Introduction
Pedersenia weberbaueri (Suess.) Holub was initially described as Iresine weberbaueri Suess. by Suessenguth (1934) from two collections of August Weberbauer who discovered the species growing near Balsas (Amazonas department) in 1904 and near Pataz (La Libertad department) in 1914. Only a handful of collections have been registered in the last century and most of them lay hidden in herbaria with erroneous determinations (Rodríguez et al. 2016).
Pedersenia weberbaueri is a leafy shrub that can reach 4 meters in height, easy to recognize by its opposite elliptic-lanceolate leaves that are densely tomentose beneath and by its striking white inflorescences that can exceed 50 cm in length (Fig. 1). The habitat bears the rain-shadow effect, for this reason is dominated by xerophytic species of Cactaceae, Fabaceae and Malvaceae (Eriotheca) agree to the dry forest zone (Young 1993). The species is locally known as “flor blanca” (Spanish; Rodríguez et al. 2016) and as “quishuar” (Quechua; Cano et al. 2010; Gonzáles et al. 2020) and has significant economic value both locally and internationally as the distinctive inflorescences are sold in local markets due to their various medicinal properties (Castillo-Vera et al. 2017; Castañeda et al. 2021; Seminario et al. 2021), and a patent has been filed for the extraction of a lipolysis promoter from the species in the United States of America (US 2007/0218108A1; Suetake et al. 2007).

Figure 1 Pedersenia weberbaueri. A. Habitat, B-C. Habit, D. Flowering branch. E. Indumentum on inflorescence, including tepals. Photos by P. Gonzáles (Gonzáles 6146).
The distribution and threat status of P. weberbaueri remains poorly understood despite the increasing extraction of the species from the wild. The species is considered Near Threatened (NT) based on a national IUCN threat assessment (Castillo-Vera et al. 2017), whereas the regional scientific community has proposed the species to be Endangered (EN) (León et al. 2006). As more collections are becoming available from across Peru, the distribution of P. weberbaueri has extended over the past decades with new reports from Amazonas, Huánuco and La Libertad (Borsch 1993; León et al. 2006) and more recently from Áncash (Cano et al. 2006) and Cajamarca (Rodríguez et al. 2016). The species seems to be restricted to Seasonally Dry Tropical Forests (SDTF) habitat within the Marañón valley (León et al. 2006; Rodríguez et al. 2016) but some authors have cited collections from up to 2,500 m elevation beyond the SDTF zone (Borsch 1993).
Species Distribution Models (SDMs; Soberón et al. 2017) have been used in recent studies to study the potential distribution ranges of rare and threatened animal and plant species in Peru (Loaiza and Gamarra 2016; Aguilar 2019; Gonzáles et al. 2019; Cotrina et al. 2021). SDMs combine species presence data (e.g. herbarium specimens) with bioclimatic, edaphic and/or topographic data to predict potential distribution areas (Peterson et al. 2011). SDMs are an extremely valuable tool for predicting the distribution of rare species in poorly collected areas such as Peru to inform conservation decision and management strategies (e.g, Särkinen et al. 2013). At the other extreme, SDMs are also a powerful tool for predicting climate change responses of entire plant communities in megadiverse regions such as the tropical Andes (Guisan et al. 2013; Fagundes et al. 2016; Sofaer et al. 2019; Tovar et al. 2022).
The objective of this work is to analyse the geographic distribution of P. weberbaueri using SDMs, to determine the current conservation status for the species, and resolve the typification and nomenclature of the species.
Material and methods
Occurrence data. We consulted herbarium collections from four Peruvian herbaria (CPUN, HUT, MOL and USM), as well as the digitised collections of 10 international herbaria (B, E, F, GH, M, MO, NY, P, US, and WAG; Thiers 2022). We also consulted the online databases of JSTOR Global Plants (https://plants.jstor.org) for all available specimens for the species, including any type material, and searched individual herbarium databases for duplicates of material cited by Suessenguth (1934) to look for lectotype material.
