Introduction
The northern Humboldt Current System exhibits some of the most productive coastal upwelling regions (Graco et al. 2007). Over the past few decades, the high biological productivity has supported the growth of a thriving fishing industry, leading to significant socio-economic impacts in important harbors like Chimbote (Guillén et al. 1977, Tresierra et al. 2007). Consequently, environmental repercussions have arisen, particularly in El Ferrol Bay, where the water and sediment quality has been adversely affected by direct discharge of waste from fish meal factories (Jacinto et al. 1996, 1997b, Orozco et al. 1997, Sánchez et al. 2008, García et al. 2019). The presence of other industrial facilities, including a steel plant, has exacerbated the environmental degradation. Moreover, anthropogenic pressures associated with agricultural runoff and inadequate management of domestic wastewater contribute to the problem (Tresierra et al. 2007). In contrast to other Peruvian bays such as Ancón (11° S), where variations in dissolved oxygen levels are attributed to natural physical processes (Tarazona et al. 2003), the pollution in El Ferrol Bay is the primary factor driving the occurrence of hypoxia in the bottom water, which can even lead to anoxia and the release of hydrogen sulfide (H2S) in certain cases (Tresierra et al. 2007). This release of H2S is lethal to organisms inhabiting the area.
Benthic communities in shallow subtidal areas, particularly along the central coast (09 - 15° S), are periodically exposed to natural oxygen deficiency events. These areas are characterized by a shallower upper boundary of the Oxygen Minimum Zone (OMZ) ranging from 30 to 40 meters (Gutiérrez et al. 2006). The presence of low oxygen conditions can potentially restrict the occurrence of certain macrobenthic organisms. However, these areas benefit from the deposition of organic-rich sediments, which support the growth of other tolerant taxa such as sulphur-oxidizing bacterial mats and meiofaunal groups (Levin et al. 2002, Gutiérrez et al. 2008, Cardich et al. 2015, Aramayo 2018). Nevertheless, the impact of oxygen depletion on benthic fauna in extremely shallow depths, outside the boundaries of OMZs, has received less attention in previous studies.
Benthic foraminifera are widely recognized as reliable indicators of the ecological condition of marine-coastal ecosystems (Balsamo et al. 2012, Schönfeld et al. 2012). The presence of their hard test, composed of materials like calcium carbonate and mineral particles, provides valuable information about past environmental conditions, as these tests can persist in sediment even after the organism's life cycle ends. In the waters off Peru, living benthic foraminifera exhibit high densities but low diversity, which is influenced by the interaction between porewater redox conditions and the quality/quantity of organic matter (Mayor 1998, Cardich et al. 2012, 2015). In shallower environments experiencing oxygen deficiency at the seafloor, lower densities and diversities of benthic foraminifera are observed (Merma-Mora 2016, 2019), and they are even present in relatively shallow layers such as the intertidal zone (Verano 1974). However, the foraminiferal communities in severely impacted marine-coastal ecosystems in Peru, including El Ferrol Bay, remain unexplored.
This study examines the precise composition and spatial distribution patterns of living (stained) benthic foraminifera in surface sediments of El Ferrol Bay, which were collected in September 2015. The primary objective is to investigate the environmental factors that influence their distribution, aiming to enhance our understanding of the species' ecology for calibration purposes and identification of sentinel species.
Material and methods
Studied area. El Ferrol Bay is situated along the northern coast of Peru, encompassing the geographic coordinates of 9.06° and 9.18° S. It is located within the Province of Santa, in the Department of Ancash. El Ferrol Bay is bordered to the north by Isla Santa-Coishco Bay and to the south by Samanco Bay. It is a partially enclosed bay, characterized by the presence of four islands: Blanca, Ferrol Norte, Ferrol Centro, and Ferrol Sur (Tresierra et al. 2007). The deepest section of the bay is located at its entrance. The bay's environmental dynamics are influenced by the input from the Lacramarca river and the presence of fish meal factories to the east, as well as the minerals dock to the north (Fig. 1).
