Introduction
Harpacticoida is arguably the most diverse order of Copepoda; it comprises 59 families, 645 genera, and over 6000 species (Ahyong et al. 2011). They are chiefly epibenthic organisms (Caramujo 2015), but they can be found in seagrass (Arunachalam & Nair 1988, Jayabarathi et al. 2012) and other aquatic (Fuentes-Reinés & Zoppi de Roa 2013a) and semi-terrestrial habitats (Gaviria & Defaye 2012, Corgosinho et al. 2017); they are also symbiotic with a wide array of vertebrates and invertebrates (Huys 2016).
The earliest records of harpacticoids from Colombia were reported from in freshwater by Thiébaud 1912, and Chappuis 1956 who reported Atheyella furhmani (Thiébaud, 1912). Since then, the Colombian epibenthic microcrustaceans have been documented from other aquatic hábitats (Noodt 1972, Löffler 1972, Michel & Foyo 1976). In the last decades, the taxonomic studies of Colombian Harpacticoida have intensified, especially in coastal marine and estuarine habitats; a significant advance has been achieved for some harpacticoid taxa (Fuentes-Reinés & Zoppi de Roa 2013a,b; Fuentes-Reinés & Gómez 2014, Fuentes-Reinés & Suárez-Morales 2014a,b, Fuentes-Reinés et al. 2015, Suárez-Morales & Fuentes-Reinés 2015, Fuentes-Reinés & Suárez-Morales 2017, Gómez & Fuentes-Reinés 2017a,b, Gómez et al. 2017, Suárez-Morales & Fuentes-Reinés 2018, Fuentes-Reinés & Suárez-Morales 2019, Fuentes-Reinés et al. 2021). Hitherto, 54 nominal species of Harpacticoida have been recorded in Colombia (Gaviria & Aranguren 2019, Gaviria et al. 2019, Fuentes-Reinés & Suárez-Morales 2019, Fuentes-Reinés et al. 2021). Of these, 20 species have been recorded from marine water (Gaviria et al. 2019 and Fuentes-Reinés & Suárez-Morales 2019).
Our goal is to document for the first time the presence of Laophonte cornuta Philippi in Colombia and confirm the occurrence of Distioculus efferata Dana, 1849 and Microsetella norvegica (Boeck, 1865) in northern Colombia.
Material and Method
Biological samples of littoral and limnetic hábitats were collected from Rodadero beach, Gaira Bay, Magdalena, northern Colombia (11°12’30.120”N, 74°13’39.13”W) during fieldwork carried out from August 2015, mainly at the inshore mangrove areas adjacent to an oyster bank. Water salinity, pH, and temperature were measured with a WTW 350i multiparameter equipment. Water samples were collected manually using a 25 L bucket at both littoral and limnetic habitats. Samples were then filtered with a plankton net (mesh size = 55 μm) and preserved in 70% ethanol. Copepods were sorted from all the samples and then processed for taxonomical identification including the examination of the whole specimen and dissection of selected appendages. Dissected appendages were mounted on slides with glycerin and sealed with Canada balsam. The specimens were measured in ventral position, from the anterior end of the rostral área to the posterior margin of the caudal rami
The specimens examined were deposited at the Centro de Colecciones Biológicas of the Universidad Del Magdalena- Colombia (CBUMAG) where they are available for consultation and/or further examination. Morphological terminology follows Huys & Boxshall (1991). The following abbreviations are used in the description: P1-P6 = first to sixth legs, EXP = exopod, ENP = endopod.
Results
The taxonomic analysis of the harpacticoid copepods collected in the surveyed area resulted in the identification of three species belonging to three families and three genera.
Order Harpacticoida Sars, 1903
Superfamily Laophontoidea T. Scott sensu Huys 1990
Family Laophontidae T. Scott 1904
Subfamily Laophontinae T. Scott 1904
Genus Laophonte Philippi 1840
1. Laophonte cornuta Philippi 1840
Material examined. Three adult females from Rodadero beach, Magdalena, Colombia (11°12’30.120”N, 74°13’39.13”W), plankton net, August 2015 to March 2016, coll. JMF-R. (CBUMAG:MEI:0005).