Global Biodiversity Information Facility (GBIF; www.gbif.org) and relevant literature were consulted to identify further locations of specimens that potentially represent P. weberbaueri (Suessenguth 1934; Rodríguez et al. 2016). All collections were taxonomically verified to avoid specimen identification errors (Goodwin et al. 2015) since it is frequently confused with the genus Buddleja (Scrophulariaceae). This process of careful taxonomic verification resulted in eliminating one specimen that represents a distinct and yet undescribed species of Pedersenia (see Discussion for details). Further collections were made by the authors during field work focused on collecting in the most poorly sampled southern section of the Marañón valley in February 2020 and March 2021. Fieldwork was carried out under the research permit issued by SERFOR with RDG N° 002-2020-MINAGRI-SERFOR-DGGSPFFS and RDG N° 602-2019-MINAGRI-SERFOR-DGGSPFFS. All newly collected specimens were deposited at USM and E, with additional duplicates placed at CPUN, MO, MOL and QCA.
All collections with detailed locality descriptions that lacked coordinate data (or had low precision coordinates; see Marcer et al. 2021; Marcer et al. 2022) were manually georeferenced (Gonzáles et al. 2018; Gonzáles et al. 2019; Cao 2021) using department, province, district, locality and habitat description together with elevation. Observed distribution maps with number of collections were made using 3 km2 (0.01°) grid cell size in DIVA-GIS version 1.4 (Hijmans et al. 2001). The final maps were made in QGIS 3.12. The final dataset included 47 herbarium collections, some with duplicate specimens distributed across several herbaria (Appendix 1).
Species distribution modelling. The machine learning algorithm based on the maximum-entropy approach (MaxEnt) version 3.4.4 (Phillips et al. 2006) was used to identify potential suitable habitat areas for P. weberbaueri. MaxEnt is one of the most widely-used algorithm for modelling plant species distributions (Baldwin 2009; Särkinen et al. 2013; Loaiza and Gamarra 2016; Gonzáles et al. 2019; Quipuscoa et al. 2019; Navarro et al. 2020; Quispe and Elías 2020; Cotrina et al. 2021; Dai et al. 2022). The widespread use of MaxEnt lies in its ease of use (Merow et al. 2013), the fact that it requires presence-only data (Phillips et al. 2006), and the high performance of the algorithm with small number of presence points (Pearson et al. 2007), making it an ideal method when working in poorly studied areas (Hernandez et al. 2008).
All georeferenced records of P. weberbaueri were used as input data (N=47) for all models. Two models were run, the first model with a larger study extent that included the whole of Peru, followed by a second model run on a smaller study extent based on key areas identified from the first model (i.e., Marañón valley). This approach of limiting the final model training extent avoids model overfitting that can lead to underestimation of species’ distribution areas (Barve et al. 2011). All models were run with default settings. Cross-validation (replicates=5) was used to evaluate model performance because it uses all the data for validation (Phillips et al. 2006). For the interpretation of the model, we used the receiver operating characteristic (ROC) and the area under the curve (AUC), where values of AUC close to 1 were taken to indicate optimal model quality, and values close to 0.5 were interpreted as poor performance equal to random (Phillips et al. 2006; Phillips and Dudík 2008; Elith et al. 2011).
