic condition of a plant that allows it to be less damaged than another of the same species under similar environmental conditions. It includes three categories: antixenosis, antibiosis, and tolerance. In antixenosis, the plant may not be preferred for oviposition, shelter, or feeding because it has certain qualities that make it a poor host (Painter 1951, Kogan & Ortman 1978, Jeevanandham et al. 2018), while antibiosis describes the adverse effects of the host plant on the biology of the insect, including death of the early stages, abnormal growth rates, failure to pupate, under size of adults (Painter 1951). Finally, resistant plants may be tolerant if they survive below levels of infestation that could kill or severely injure susceptible ones.
Studies have shown that Bemisia tabaci is affected by the physical characteristics of the leaf surface, such as villi, glandular trichomes, and leaf shape (Ballina-Gomez et al. 2013, Al-Aloosi et al. 2020, Sripontan et al. 2022). It is also known that pepper varieties and cultivars have different chemical composition, antioxidant and allelochemical compounds that influence resistance against phytophagous insects (Sripontan et al. 2022). Particularly, domesticated plants and wild relatives of Capsicum can be important sources of resistance to reduce damage by phytophagous (Ballina-Gomez et al. 2013, Tripodi & Kumar 2019). Therefore, the local germplasm of domesticated chillies and peppers could be sources of resistance especially in Ecuador, which is a country of high diversity of Capsicum species. Consequently, the objective of this study was to evaluate the response of Ecuadorian Capsicum genotypes to B. tabaci infestations.
Material and methods
The experiment was conducted from February until May 2018 inside a shade house of 1625 m2 (25 m x 60 m) built with high-density polyethylene shade cloths and a transparent polyurethane roof, at the Experimental Station “La Teodomira”, Faculty of Agricultural Engineering, Universidad Técnica de Manabí (UTM), Lodana, Manabí province, Ecuador (coordinates: 01° 09’ 51” S and 80° 23’ 24” W), altitude 60 meters above sea level). Inside the shade house, the average temperature was 26.25°C and the relative humidity was 79.75%. The life zone corresponds to a tropical dry forest.
Capsicum genotypes native to Ecuador (73 accessions) were evaluated, corresponding to C. annuum, C. baccatum, C. frutescens, C. pubescens and C. chinense (Syn. C. sinense Murray), from INIAP genebank originally collected from different provinces of Ecuador (Monteros-Altamirano et al. 2018). The accessions are being characterized morphologically and molecularly, whose genebank codification (numbers), respective species classifications as well as the provinces where the accessions were collected are presented in Table 1.
Table 1 List of Ecuadorian Capsicum spp. accessions from INIAP’s genebank evaluated in the present study.
Seeds of each genotype were sown in 50-well germination trays (one seed per well) with peat moss where they remained for 30 days. Subsequently, plantlets were transplanted in the shade house at 1.30 m between rows and 0.70 m between plants (10 plants per genotype) in a completely randomized design. Irrigation was carried out twice a week for 15 minutes at the beginning of the cycle (first 35 days) and later for 30 minutes through a drip system with 0.02 mm tapes, located every 20 cm and a capacity of 3 L. hour-1. Following previously established methodologies to induce populations of whiteflies through insecticide applications in tomato crops (Chirinos et al. 1996, Geraud-Pouey et al. 1996), all plants by Capsicum genotypes were sprayed with insecticides; four applications were made every 15 days starting with the transplant. The first two sprays were conducted with imidacloprid (15 days) and thiamethoxam (30 days) at doses of 2 cc.L-1. For the other two applications chlorpyrifos (2.5 cc.L-1) and Thiocyclam hydrogen oxalate (1 g.L-1) were used, following local recommendations.
After insecticide spraying, observations were made on leaves to corroborate the establishment of B. tabaci individuals in all genotypes, which occurred approximately one month after the last spraying. Since then, the populations were monitored weekly on the leaves in each genotype until 90 days. The populations of B. tabaci were counted on four random leaves in four plants per genotype, two leaves in the upper layer and two in the middle layer of the plant. The leaves were kept in transparent plastic bags, labelled for each genotype, and taken to the Entomology Laboratory of the Faculty of Agricultural Engineering, Universidad Técnica de Manabí. There were observed under a 10 - 40X magnification stereoscope Carl-Zeiss®️ brand, counting per leaf the number of: eggs, nymphs, and adults, of B. tabaci.