Diagnosis. Female. Total body length of Colombian female specimens = 532 -546 μm (n = 3, average length = 536 μm), measured from anterior margin of cephalothorax to caudal rami. Habitus fusiform (Fig. 1A), urosome 5-segmented (Fig. 1B), anal operculum rounded with a small acute medial projection (Fig. 1C), caudal rami about 3 times as long as wide (Fig. 1D). Antennules 4- segmented (Fig. 1E), first two segments with acute outer thorn-like process (largest on segment 2, Fig. 1E-F). Antenna robust (Fig. 2A), allobasis with single seta, exopod 1-segmented, with four pinnate setae (Fig. 2B). Endopod with spinule row along outer margin bearing 2 spines and 1 long, slender seta laterally (the small spine is not shown); apically with 2 spines, 3 pinnate geniculate setae (Fig. 2A).
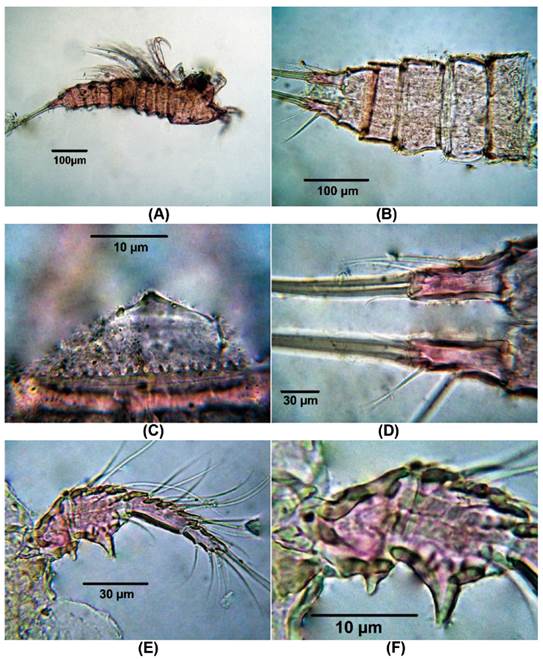
Figure 1 Laophonte cornuta Philippi, 1840. Female from Rodadero beach. (A) Habitus, (B) Urosome, (C) Anal operculum, (D) Caudal rami, (E) Antennule, (F) Idem, first and second segment.
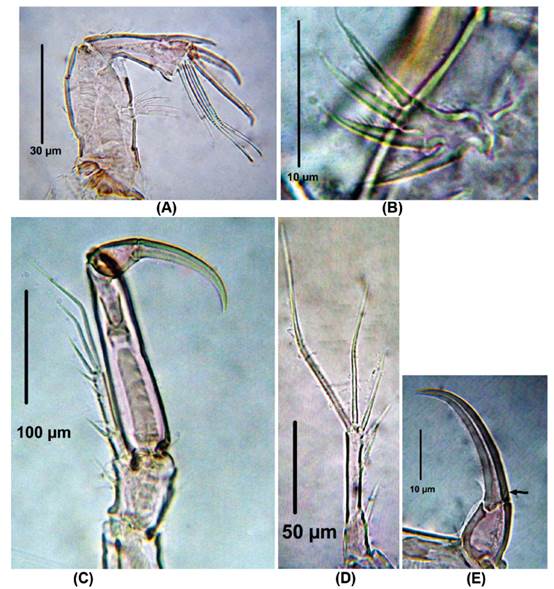
Figure 2 Laophonte cornuta Philippi, 1840. Female from Rodadero beach. (A) Antenna, (B) Antennal exopod, (C) P1, D. P1EXP, (E) Second segment of P1ENP (the arrow shows small seta).
P1. EXP and ENP 2-segmented (Fig. 2B,). First and second exopodal segment with one outer and five elements, respectively (Fig. 2B, C). ENP1 about four times as long as wide, smooth; ENP2 about 1.5 times as long as wide, with single apical seta and one strong claw (Fig. 2D).