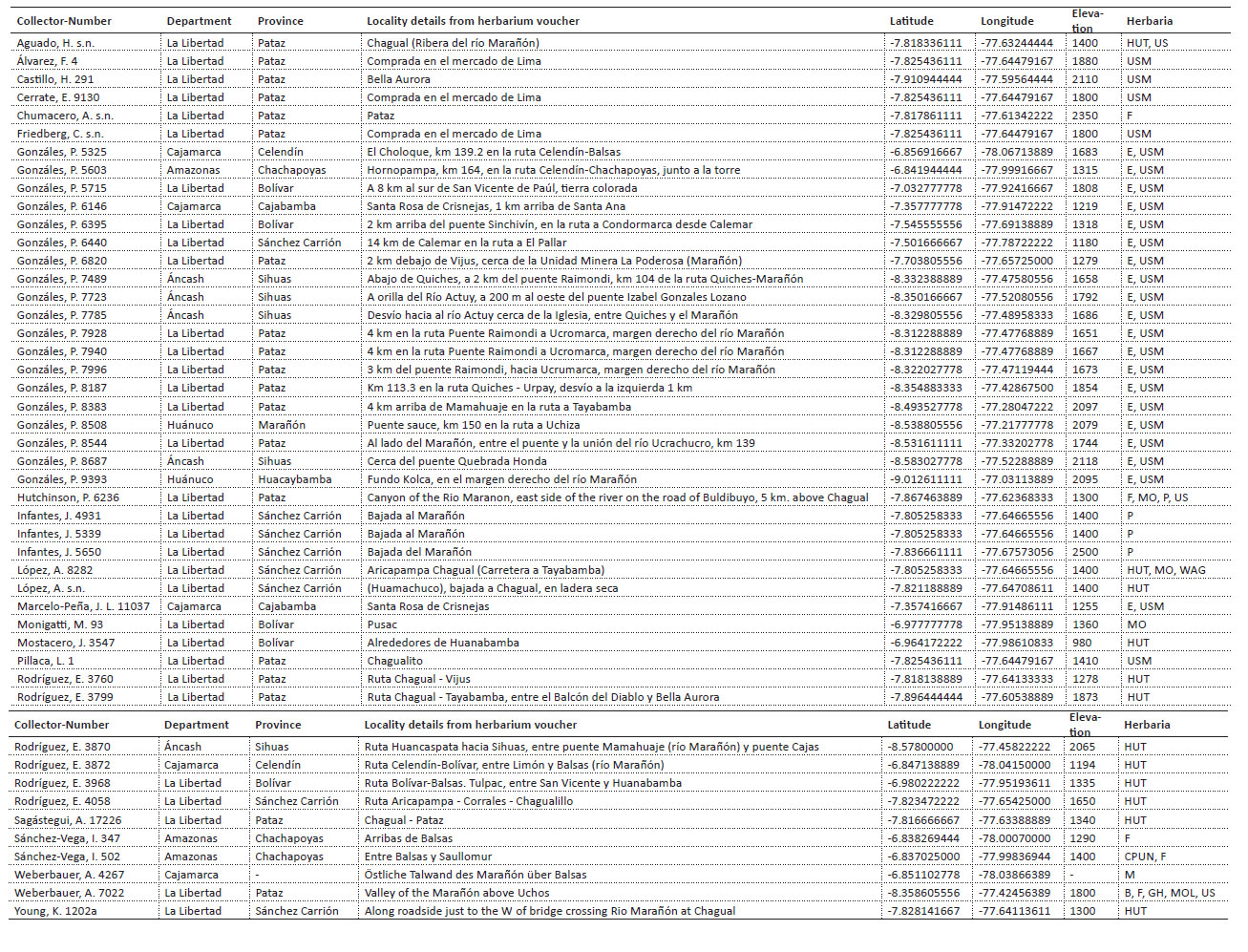
The first model was run using Peru as the study extent with 11 bioclimatic predictor variables at 30 arc second spatial resolution (ca. 1 km2) from the WorldClim (Hijmans et al. 2005; Fick and Hijmans 2017) global interpolated temperature and precipitation data derived from satellite data and corrected based on weather station data from 1970-2000 (Hijmans et al. 2005; Fick and Hijmans 2017). The 11 chosen variables were not highly correlated (r <0.7; Table 1) to avoid collinearity between predictor variables (Dormann et al. 2013) and included seven temperature (BIO2 Mean diurnal range, BIO3 Isothermality, BIO4 Temperature seasonality, BIO5 Max temperature of warmest month, BIO6 Min temperature of coldest month, BIO7 Temperature annual range, and BIO9 Mean temperature of driest quarter) and four precipitation variables (BIO15 Precipitation seasonality, BIO16 Precipitation of wettest quarter, BIO17 Precipitation of driest quarter, and BIO18 Precipitation of warmest quarter).
Table 1 Collinearity between the bioclimatic predictor variables. Pearson correlation between all bioclimatic variables from Peru (above cross) and the Marañón watershed (below cross), with variance inflation factors (VIFs) shown in bold for the nine bioclimatic variables used in the second model. Variables used in the first model are shown in grey highlights.
