Introduction
Trehalose is a disaccharide that consists of two glucose molecules (Sakaguchi 2020). This molecule has been related in various studies for its ability to protect microorganisms against various types of stress as osmotic stress and cold and heat shock (Sakaguchi 2020, Avonce et al. 2006, Kandror et al. 2002), various studies have reported that the accumulation of this sugar begins in the stationary phase (Crowe et al. 2001, Vanaporn & Titball 2020, Reina-Bueno et al. 2012); however, the exact mechanism of action in vivo has not been fully elucidated. One of the peculiarities of this carbohydrate is the reversible change of its structure depending on the physical-chemical conditions to which the molecule is exposed (Jain & Roy 2009).
There are several theories about the mechanism of action of this molecule, such as proteins stabilization through vitrification, that is, trehalose encapsulates the protein in a way that protects it against external damage, maintaining its functional native structure (Elbein et al. 2003, Jain and Roy 2010). The second mechanism that explains its bioprotective capacity is that trehalose can replace water, helping to maintain the molecular structure of proteins against desiccation or preventing the formation of crystals at low temperatures (Schlichter et al. 2001). The third mechanism is the preferential exclusion, since this carbohydrate has a stable bond with water molecules, this bond being even stronger than the interaction between water molecules, preventing the formation of water crystals (Crowe et al. 2001, Sampedro & Uribe 2004). In addition, it has been reported that the protection mechanism of this molecule at low temperatures also involves the stabilization of cell membrane proteins (Chiu et al. 2011).
The trehalose was first described in acidophiles (Liljeqvist et al. 2015), from a metagenomic study of microbial communities of acid mine drainage at low temperature (6 - 10 °C). It is also mentioned that the response to stress caused by low temperatures includes modifications in the membrane to alter its fluidity, changes in the conformation of DNA and RNA, proteins conformation and trehalose synthesis was postulated as a possible cryoprotectant (Liljeqvist et al. 2015, Phadtare 2004).
The presence of multiple pathways of trehalose synthesis in A. ferrivorans would be involved in resistance to low temperatures (Liljeqvist et al. 2015, Guerra-Bieberach et al. 2017). Acidithiobacillus ferrooxidans exhibits the TreT and TreP pathways, while A. ferrivorans exhibits the complete trehalose synthase pathway (TS), TreY/TreZ, and TreT pathway. Additionally, the latter presents the trehalose-6-phosphate synthase (TPS) gene, which participates in the TPS/trehalose-6-phosphate phosphatase (TPP) pathway (Tran et al. 2017).
Operations and mining projects carried out at high altitudes are subject to low ambient temperatures (Bao 2019), which represent a major obstacle for bacterial leaching, given that bacteria are organisms particularly sensitive to extreme temperature variations.
The aim of the present study was to quantify trehalose by an enzymatic method in Acidithiobacillus ferrooxidans ATCC 23270 and Acidithiobacillus ferrivorans CF27 during the oxidation of ferrous ion, zinc sulfide and copper sulfide at temperatures of 28 °C and 15 °C to evaluate if there is a relation with the trehalose production and the ability to survive low temperatures.
Material and methods
Effect of culture medium on growth rate in A. ferrooxidans ATCC 23270 and A. ferrivorans CF27. Acidithiobacillus ferrooxidans ATCC 23270 and A. ferrivorans CF27 strains from the Laboratory of Molecular Biology and Biotechnology from the Universidad Nacional Mayor de San Marcos (UNMSM) were reactivated in 100 mL of modified 9K medium (Amaro et al. 1991, Guiliani and Jerez 2000, Arredondo et al. 1994) until reaching the Log phase. Then, the cells were concentrated and washed with acid water (pH 2.0) (Mamani et al. 2016) and cultured in three liquid culture media: modified 9K medium, modified 9K with ZnS (0.5%) and with CuS (0.5%) (Guerra-Bieberach et al. 2017, Ramírez et al. 2004) as a unique source of energy; with an initial inoculum at 10% and incubated at 28 °C in a shaker at 200 rpm to adapt the strains to each medium. When the cells reached an approximate concentration of 1.0x108 cells/mL, the cells were harvested by centrifugation to initiate the respective growth kinetics. All tests were performed in triplicate and a culture without inoculum was prepared as a negative control. Microbial growth was monitored by counting cells in a Petroff-Hausser chamber (Meruane & Vargas 2003, Osorio et al. 2019) using a Leica DM750 microscope (Leica, Wetzlar, Germany).