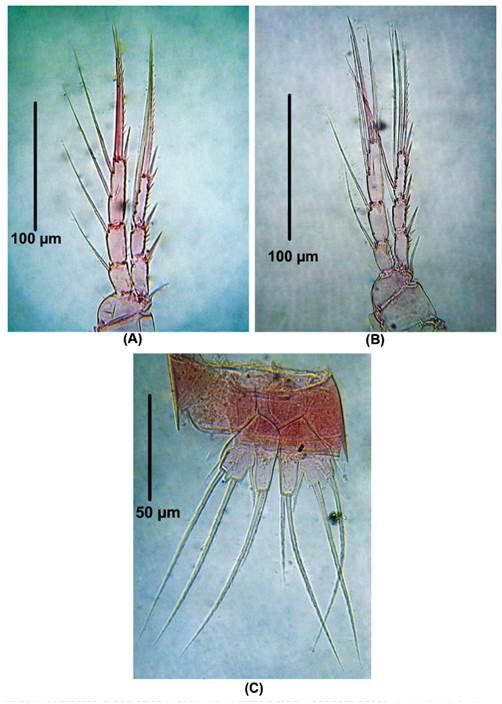
P2 (Fig. 3A). EXP 3-segmented. First exopodal segment without inner seta, second exopodal segment with inner seta and third segment with six elements. ENP 2-segmented. First segment with one inner seta, second segment with four elements.
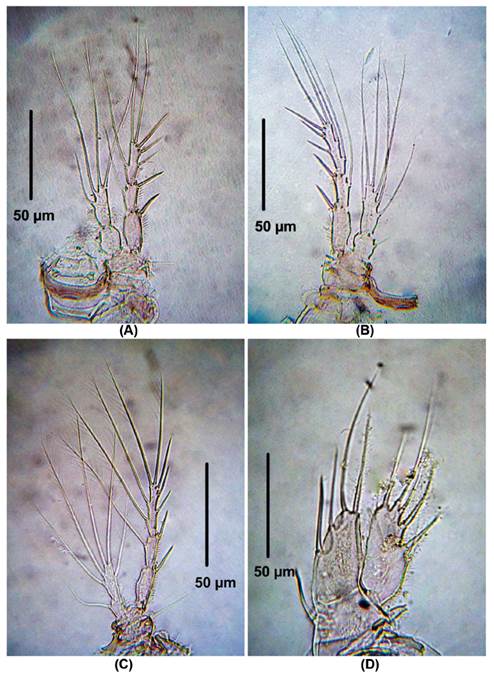
Figure 3 Laophonte cornuta Philippi, 1840. Female from Rodadero beach. (A) P2, (B) P3, (C) P4, (D) P5.
P3 (Fig. 3B). EXP 3-segmented. First exopodal segment without inner seta, second exopodal segment with inner seta, third segment with seven elements. ENP 2-segmented. First segment with one inner seta, second segment with six elements.
P4 (Fig. 3C). EXP as in P3. ENP 2-segmented. First segment with one inner seta, second segment with five elements.
P5 (Fig. 3D). EXP and ENP separated. EXP with six elements. Baseoendopod with moderately long setophore bearing a basal seta. Basoendopodal lobe with two apical and two inner setae.
Remarks. There are three previous attempts to define the species groups within the genus. The first, by Sewell (1940), divided the genus in three groups based on the number of P1 segments, (group I: with 3 segments, group II: with 2 segments, group III: with 1 segment), and subdivided each group according to the number of antennulary segments and female P5 armature formula Laophonte cornuta was included in Group II, iv. Subsequently. Nicholls (1941) subdivided the genus into five subgenera based mainly on the setation of the female P3 ENP2. Lang (1948) disagreed with Nicholls (1941), and characterized Laophonte by the following characters: body cylindrical or sometimes dorso-ventrally flattened , cylindrical caudal rami, female antennule with four to seven segments, with aesthetasc on the third or fourth segments, antennal exopod with four setae, P1EXP 2 or 3 segmented, P2-P4EXP with three segments in both sexes, female P2-P3 two-segmented; he divided the genus in seven species groups: L. cornuta-group, L. serrata-group, L. depressa-group, L. setosa-group, L. inornata group, L. denticornis-group, and L. inopinata-group. Laophonte cornuta is included in the first group. The specimens of Laophonte cornuta examined (three adult females) agree with previous descriptions and illustrations (Sars 1907, Lang 1948, 1965, Gómez et al. 2006, and Sham et al. 2020); we also followed the taxonomic keys by Wells (2007) and Fiers (1986). This species can be easily recognized by a unique combination of characters including: 1) antennal exopod one-segmented with four developed setae, 2) four-segmented female antennule, with acute thorns on segments I and II, 3) caudal rami three times as long as wide, 4) P1-P4 EXP and ENP 2 and 3 segmented respectively, 5) P5 EXP and basoendopod with six and five setae respectively. These distinctive characteristics are present in the specimens from Colombia, thus confirming its identity.