The second model was run using the Marañón watershed as a study extent based on results from the first model that indicated that most suitable habitat area for the species are found within the long and narrow river valley. A different set of predictor variables was used from the WorldClim (Hijmans et al. 2005; Fick and Hijmans 2017) at 30 arc second spatial resolution (ca. 1 km2) for the second model based on variance inflation factor (VIF). VIF is based on correlation coefficient (R2) estimated from regressions for all predictors, implemented via the 'sdm' package in R (version 4.2.1) (Table 1). Nine bioclimatic variables were chosen with the greatest contributing power to the model, including four temperature variables (BIO2 Mean diurnal range, BIO3 Isothermality, BIO4 Temperature seasonality, BIO8 Mean temperature of wettest quarter), and five precipitation variables (BIO13 Precipitation of wettest month, BIO14 Precipitation of driest month, BIO15 Precipitation seasonality, BIO18 Precipitation of warmest quarter, and BIO19 Precipitation of coldest quarter). Results from the second model were used to produce a final potential distribution map for P. weberbaueri using the logistic output format, where all areas with relative suitability were showed for the second model as potential areas of occurrence for the species.
Conservation status. The conservation status of P. weberbaueri was assessed according to the IUCN (2012; IUCN 2019) following criteria B on geographic distribution based on Extent of Occurrence (EOO; criterion B1) and/or Area of Occupancy (AOO; criterion B2). EOO and AOO were calculated using GeoCAT (http://geocat.kew.org) using minimum convex polygon with a cell width of 2 km (Bachman et al. 2011). We collated information regarding the number of known locations (defined as geographically or ecologically distinct area in which a single threatening event can rapidly affect all individuals of the species present), population fragmentation, and population decline (observed, estimated, inferred, or projected) following the guidelines.
Results
Distribution. Pedersenia weberbaueri is currently known from a total of 50 collections, all from the Marañón SDTF (Fig. 2). The first collection of the species was made in 1904 by August Weberbauer around Balsas in the department of Amazonas. Most of the collections (>50%) have been made since 2000. Populations are known from five departments with La Libertad having the most collections (32) followed by Cajamarca (7), Áncash (5), Amazonas (3) and Huánuco (2). The provinces within these departments that have the most collections are Pataz (19), Sánchez Carrión (8) and Bolívar (5), all in the department of La Libertad. All collections come from the southern part of the narrow river valley, with no collections from the northern SDTF of the Marañón (Fig. 2C). Thirteen collections (28%) come from Chagual (Pataz, La Libertad) from an area of only 9km2 seen as a hotspot of collections in the collection density map (Fig. 2C).
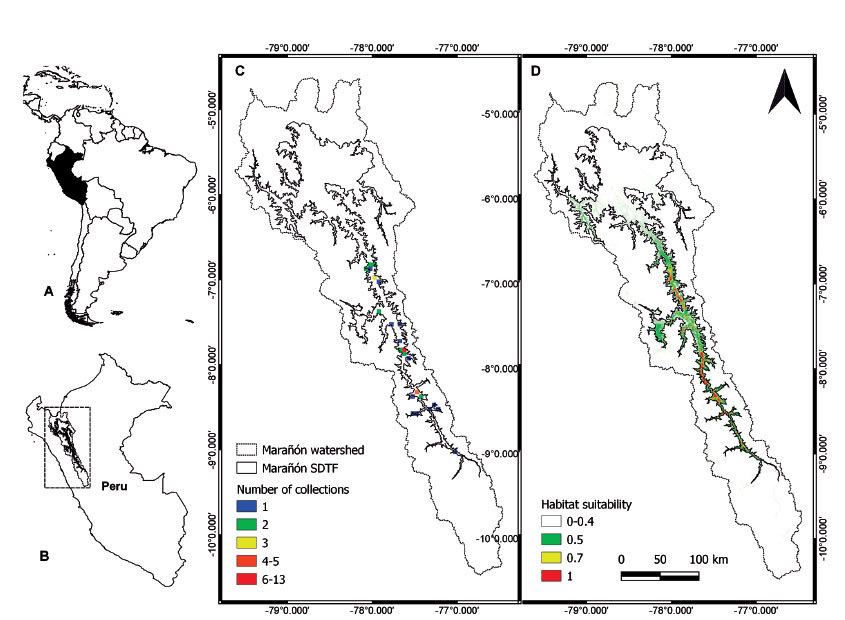
Figure 2 Distribution of Pedersenia weberbaueri. A. Location of Peru (black) within South America. B. Location of the Marañón watershed (rectangle) in northern Peru. C. Observed distribution of P. weberbaueri based on herbarium collections; number of collections is shown at 3 km2 grid cell resolution. D. Potential distribution of P. weberbaueri based on habitat suitability results from the final MaxEnt model.