Effect of temperature on doubling time in A. ferrooxidans ATCC 23270 and A. ferrivorans CF27. The effect of temperature in each strain was evaluated at 28 and 15 °C, therefore growth kinetics were performed according to the methods described above. The tests were conducted in triplicate, using a culture without inoculum as a negative control and for the kinetics at 15 °C, each strain was adapted (Zhou et al. 2017) and the microbial growth was monitored as described above. The doubling time for each condition were compared (Ccorahua-Santo et al. 2017) using the one-way ANOVA test followed by Tukey’s test using GraphPad Prism version 6.0.0 for Windows (www.graphpad.com). The differences were considered to be significant at P < 0.05 (Ueoka et al. 2016).
Adaptation of the enzymatic method for trehalose quantification. The quantification of trehalose was made following an enzymatic method described by Reina-Bueno et al. (2017, 2012) involving a first reaction with trehalase (Sigma-Aldrich T8778) that results in the release of two molecules of glucose and a second reaction where the released glucose is quantified.
We use different concentrations of commercial trehalose from Sigma-Aldrich T9531 (0, 25, 50, 100, 150, 200, 250, 500, 1000 µM) and the final volume of the second enzymatic reaction was modified from 1 mL to 350 µL to read the results in a microplate lector. Samples were measured every 10 minutes at a wavelength of 420 nm and depending on the trehalose content, the sample turned a pink or purple colour.
Also, a quantification of glucose was made using concentrations of glucose prepared in triplicate (0, 50, 100, 200, 400, 600, 800 and 1000 µM), for which the same trehalose quantification enzymatic protocol was followed to quantify the amount of glucose that could be present in samples.
Quantification of trehalose in Acidithiobacillus by the adapted enzymatic method. The quantification of trehalose was carried out in triplicate and using a control without inoculum as negative control. The culture was harvested by centrifugation during the early stationary phase of growth and then was washed with acid water to remove as much jarosite as possible (Guerra-Bieberach et al. 2017). Then, the pellet was resuspended in 500 µL of sodium acetate buffer (25 mM, pH 5.6) and the cells were disrupted by sonication (15 seconds on and 30 seconds off pulse; for 15 cycles) (Kaksonen et al. 2021).
The lysed cells were centrifuged at 3600 rpm for 6 minutes to separate the intracellular content of the cell debris and 100 µL of the supernatant was used to determine the trehalose content by the enzymatic method and a sample without the addition of trehalase was used to quantify glucose content. The results were expressed in µmol of trehalose per mg of protein (Reina-Bueno et al. 2012, Galleguillos et al. 2018) and the protein quantification was performed following the Bradford method (Bradford 1976, Kruger 1994).
Results
Effect of culture medium on doubling time. The biomass was higher in modified 9K medium with CuS, being 1.78x108 cells/mL for A. ferrooxidans ATCC 23270 and 2.28x108 cells/mL for A. ferrivorans CF27 at the early stationary phase, but the time to reach the stationary phase was delayed from 2 days to 7 days (Fig. 1).

Figure 1 Growth kinetics of A. ferrooxidans ATCC 23270 (█) and A. ferrivorans CF27 (●) using modified 9K medium, modified 9K medium with ZnS (0.5%) and with CuS (0.5%) at 28 °C (A, C, E) and 15 °C (B, D, F). Measurements were performed in triplicates; error bars indicate standard deviations from the mean value.
Also, the doubling time in modified 9K medium is less than the other two culture media in both strains, 0.53 days in A. ferrooxidans and 0.92 days in A. ferrivorans (p=0.99), for the culture medium with ZnS was 2.52 days and 2.32 days (p>0.99) and with CuS was 4.65 days and 3.65 days, respectively (p=0.48).
Effect of temperature on doubling time. The doubling time at 15 °C in modified 9K medium was 2.59 days for A. ferrooxidans ATCC 23270 and 3.42 days for A. ferrivorans CF27. For the medium with CuS was 18.85 and 15.97 days, respectively. Comparing with the doubling time at 28 °C, we found significant differences with a p<0.05 in all the conditions mentioned (Fig. 2).

Figure 2 Comparison of doubling time in A. ferrooxidans ATCC 23270 and A. ferrivorans CF27 at 28°C and 15°C for each tested media. The bar graph shows the mean of the doubling time and error bars indicate standard deviations from the mean value (ns is not significant, *p < 0.05, **p < 0.005, ***p < 0.0005 and ****p < 0.0001).