Morphological data on this species have been provided from the United States (Lang 1965), Argentina (Pallares 1968), Japan (Ito 1968), Andaman and Nicobar Islands, India (Wells & Rao 1987), Monaco (Apostolov 1990), France (Gómez & Boyko 2006), Easter Island, Chile (Gómez & Boyko 2006), China (Sham et al. 2020). Our specimen agrees with these morphological descriptions except for several subtle differences: (i) anal operculum with a short projection in Colombia populations (Fig. 1C), whereas specimens from Ester Island (Gómez & Boyko 2006, Figs. 1A - B, 2E - F), Monaco (Apostolov 1990, fig. 4), France (Gómez & Boyko 2006, Figs., 11A, 12A, 13A), China (Sham et al. 2020, fig. 2D) bear a long projection, (ii) P1ENP1 length/width ratio about 4.0 in specimens from Colombia (Fig. 2C) and Ester Island (Gómez & Boyko 2006, fig. 6) vs. 3.6 in Monaco populations (Apostolov 1990, fig. 2). (iii) P1ENP2 length/width ratio 1.5 in specimens from Colombia (present data, figs. 2C,E), and Ester Island (Gómez & Boyko 2006, fig. 6A) vs. 0.52 in Monaco population (Apostolov 1990, fig. 2), (iv) P5ENP almost reaching the end margin of EXP in Colombian populations (Present data, fig. 3D) while in the specimen form Ester Island (Gómez & Boyko 2006, fig. 3B), France (Gómez & Boyko 2006, fig. 13D), Monaco (Apostolov 1990, fig. 3), China (Sham et al. 2020, fig. 3) it reaches the half of EXP ramus.
Our observations of the Colombian population of L. cornuta confirm its wide morphological intraspecific variability: the projection of the anal operculum varies from a plain operculum to a large spiniform structure (Lang 1965, Wells & Rao 1987, present data, Fig. 1C). Members of the family Laophontidae also show a wide range of variability (Wells 2007).
Distribution. Laophonte cornuta has a wide distribution; it has been reported from the Indian Ocean, North Sea, North Atlantic Ocean, and the Economic Exclusive Zone of New Zealand (Shan et al. 2020). This is its first record from Colombia.
Family Miraciidae Dana, 1846 Genus DistioculusHuys & Böttger-Schnack, 1994
2. Distioculus minor (Scott T., 1894)
Material examined: 2 adult males from Roda
dero beach, Magdalena, Colombia (11°12’30.120"N, 74°13’39.13"W), plankton net, August 2015 - March 2016, coll. JMF-R. (CBUMAG:MEI:091 - 0907).
Morphology of male Colombian specimens. Male. Body length = 854 - 896 μm (n = 2, average length = 875 μm). Habitus cyclopiform (Fig. 4A), cephalothorax tape-ring posteriorly, caudal rami 2.6 times as long as wide (Fig. 4B). Antennules 10-segmented, haplocer, geniculation between seventh and eighth segments (Fig. 4C). Antenna represented by short smooth coxa , basis about 2.8 times as long as wide, exopod 1-segmented with two setal elements, endopod with seven elements (Fig. 4D).
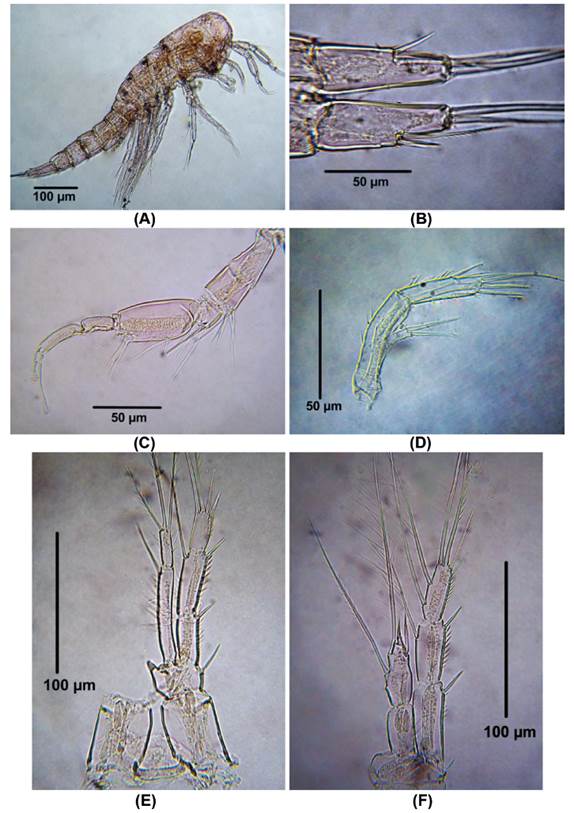
Figure 4 Distioculus minor (Scott T., 1894) male from Rodadero beach. (A) Habitus, (B) caudal rami, (C) Antennule, (D) Antenna, (E) P1, (F) P2.