Other collection hotspots include Quiches-Urpay further south (Áncash and La Libertad) and Balsas in the north (Amazonas; Fig. 2C). The species grows between 980-2,500 m elevation, with most collections found between 1,300-1,800 m (Fig. 3). Field work by the authors between 2020-2021 throughout the Marañón valley showed an upper limit of 2,235 m.
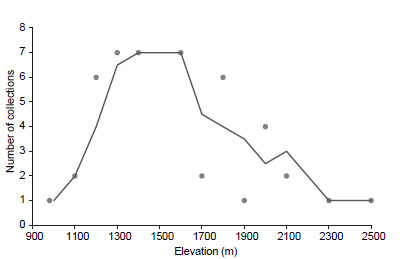
Figure 3 Elevational range of Pedersenia weberbaueri based on herbarium collections, showing number of collections known from different elevations. The curve indicates the mean trend.
Results from the final SDM showed good model performance based on AUC value >0.988 and indicate most of the southern sections of the Marañón valley SDTF as highly suitable (>0.5) for P. weberbaueri (Fig. 2D). Many of the areas within Marañón valley SDTF lacking collections are highlighted as highly suitable for the species (Fig. 2C-D). Only a few small areas outside of the Marañón watershed were predicted as highly suitable (>0.7) in the first model (Fig. 2D), but these are considered highly unlikely to represent true presences because the physical isolation of the Marañón SDTF surrounded by high mountain peaks (>5,000 m) preventing dispersal. The SDMs identified temperature seasonality (BIO4) and mean temperature of wettest quarter (BIO8) as the most important climatic variables contributing to the final model. Other important variables included isothermality (BIO3), precipitation of warmest quarter (BIO18) and precipitation of coldest quarter (BIO19).
Conservation status. Pedersenia weberbaueri has an estimated extent of occurrence (EOO) of 5182 km2 and estimated area of occupancy (AOO) of 116 km2. The species occupies a narrow geographic range in northern Peru between 6.8°-9.0° S and 77.0°-78.1° W. The Marañón SDTF is under threat from increased agricultural development, overgrazing and proposed dam developments (Finer and Jenkins 2012). Based on the AOO, and considering the overall declined habitat quality, the absence of any known population within a protected area (Fig. 4C), and the continuing decline in the number of mature individuals due to overextraction for medicinal use, we assign this species as Endangered (EN; B2ab(iii,v)) status to this species. The EOO would merit Vulnerable (VU; B1ab(iii,v)) status, but we follow the recommendation of the IUCN (IUCN 2012; IUCN 2019) to assign the highest category. Below we propose the supporting text for an assessment to be published on the IUCN Red List.
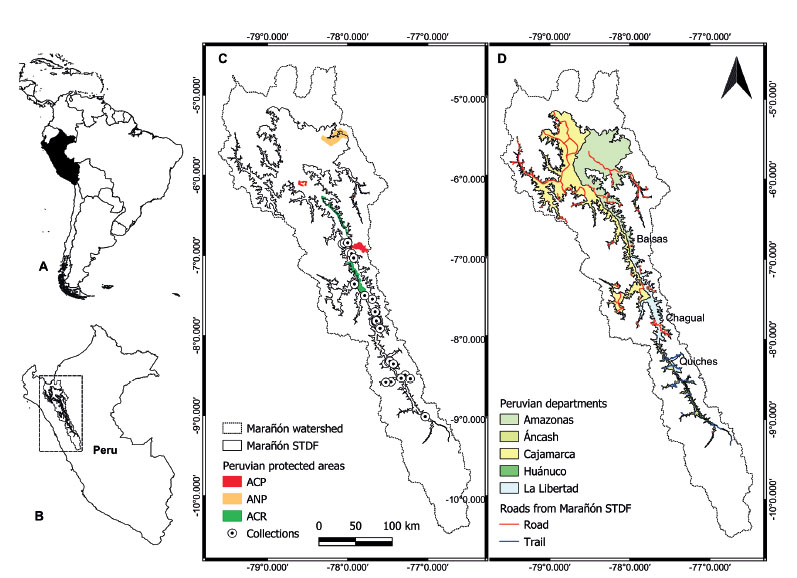
Figure 4 Study area and distribution of Pedersenia weberbaueri. A. The location of Peru (black) within South America. B. Location of the Marañón watershed (rectangle) in northern Peru. C. Distribution of P. weberbaueri within the Marañón Seasonally Dry Tropical Forests (SDTF) within the watershed based on herbarium collections. Peruvian protected areas are highlighted on the map in colours (ACP=Área de Conservación Privada; ANP= Áreas Naturales Protegidas, and ACR= Área de Conservación Regional). D. Accessibility within the Marañón SDTF across the different departments based on road and trail networks.