The number of eggs, nymphs and adults per leaf was analysed by ANOVA (p < 0.05). The comparison of means by genotype were evaluated with the Scott-Knott test (p < 0.05) and by species with the LSD Fischer test (p < 0.05). A dendrogram including the average of the total number of individuals of B. tabaci per leaf by genotype was plotted to establish similarity relationships based on infestations, using the unweighted arithmetic average method and Euclidean distance. The analyses were performed using the statistical software InfoStat professional version 2019 (Di Rienzo et al. 2019).
Results
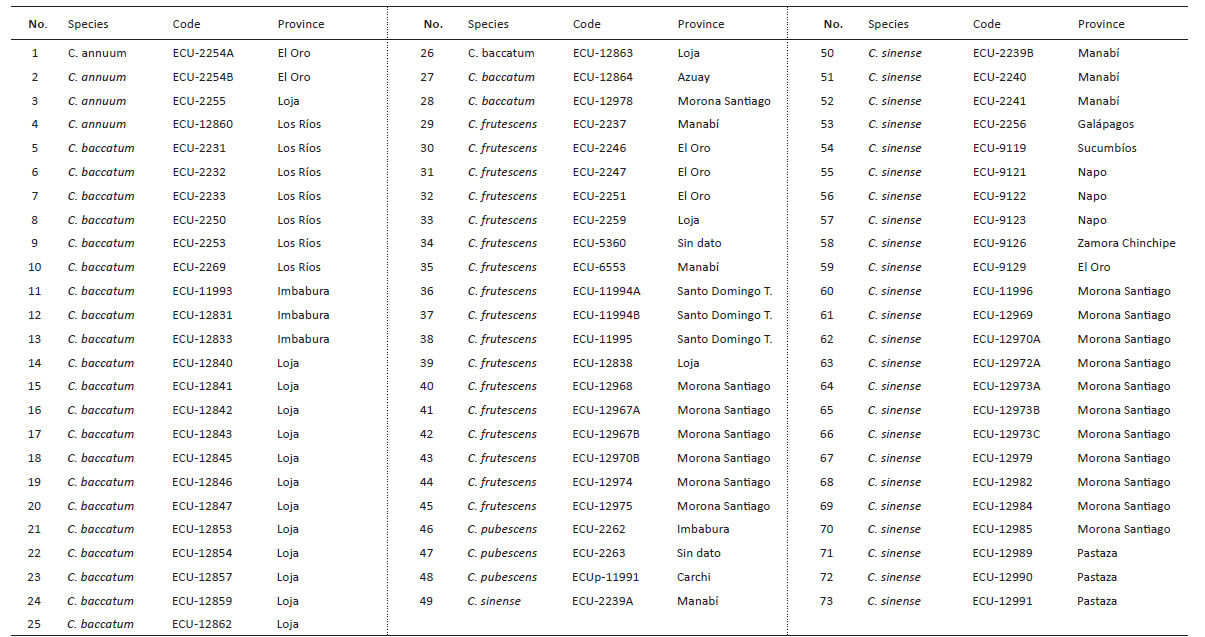
In relation to number of adults, the Scott-Knott test determined three groups according to media differentiation (a, b, and c) (Fig. 1, p < 0.05).
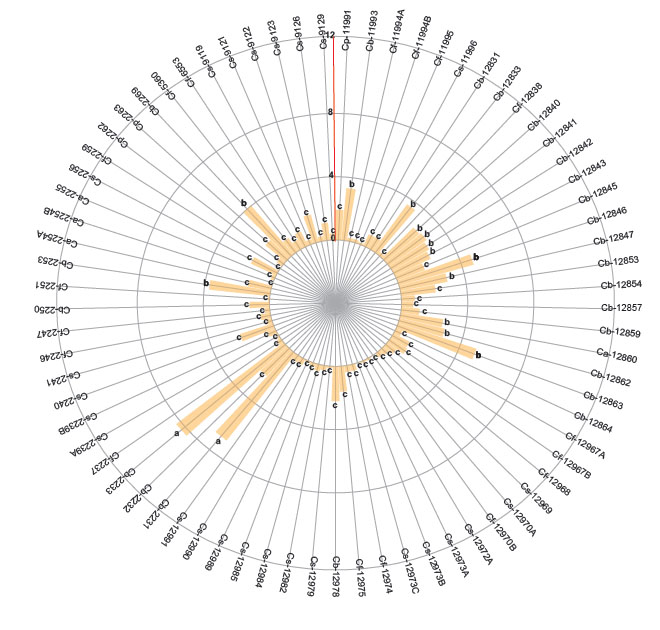
Figure 1 Number of adults of Bemisia tabaci evaluated in the 73 genotypes of the five cultivable Capsicum species. C. annuum (Ca); C. baccatum (Cb); C. frutescens (Cf); C. pubescens (Cp); C. sinense (Cs). The bars represent the means by genotype. Mean followed by different letters in each bar is significantly different at 0.05 level of significance using Scott-Knott test (p < 0.05).