There are also significative differences in the doubling time of A. ferrivorans CF27 in 9K modified medium with ZnS being 5.32 days comparing at 28 °C (p<0.05). In contrast, there are no significative differences on the doubling time in A. ferrooxidans ATCC 23270 (3.54 days) in modified 9K medium with ZnS at 15 °C comparing at 28 °C (p=0.54).
Also, we report a higher biomass of A. ferrivorans CF27 at the medium with CuS at 15 °C (2.92x108 cells/mL) and a doubling time of 15.97 days, less than A. ferrooxidans ATCC 23270 (p<0.05) that would give an indication of a better adaptation (Fig. 1).
Adaptation of the enzymatic method for trehalose quantification. A minimum quantifiable concentration of trehalose was determined for the enzymatic method of 50 µM due to the modification of the final volume of reaction, lower values were not significant with respect to the values presented by the blank.
Also, it was verified that the incubation time of 30 minutes at 37 °C is appropriate for the second enzymatic reaction, observing the highest absorbance values, despite having varied the final volume of the second reaction (Fig. 3). From the quantified trehalose values, a standard curve was obtained from 50 uM to 1000 uM of trehalose (Fig. 4). The equation was used to calculated the trehalose content with the enzimatic method.

Figure 3 Determination of the incubation time of the second reaction for trehalose quantification. The measurements were performed every ten minutes in triplicates at 420 nm.

Figure 4 Linear regression of trehalose concentrations at 420 nm by enzymatic method of detection. Measurements were performed in triplicates; error bars indicate standard deviations from the mean value.
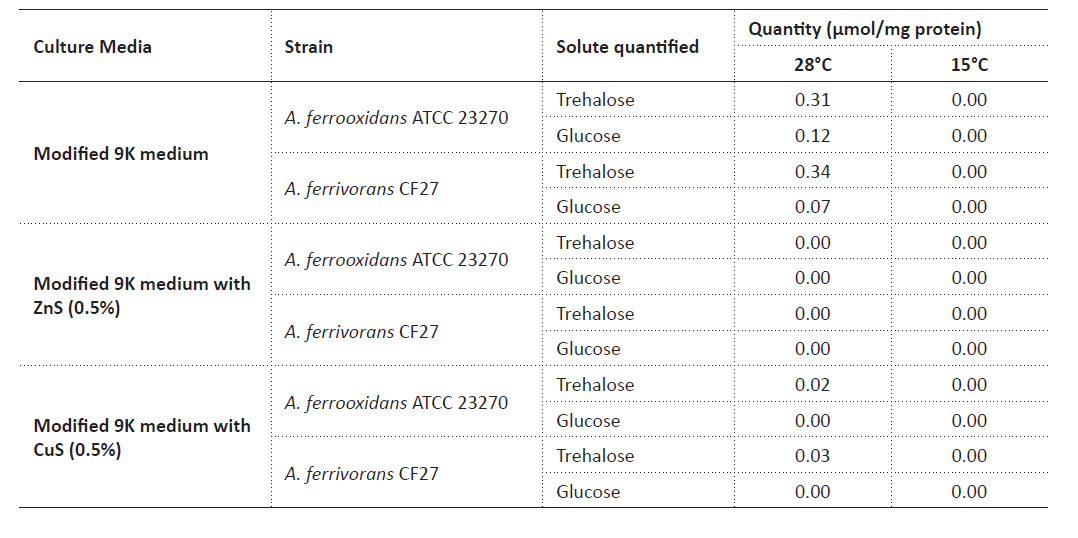
Quantification of trehalose in Acidithiobacillus by the adapted enzymatic method. The highest accumulation of trehalose was observed in modified 9K medium for both strains, being more than ten times the value with CuS. There is also a detection of glucose in both media. At 15 °C, there was no trehalose or glucose detected in any culture media (Table 1).
Discussion
At a temperature of 15 °C, there was an increase in cell concentration in all culture media as compared to 28 °C. CuS culture medium showed a higher number of cells at both temperatures, while the modified 9K medium had a lower cell concentration. This could be attributed to the rapid increase in the concentration of Fe+3 ions in the medium caused by the oxidation of ferrous iron to ferric ion. It is known that the latter negatively affects the growth of cells (Saavedra et al. 2020).
Furthermore, there was a slower growth observed along with an increase in the doubling time at 15°C, which is the opposite of the observations made at 28°C for all three cultured media analyzed. This behaviour has been observed in various species of Acidithiobacillus, including Acidithiobacillus ferrivorans, which is referred to as psychrotolerant, rather than psychrophilic. (Guerra-Bieberach et al. 2017, Hallberg et al. 2010, Escobar et al. 2009).