Geographic range information. Pedersenia weberbaueri is confirmed as an endemic to the dry forest of northern interandean Peru (Fig. 2) and known only from Amazonas, Áncash, Cajamarca, Huánuco, and La Libertad departments between 980 and 2,235 m (see Discussion below) from five locations. The area of occupancy AOO is estimated at 116 km2.
Habitats and ecology information. This evergreen woody shrub species of the Amaranthaceae family grows in dry, rocky, stony, and clay places, roadsides, and inhospitable and inaccessible slopes (Fig. 3), associated with deciduous tree species such as Eriotheca discolor (Kunth) A. Robyns and columnar cacti such as Armatocereus rauhii Backeb. and Browningia pilleifera (F. Ritter) Hutchison (Rodríguez et al. 2016). There is a continuing decline in the extent and quality of its habitat due to extension of agricultural frontiers, over-grazing and dust pollution on roads.
Threat information. Four out of the five known subpopulations are at risk from over-harvesting by the local community. The inflorescences of the species are harvested for their medicinal properties, and this practice has grown to become the species' main threat (Rodríguez et al. 2016). The collection of the inflorescences is likely to negatively impact regeneration of individual plants, local recruitment as well as gene flow between individuals, and therefore negatively influence genetic diversity and viability of populations and the species.
Population structure. There is no data on population size and trends for this species. It is frequent in suitable habitat; however, the population is considered severely fragmented as it occurs in small patches (ca. 1 km2). There is a continuing decline in the number of mature individuals due to the extraction of the plant from the wild for medicinal use, as despite not extracting the entire plant, a large percentage of individuals die due to the collection practices (Rodríguez et al. 2016).
General notes regarding trade and use of this species. The species is harvested locally because it possesses medicinal properties that make it valuable locally and regionally. Properties attributed to this species include healing inflammation of the ovaries, uterus, bladder, kidneys, prostate, urinary tract, and digestive system (Cano et al. 2006; Rodríguez et al. 2016).
Future projects should collaborate with local communities to create guidance on best practices for sustainable harvesting with self-regulated quotas and communities should be encouraged to help conserve dry forest habitat. It may also be viable for some communities to trial growing the plant ex situ for harvesting.
Conservation assessment. Pedersenia weberbaueri is considered as Near Threatened (NT) following the national IUCN Red List assessment (Castillo-Vera et al. 2017) and is protected by legislation in Peru (Law No 043-2006-AG). However, León et al. (2006) assessed this species as Endangered (EN, B1a) under the global IUCN Red List Criteria. Here we re-assess the species’ status and consider it as Endangered (EN, B2ab(iii,v)) under the global IUCN Red List Criteria as no subpopulation is located within an existing protected area (Fig. 4C).
Rationale for the Red List assessment. Pedersenia weberbaueri is endemic to dry forest of northern interandean Peru where it has a restricted distribution (EOO = 5,182 km2). The population is severely fragmented given that it occurs in isolated patches along a narrow valley. There is a continuing decline in the number of mature individuals due to the extraction for medicinal use. It is therefore listed as Endangered (EN).