The first group (a) identified high number of adults of B. tabaci in two genotypes of C. baccatum (2231 and 2233 codes). The second group included 15 accessions of C. baccatum and one accession of C. annuum. The third group with the least number of adults included 25 accessions of C. sinense, 10 of C. baccatum 16 of C. frutescens and 3 of C. annuum. Regarding the higher number of eggs (range or group “a”) (Fig. 2, p < 0.05) were found in 4 accessions of C. baccatum (12859, 12847, 12845, 12842) one accession of C. pubescens (11991) and one C. baccatum (2269). The second group includes 14 accessions of C. baccatum and one accession of C. sinense. Finally, the third group with the lesser number of eggs includes 24 accessions of C. sinense, 4 of C. baccatum, 17 of C. frutescens, 3 of C. annuum and 2 of C. pubescens. Average of the number of nymphs are also separated in three groups according to the Scott-Knott test (Fig. 3, p < 0.05).
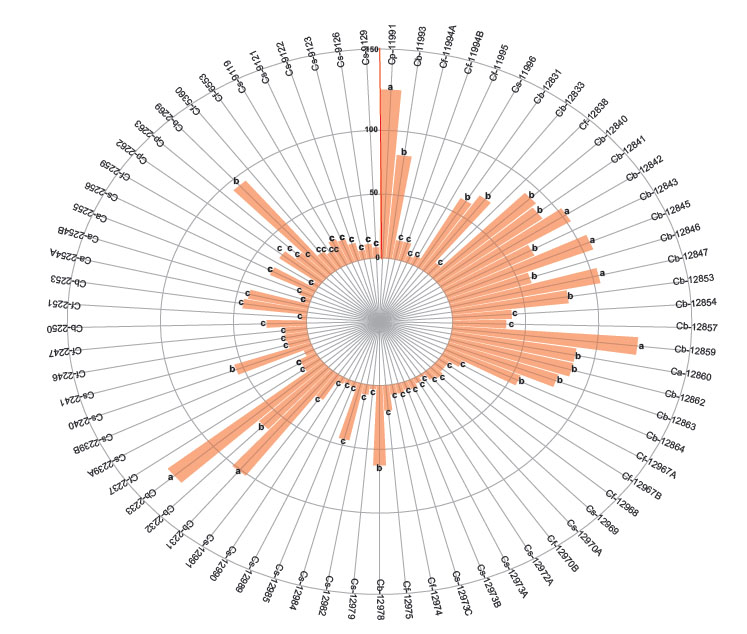
Figure 2 Number of eggs of Bemisia tabaci evaluated in the 73 genotypes of the five cultivable Capsicum species. C. annuum (Ca); C. baccatum (Cb); C. frutescens (Cf); C. pubescens (Cp); C. sinense (Cs). The bars represent the means by genotype. Mean followed by different letters in each bar is significantly different at 0.05 level of significance using Scott-Knott test (p < 0.05).
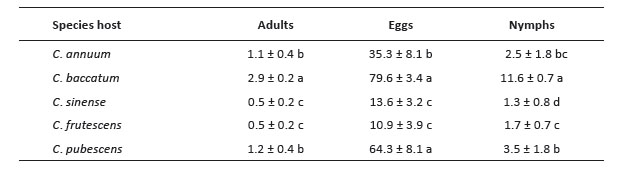
The first group with the higher number of nymphs are 3 accessions of C. baccatum (12853, 12845 and 2232). The second group includes other 3 C. baccatum accessions (12847, 12842, 12831) and the third group with the lesser number of nymphs includes 17 accessions of C. baccatum, 25 of C. sinense, 17 of C. frutescens, 4 of C. annuum and 3 of C. pubescens (Fig. 3). When comparing the variables by species according to the differences in the degrees of significance, the number of adults, eggs, and nymphs of B. tabaci were significantly higher in C. baccatum and lower in C. frutescens and C. sinense (Table 2).
Table 2 Average of the number of adults, eggs, and nymphs of Bemisia tabaci per leaf in accessions of Capsicum species. Means ± standard error. Mean followed by different letters in each column is significantly different at 0.05 level of significance using Fischer test (p < 0.05).