A higher cell concentration and a shorter doubling time were observed in A. ferrivorans CF27 when using CuS as the sole energy source at 15 °C. This could be attributed to an additional mechanism of copper tolerance, as this strain possesses a higher copy number of genes associated with copper resistance (operon cus) compared to A. ferroxidans ATCC 23270 (Tran et al. 2017, Talla et al. 2013). This difference could explain the shorter doubling time and higher biomass observed at 15°C, a condition that imposes low temperature stress on both strains.
Despite having made a volume variation of the enzymatic method, the incubation time for the second enzymatic reaction was maintained at 30 minutes at 37 °C. This variation allowed the quantification from 50 µM of trehalose, unlike the original method that detected from 1 mM of trehalose (Reina-Bueno et al. 2012).
This method is cost-effective compared to other trehalose quantification methods that used specialized equipment, such as mass spectrometry that can quantify from 0.1 µM but use a triple quadrupole mass spectrometer with an electrospray ionization source (Kretschmer et al. 2016) and HPLC-RID that quantify from 2.2 mM but need a Refractive Index Detector (Hayner et al. 2017), trehalose method can quantify from 50 µM of trehalose and only need a plate reader. This point is important, since the biomass obtained from Acidithiobacillus is relatively smaller due to its small size of 1.0 - 1.68 µm length (Zhang et al. 2020) compared to other bacteria as E. coli with 2.0 µm length (Riley 1999).
Compared to other trehalose quantification methods that require specialized equipment, such as mass spectrometry that can quantify as low as 0.1 µM but requires a triple quadrupole mass spectrometer with an electrospray ionization source (Kretschmer et al. 2016) and HPLC-RID that can quantify as low as 2.2 mM but requires a Refractive Index Detector (Hayner et al. 2017), the trehalose method is a cost-effective alternative. This method can quantify trehalose from as low as 50 µM and only requires a plate reader. This is an important point, given that the biomass obtained from Acidithiobacillus is relatively smaller due to its size, which ranges from 1.0 to 1.68 µm in length, as compared to other bacteria such as E. coli, which has a length of 2.0 µm (Riley 1999).
Despite several previous studies that related the cryoprotective effect of trehalose in acidophilic bacteria (Liljeqvist et al. 2015), the results about the trehalose quantification at 15 °C show that there was very little production of trehalose in all the culture medium used, and therefore it would have a function other than cryoprotection. Trehalose quantification in 9K medium at 28 °C, with the presence of 0.31 µmol trehalose/mg protein for A. ferrooxidans ATCC 23270 and 0.34 µmol trehalose/mg protein for A. ferrivorans CF27, shows that the amount of trehalose is slightly higher in A. ferrooxidans (Table 1) but the variation is not significant between one strain and another (p-value = 0.10).
In addition to the low temperatures resistant, other probable function of this carbohydrate would be related to a further type of protection. Galleguillos et al. (2018) quantified trehalose in acidophiles and also observed an increase of trehalose using a culture medium with iron and sulfur ions and report in A. ferrooxidans 0.01 µmol trehalose/mg protein without the addition of any solute, 0.06 when adding 300 mM MgSO4 in the medium with ferrous sulfide (50 mM) and 0.05 in the medium with elemental sulfide (1%); however, they do not report trehalose in A. ferrivorans (Galleguillos et al. 2018). Other study proposed that trehalose biosynthesis in acidophiles, including the genus Acidithiobacillus, is related to tolerance mechanism to NaCl (Rivera-Araya et al. 2020).
A more recent study was conducted to analyze the variation in gene expression related to trehalose production in Leptospirillum ferriphilum using qPCR and GC-MS techniques. The study employed a medium supplemented with ferrous sulfate and high salinity conditions. The results confirmed an increase in the expression of genes related to trehalose production and subsequently, an increase in trehalose production itself (Rivera-Araya et al. 2019). These findings were consistent with the proposals made by Galleguillos et al. (2018).
A previous study in our laboratory in Acidithiobacillus ferrivorans PQ33 in 9K medium at 5 °C shows that the treS gene is not overexpressed; conversely, it shows a significant decrease in the expression of this gene with respect to the optimum growth temperature of this strain (21 °C). While treZ gene shows a slight decrease in its expression, which is not significant compared to that quantified at 21°C (Guerra-Bieberach et al. 2017).












 uBio
uBio