Taxonomy. Pedersen (1997) re-surrected the genus Trommsdorffia, now recognized as Pedersenia (see below under Nomenclature), based on pollen characters (Pedersenia argentata (Mart.) Holub; Pedersenia canescens (Humb. & Bonpl. ex Willd.) Holub; Pedersenia cardenasii (Standl.) Holub; Pedersenia costaricensis (Standl.) Holub; Pedersenia hassleriana (Chodat) Pedersen; Pedersenia macrophylla (R.E. Fr.) Holub; Pedersenia pulverulenta (Mart.) Holub; Pedersenia volubilis Borsch, T. Ortuño & M. Nee; and Pedersenia weberbaueri (Suess.) Holub). The nine species now part of Pedersenia were previously considered part of Iresine, a morphologically closely related genus (Borsch et al. 2011). Both genera develop conspicuous white trichomes at the abaxial side of the tepals at maturity, serving for dispersal of the deciduous mature flowers that contain the fruits inside, but molecular data strongly supports the separation of the two genera (Borsch et al. 2011). Differences between Pedersenia and Iresine include tepal trichomes present on abaxial surface and capitate to bilobed stigmas in Pedersenia vs tepal trichomes restricted to the base and two, elongate, filiform branches stigmas in Iresine (Pedersen 1997; Borsch et al. 2011). While we lack molecular data to assign P. weberbaueri to a genus, morphology clearly supports it as member of Pedersenia.
Nomenclature. Pedersenia weberbaueri (Suess.) Holub, Preslia 70(2): 181 (1998).
Basionym: Iresine weberbaueri Suess., Repertorium specierum novarum regni vegetabilis 35: 323. 1934. Type: Östliche Talwand des Marañón, über Uchos, Depart. La Libertad, Prov. Pataz, 1,800-1,900 m, 26 Jul 1914, A. Weberbauer 7022 (Lectotype, designated here: B [B 10 0242394!]; isolectotypes: GH00037120!, MOL00000666!, US 1444981, V0429663F!). Syntype: Östliche Talwand des Marañón über Balsas, Depart. Amazonas, Prov. Chachapoyas, 1000 m, Jun 1904, A. Weberbauer 4267 (M M0241875!, fragment only).
The generic position of Pedersenia weberbaueri (Suess.) Holub has suffered a complicated taxonomic history. Based on the characteristics mentioned in the protologue and without seeing the type specimens, Pedersen (1997) considered Iresine weberbaueri Suess. within the section Trommsdorffia, a taxon that he re-established at its generic level Trommsdorffia Mart. (Martius 1825) and then made the new combination to Trommsdorffia weberbaueri (Suess.) Pedersen. However, the generic name Trommsdorffia had already been used by Bernhardi (1800) for a Compositae genus. Holub (1998) identified this homonymy and substituted the illegitimate Trommsdorffia Mart (1825) with a new generic name Pedersenia Holub, making a new combination Pedersenia weberbaueri (Suess.) Holub. The basionym was incorrectly cited by Holub as Trommsdorffia weberbaueri Suess., although with correct bibliographic data for the protologue of I. weberbaueri (Holub 1998); this error does not, however, invalidate the new combination according to ICN Art. 41.6 (Turland et al. 2018).
Suessenguth (1934) described I. weberbaueri from collections 4267 and 7022 of August Weberbauer that were housed in the Berlin (B) and Gray herbarium (GH), but he did not know about the duplicates of 7022 housed in F, MOL and US. The only specimen of Weberbauer 4267 is missing, presumed to have been destroyed during the bombing of B during World War II. Physical fragments of the collection remain, however, accompanying a nostalgic photograph in herbarium M of what once existed in the Berlin herbarium (B). Fortunately the Weberbauer 7022 collection from herbarium B was saved from this botanical tragedy together with the 20,000 types that were saved from the war (Hiepko 1978).
There are no indications by Karl Suessenguth which of the two cited collections should be taken as the type for I. weberbaueri and he did not specify a holotype. Of the two collections, Weberbauer 7022 represents better material as Weberbauer 4267 is known from a fragment only. Five duplicates of Weberbauer 7022 exist, but only two of these (B and GH) were seen/known by the original author. Duplicate of Weberbauer 7022 at Berlin (B 10 0242394) is chosen because the specimen has a complete stem with branches and inflorescences.