The dendrogram constructed by genotype including average of density per leaf of all individuals of B. tabaci shows three groups with similar genotypes depending on the level of pest infestation (Fig. 4). A first group (right to left) made up of 14 genotypes that showed a high infestation, of which 13 belong to C. baccatum, one to of C. annuum. The second group is made up of 6 genotypes of C. baccatum. A third grouping includes those genotypes of Capsicum spp. with lower infestations including all genotypes of C. frutescens and C. sinense, as well as three of the four genotypes of C. annuum, two of C. pubescens and three of C. baccatum.
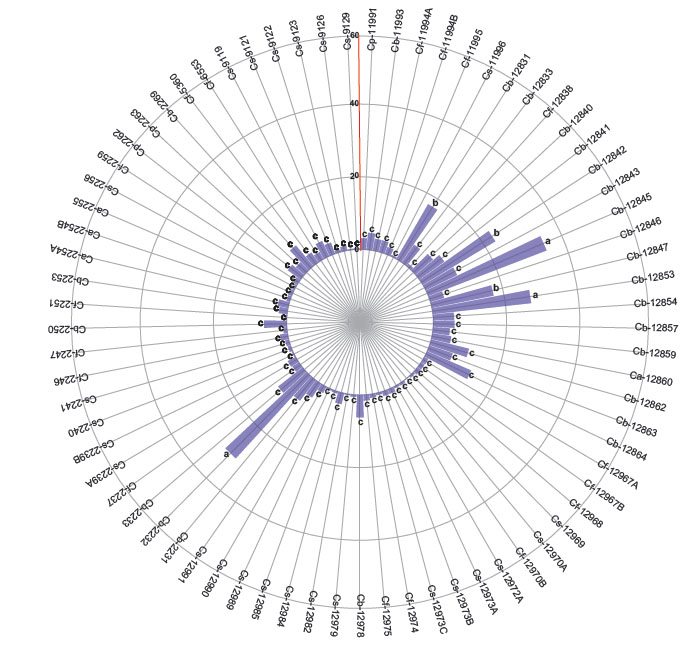
Figure 3 Number of nymphs of Bemisia tabaci evaluated in the 73 genotypes of the five cultivable Capsicum species. C. annuum (Ca); C. baccatum (Cb); C. frutescens (Cf); C. pubescens (Cp); C. sinense (Cs). The bars represent the means by genotype. Mean followed by different letters in each bar is significantly different at 0.05 level of significance using Scott-Knott test (p < 0.05).
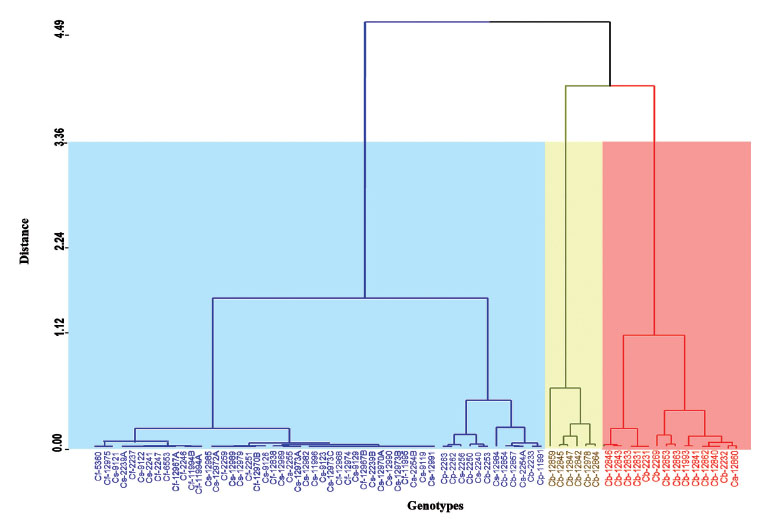
Figure 4 Dendrogram of 73 genotypes of Capsicum spp. in relation to the infestation of Bemisia tabaci. C. annuum (Ca); C. baccatum (Cb); C. frutescens (Cf); C. pubescens (Cp); C. sinense (Cs). Each color includes the genotypes with infestations similar to the distance at which the dendrogram was cut.