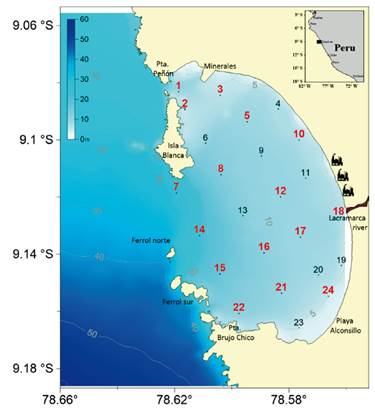
Figure 1 Hydrographic sampling sites in El Ferrol Bay, September 2015. Sediment sampling sites are indicated in red numbers.
In September 2015, a bio-oceanographic monitoring campaign was conducted to characterize the physical, chemical, and biological conditions of the water column and substrate at 24 stations (Fig. 1, Table 1). Hydrographic information was obtained by collecting seawater at standard depths using a 5 L Niskin bottle. From these samples, dissolved oxygen was determined onboard by performing titrations according to the Winkler method modified by Carrit & Carpenter (1966) and pH with a Mettler Toledo potentiometer. Samples for nutrients were frozen until laboratory processing. Temperature was recorded at each point with a mercury thermometer.
Table 1 General information about sampling sites, in El Ferrol Bay, September 2015. Sampling sites for foraminiferal fauna are denoted by bold numbers.
Sediment sampling. Benthic foraminifera were obtained from depths ranging from 4.5 to 27 m (Fig. 1) using a 0.05 m2 van Veen grab. The surface sediment (in contact with the sediment-water interface) was subsampled by scrapping with a spatula. The sediment collected was immediately stored in jars and fixed with a 10% formaldehyde solution buffered with sodium tetraborate (borax). Sediment samples were also collected for the determination of total organic matter (TOM) and grain size (approximately 10 g of wet sediment) and for phytoplankton pigments (chlorophyll-a) (less than 0.5 g of wet sediment) and were stored in tightly sealed plastic bags and aluminium foil packages, respectively, and frozen at -20 °C. The remaining sediment was sieved in a 500 μm mesh bag and the retained material was preserved in jars with formaldehyde at the same concentration.
Benthic foraminifera. In the laboratory, 30 mL of a Rose Bengal solution (1 g/L) was added to the samples and stored for at least two weeks. When processing, sediment volume was determined according to the protocol of Rathburn & Corliss (1994) and Cardich et al. (2012). Sediments were divided into the fractions >150 μm and 63-150 μm by wet-sieving. Specimens were observed and photographed under a NIKON SMZ18 stereo microscope with a NIKON DS-FI3 camera (DS-L4).
For ecological studies on foraminifera, Rose Bengal is used as a vital staining method (Walton 1952) because it is practical and has low cost, although its research efficiency depends on the researcher’s ability to obtain an adequate count. In fact, this technique can generate false positives (Corliss & Emerson 1990, Bernhard 2000), which can be overcome applying the criteria used by Tapia et al. (2008) and Cardich et al. (2012) regarding staining in the chambers. The tonality in the staining of the chambers is another criterion to be considered (Schönfeld et al. 2012). In the case of the porcelaneous forms, having an opaque shell, it was necessary to break the shell to confirm the filling and shade of the protoplasm.
Systematics followed the classification of Sen Gupta (2003) and literature from the region was used to reach the lowest possible taxonomic level (Verano 1974, Resig 1990, Mayor 1998, Cardich et al. 2012, 2015, Merma-Mora 2016). For soft-shelled foraminifera (family Allogromiidae), morphotypes mentioned in Cardich et al. (2012) were used.
Physico-chemical parameters in bottom water. The quantification of nutrients was performed using colorimetric techniques (Strickland & Parsons 1972). Bottom water parameters temperature (T), dissolved oxygen (DO), nitrates (NO3), phosphates (PO) and pH were considered (Table 2).
Sediment parameters. The content of pigments such as chlorophyll-a (chl-a) and phaeopigments (phaeo) was estimated through the fluorometric method described in Gutiérrez et al. (2000) and Aramayo et al. (2021). The chlorophyll-a and phaeopigments ratio (chl-a/phaeo) was calculated as a descriptor of the available labile food. The total organic matter and carbonate content was obtained with the weight loss on ignition method (Dean 1974). Finally, the particle size analysis was based on sieving for the gravel, sand, and mud (silt+clay) fraction according to Ingram (1971).