Discussion
Pedersenia weberbaueri is a species that has remained in the shadowed corners of botanical knowledge due to the small number of collections and misidentification (Rodríguez et al. 2016). The high demand for P. weberbaueri as a medicinal plant (Suetake et al. 2007; Castillo-Vera et al. 2017; Seminario et al. 2021) and its depredation in wild state has captured the attention and concern of the scientific community (Rodríguez et al. 2016; Briers 2018). This concern has encouraged further study and field work over the recent years. The new collections add valuable data on the geographic distribution of the species.
We confirm that P. weberbaueri is endemic to Marañón SDTF to a narrow strip (<3 km) on both flanks of the Marañón river from Cajamarca and Amazonas regions in the north to Ancash and Huánuco in the south based on both observed and modelled distribution. The species is known from a total of 50 collections, a number much increased thanks to our intensive field work efforts in 2020 and 2021. The current collections are not equally distributed across the valley, where collections are limited to roads, trails, and access points. This is exemplified by La Libertad which has the highest number of collections with four main road crossing across the river, compared to other departments that have between one or two crossings (Fig. 5D).
The modelled distribution of P. weberbaueri shows that there are suitable areas for the species throughout the southern section of the Marañón valley. The models predicted small areas of suitable habitat outside of the Marañón watershed, where the species has not been able to reach so far due to the physical isolation provided by the 5000 m mountains that surround the valley. These high peaks with moister and colder conditions are acting as effective dispersal barriers to this dry adapted species. Unverified herbarium collections have been made in Huancabamba (Emperaire and Friedberg 1990)(Emperaire & Friedberg 1990), but our modelled distribution shows low habitat suitability in these areas (<30%). Because our SDMs were based on taxonomically verified specimen data with high precision coordinates, we believe that the modelled distribution closely resembles of the suitable habitat areas of the species within the Marañón valley.
Our results show that P. weberbaueri is currently limited to elevations between 900-2,235 m. We are aware of two specimens presumably collected from higher elevations (Chumacero s.n . and Infantes 5650), but these collections are not verifiable due to vague locality information. The elevation range in any given section of the valley is much narrower, however, if we consider the variation in valley depth along its latitudinal position. The deepest part of the southernmost side of the Marañón valley (corresponding to the SDTF) is at 2,500 m while that of the northernmost is at 500 m. This shows that the southernmost populations of P. weberbaueri occupies higher elevation, but are similarly close to the bottom of the river valley growing in close proximity to the river. In fact, our field work showed that the local elevational range of P. weberbaueri is <1,000 m at any given locality. This finding is highly concerning as species with narrow elevational ranges may be thermally specialized and vulnerable to global warming (Laurance et al. 2011).
Our study also highlights a potential cryptic and undescribed species in Pedersenia based on the collection of Acevedo-Rodriguez 11149 (US, NY) identified as P. cf. weberbaueri from Bolivia. Examination of this specimen based on digital images indicate that it represents a yet undescribed species morphologically closely related to P. weberbaueri (see Borsch et al. 2011). This pattern of narrowly restricted con-generics reflects previous studies of other Andean SDTF groups that have similarly narrow ranges and many undescribed species, and include the description of even new genera endemic to individual valley systems (Lewis et al. 2010; Pennington et al. 2010; Särkinen et al. 2011; Särkinen et al. 2012; Gagnon et al. 2015).
In conclusion, P. weberbaueri is a species with small latitudinal, longitudinal, and elevational distribution range. The small geographic and ecological range puts the species to a higher risk category as demonstrated by our IUCN threat assessment of the species as Endangered (EN), due to the high degree of subjected threat from indiscriminate extraction and also by the propose of construction many dams throughout the valley (Finer and Jenkins 2012; Marcelo-Peña et al. 2016). Finally, we are concerned that P. weberbaueri is neglected by the Peruvian protected areas system, as there is no known population within the two recently created Regional Conservation Areas (ACRs) within the Marañón valley, Bosques Tropicales Estacionalmente Secos del Marañón and Bosques Secos del Marañón (Fig. 5C).












 uBio
uBio