P1 (Fig. 4E). EXP 3-segmented. First exopodal segment without inner seta, second segment with inner seta, third exopodal segment with four elements. ENP 2-segmented, first endopodal segment long, almost reaching insertion point of inner seta of EXP, with one inner seta. Last endopodal segment with three elements.
P2 (Fig. 4F). EXP 3-segmented. First exopodal segment without inner seta, second segment with inner seta, third exopodal segment with six elements. ENP 2-segmented, first endopodal segment lacking inner seta, second endopodal segment with 2 inner seta, one apical spine and one outer element.
P3 (Fig. 5A). EXP 3- segmented. First exopodal segment without inner seta, second segment with inner seta, third segment with seven elements. ENP 3-segmented, first endopodal segment without inner seta, second segment with two inner setae, third segment with five elements.
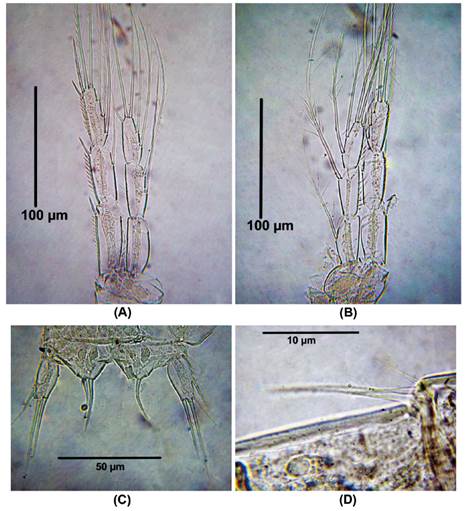
Figure 5 Distioculus minor (Scott T., 1894) male from Rodadero beach. (A) P3, (B) P4, (C) P5, (D) P6.
P4 (Fig. 5B). EXP 3-segmented. First exopodal segment without inner seta, second segment with inner seta, third segment with seven elements. ENP 3-segmented, first and second endopodal segment with one inner setae respectively, third segment with five elements.
P5 with four and two elements on the EXP and ENP, respectively (Figure 5C).
P6 represented by 3 setal elements (Figure 5D).
Remarks. This species was originally described as Miracia minor by T Scott 1894, and its identification was confused because of an incomplete original description (T Scott 1894) and subsequent repetition by other authors. This species was later transferred to the genus Distioculus by Huys & Böttger-Schnack 1994.
Distioculus minor (Scott T. 1894) is the only species of the genus (Walter & Boxshall, 2022) and the specimens examined (two adult males) agree with previous descriptions and illustrations (Lang 1948, Boxshall 1979 (as Miracia minor): Huys & Böttger-Schnack 1994). The male of this species can be easily recognized by a unique combination of characters including: 1) ENP and EXP of P5 with 2 and 4 elements, respectively, 2) P2-P4 ENP1 lacking an inner seta, 3) P3-P4 EXP3 armed with two spines. These distinctive characteristics were observed in the specimens from Colombia thus confirming its identity.
Distribution. Distioculus minor has been recorded in the subtropical and tropical zones of all oceans, approximately between 40° N and 40° (Steuer 1935, Steuer & Hentschel 1937). In Colombia, this species has been reported off San Andrés Island (Martínez-Barragán et al. 2009) but illustrations and morphologic comparisons among the Colombian populations have not been yet provided for this species. The present work is the first documented, illustrated record of this planktonic species in Colombia and Magdalena.