Discussion
Resistance of C. annuum genotypes to B. tabaci infestations has been proven in field and laboratory research carried out in different geographical areas e.g. Al-Aloosi et al. (2020) in field conditions at Iraq, evaluated a local variety and two commercial varieties of C. annuum “Anaheim chili” and “Aleppo”; the last one showed the lowest infestations by B. tabaci, whose resistance is attributed to the presence of secondary metabolites and other factors, such as the high density of trichomes, thickness and colour of the leaves. Jeevanandham et al. (2018) in a study conducted under shade house conditions, detected fewer adults and eggs of B. tabaci in 4 genotypes of C. annuum suggesting their strong antixenotic and antibiotic effects. Free-choice tests conducted inside entomological boxes evaluated the resistance of C. annuum genotypes collected in south-eastern Mexico, to B. tabaci (Ballina-Gomez et al. 2013, Chan et al. 2014). Of the twelve genotypes evaluated by Ballina-Gomez et al. (2013), three (Blanco, Bolita and Pico Paloma) showed low egg hatching and little or no survival of B. tabaci nymphs, mentioning that resistance could be associated with antibiosis due to low nutritional quality or toxic secondary metabolites. Chan et al. (2014) assessed 14 genotypes, in which the one collected in a wild habitat (Maax ik), showed the least attraction of adults and low preference for oviposition.
Besides C. annuum, resistance to B. tabaci has also been examined in genotypes of the other species such as C. frutescens, C. pubescens, and C. sinense. Pantoja et al. (2018) in a B. tabaci oviposition preference test found a small number of adults and eggs on some accessions of C. annuum, C. frutescens, C. pubescens, and C. sinense, conferring resistance to the infection due to antixenosis. Sripontan et al. (2022) evaluated in entomological boxes resistance to B. tabaci of C. annuum, C. frutescens and C. sinense cultivars; small number of adults of B. tabaci were found in cultivars of C. annuum while those of C. frutescens and C. sinense presented a higher number of both adults and eggs. Firdaus et al. (2011) evaluated the resistance of forty-four genotypes of C. annuum, C. baccatum, C. frutescens and C. sinense species, finding a high negative correlation between the number of adults and eggs of B. tabaci and two characteristics of the leaf (the density of glandular trichomes and the thickness of the cuticle). Additionally, C. annuum was the species that developed the lowest whitefly populations. Kumar et al. (2020) evaluated the resistance of 125 Capsicum genotypes and associated the non-preference of B. tabaci to the presence of glandular and non-glandular trichomes, as well as the flavonoids contained in the plants.
The results presented allow to identify three situations among the native Capsicum of Ecuador evaluated to the infestation of B. tabaci: One, in which a high population density of B. tabaci was observed in 76% of the C. baccatum genotypes; two, in which most of the evaluated genotypes of C. annuum and C. pubescens showed low populations; and third, all genotypes of C. frutescens and C. sinense showed low whitefly infestations.
Genetic variability has been detected in Capsicum accessions and hybrids, which present distinct morphological and molecular characteristics (Costa et al. 2016, Cardoso et al. 2018, Tripodi & Kumar 2019). This could explain the different whitefly infestation rates in genotypes of C. baccatum and C. annuum. Low infestation in C. pubescens could be associated with the pubescence of the leaves; however, it is not ruled out that the presence of volatile compounds, high concentrations of capsaicinoids in this species as well as C. frutescens and C. sinense have affected the colonization of B. tabaci populations on the evaluated accessions.
The high attraction of B. tabaci for C. baccatum genotypes, as well as the low infestation in all the genotypes of C. frutescens and C. sinense is demonstrated in this study. The non-preference of adults and the scarce oviposition of B. tabaci on genotypes of C. frutescens and C. sinense suggest resistance due to antixenosis that could later have affected the lower number of nymphs. Nevertheless, antibiotic effects of the genotypes on B. tabaci nymphs cannot be ruled out. The term antixenosis was proposed by Kogan and Ortman (1978) and defined as the resistance mechanism employed by the plant to deter colonization by an insect and may include morphological changes, such as subtle variations in plant surface colour, waxy or hairy leaves, and flavour, as well as defensive exudations of gums or resins.
Tripodi and Kumar (2019) reported that an important number of accessions of cultivated and wild species are stored in genebanks around the world, which represent a valuable resource for breeding to transfer traits related to resistance to selected cultivars. Certainly, these results can be used as a basis for genetic improvement for the resistance of Capsicum species to B. tabaci.
This research shows the high attraction of the whitefly B. tabaci, towards evaluated genotypes of C. baccatum. Likewise, results indicate the non-preference of adults and the scarce oviposition of B. tabaci over genotypes of C. sinense and C. frutescens, suggesting resistance due to antixenosis. These results could guide breeding programs for the resistance to B. tabaci infestations of Capsicum species.












 uBio
uBio 