Family Ectinosomatidae
Genus Microsetella Brady & Robertson, 1873
3. Microsetella norvegica (Boeck, 1865)
Material examined: 8 adult females from Rodadero beach, Magdalena, Colombia (11°12’30.120”N, 74°13’39.13”W), plankton net, coll. JMF-R,August 2015 - March 2016. (CBUMAG:MEI:0908)
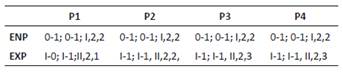
Diagnosis. Body length of Colombia female specimens = 504 - 616 μm (n = 8, average length = 537 μm). Habitus fusiform (Figs. 6A, B), caudal rami short (Fig. 6C), each with long apical caudal seta. Antennules 6-segmented, third segment longest, with aesthetasc (Fig. 6D). P1-P4 EXP and ENP 3-segmented (Figs. 6E-F, 7A-B) with setal formula as follows in Table 1.
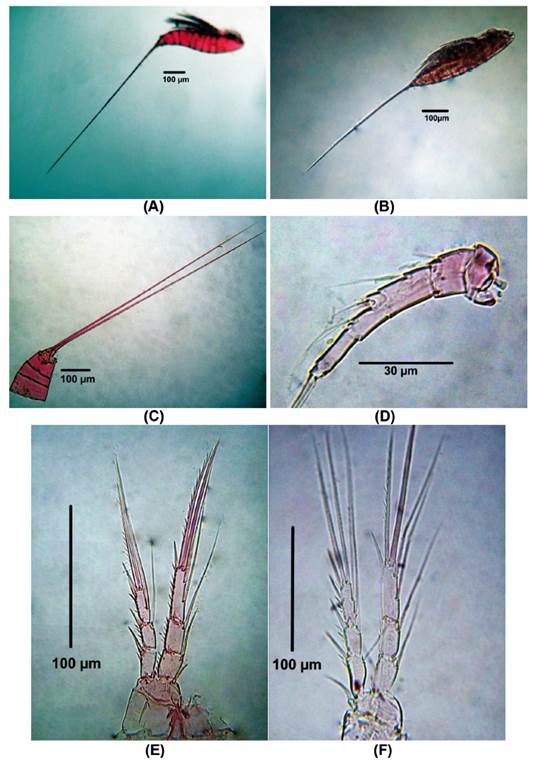
Figure 6 Microsetella norvegica (Boeck, 1865) female from Rodadero beach. (A - B) Habitus, (C) Urosome, (D) Antennule, (E) P1, (F) P2.
P5 (Fig. 7C). Inner expansion of baseoendopod with 2 subequally long thick elements. Exopod with one ventral surface and 3 setal elements (innermost seta shortest)
Remarks. The specimens of Microsetella norvegica examined agree with the descriptions and illustrations by Lang (1948) and Boxshall (1979). This species can be easily distinguished from M. rosea (Dana 1847), its only known congener, by a unique combination of characters including: 1) inner seta on distal margin of caudal ramus usually about twice as long as body, 2) inner seta on female P5 baseoendopod less than half as long as outer seta. These distinctive characteristics were observed in the specimens from our samples, thus confirming its presence in Colombia. This species closely resembles M. rosea (Dana 1847), but they can be separated by several other characters: 1) body length, M. norvegica is smaller (350 - 570 μm, Boxshall 1979, Present data, 504 - 616 μm, Figs. 6A-B) than M. rosea (640 - 850 μm, Boxshall 1979), 2) the inner seta on female P5 basoendopod reaches less than half of the outer seta in M. norvegica (Boxshall 1979, fig. 2I, Present data, Fig. 7C), whereas in M. rosea the inner seta reaches almost the distal end of the outer seta (Boxshall 1979, fig. 2N), 3) the innermost caudal seta is about twice as long as body in M. norvegica (Fig. 6A) whereas it is about as long as body in M. rosea (Wells 2007).
Distribution. This epiplanktonic species has a wide distribution in all oceans (Razouls et al. 2005-2022). In Colombia, it has been hitherto recorded only from Cispatá Bay (Fisco 2006), but the record was considered doubtful (Gaviria et al. 2019) because of the lack of illustrations and morphologic comparisons. This work provides the first illustrated record of this species in Colombia and Magdalena.
Ecology. Specimens herein mentioned were found at a depth of 0.70 m where water temperature varies over the seasons in the range of 30 - 32 °C, salinity is 36.1 psu, and pH 8.3.












 uBio
uBio