Data analysis. Foraminiferal density was standardized to a volume of 50 cm3 of sediment. Species richness (S), defined as the number of taxa, Shannon-Wiener (H), Fisher's (α) and Pielou evenness (J') indices were calculated based on untransformed data. Sedimentary variables were plotted by isoline maps, using the krigging option as an interpolation method.
The Principal Coordinates Analysis (PCoA) ordination method was employed to graphically visualize similarities in distribution species among sites, using the Spearman Rank Correlation as a measure of resemblance prior to fourth root transformed on quantitative foraminiferal data. Inner- and outer bay were defined using the 10 m isobath as a boundary. To explore associations between foraminifera and environment, the distance-based linear model (DistLM) was used. This multivariate technique assesses the contribution of the environmental variables included in this study to the variability observed in the assemblage structure of benthic foraminifera. Estimates of the fitted model were obtained by selecting the forward procedure and Akaike information criterion (AIC). The model was visualized in a multidimensional space using distance-based redundancy analysis (dbRDA). All analytical procedures were performed using PRIMER 6.0 and PERMANOVA+ (Primer-e).
Results
Bottom environmental variables. Dissolved oxygen fluctuated between 0.75 and 5.49 mL/L with the highest values in front of Lacramarca river and the lowest at the deeper locations (Table 2). All sites were above a pH of 7.7, with lower values in the south of Isla Blanca. Most sites studied showed high concentrations of nitrates (>10 μM) with the lowest in sites 10 and 11 (<2 μM) while a narrow range of phosphates did not exceed 3 μM in all sites of the bay (Table 2).
Table 2 Environmental and sedimentary parameters at the bottom water and at the surface sediment: temperature (°C), dissolved oxygen (mL/L), pH, nitrate (µM), phosphate (µM), chl-a/phaeo ratio, percentages of total organic matter, gravel, sand, mud and carbonates (CaCO3).
| Site | Water depth (m) | Bottom water | Surface sediment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | DO (mL/L) | pH | NO3 (μM) | P (μM) | chl-a/phaeo | TOM (%) | Gravel (%) | Sand (%) | Mud (%) | CaCO3 (%) | |||||
| 1 | 9 | 17.3 | 1.36 | 7.81 | 18.36 | 2.52 | 0.15 | 8.58 | 2.2 | 81.33 | 16.47 | 22.66 | |||
| 2 | 7 | 17.4 | 1.36 | 7.82 | 16.97 | 2.41 | 0.17 | 9.04 | 0 | 45.07 | 54.43 | 12.3 | |||
| 3 | 6 | 18.4 | 3.89 | 8.04 | 7.83 | 1.52 | 0.14 | 3.52 | 0 | 17.32 | 82.68 | 5.82 | |||
| 4 | 7.5 | 18.1 | 2.08 | 7.95 | 7.57 | 1.24 | -- | -- | -- | -- | -- | -- | |||
| 5 | 8.5 | 17.9 | 1.96 | 7.88 | 12.34 | 2.35 | 0.26 | 3.62 | 0 | 86.22 | 13.78 | 4.88 | |||
| 6 | 10.5 | 17.1 | 1.67 | 7.82 | 14.04 | 2.33 | -- | -- | -- | -- | -- | -- | |||
| 7 | 27 | 16.8 | 0.75 | 7.78 | 21.79 | 2.93 | 0.18 | 3.25 | 0 | 82.28 | 17.72 | 3.21 | |||
| 8 | 11 | 17.1 | 1.42 | 7.84 | 17.3 | 2.56 | 0.27 | 3.37 | 0 | 66.64 | 33.36 | 2.47 | |||
| 9 | 9 | 17.3 | 1.9 | 7.86 | 15.24 | 2.48 | -- | -- | -- | -- | -- | -- | |||
| 10 | 7 | 19.4 | 4.12 | 8.09 | 1.72 | 1.07 | 0.16 | -- | -- | -- | -- | -- | |||
| 11 | 7 | 18.8 | 3.17 | 8.00 | 1.99 | 1.24 | -- | -- | -- | -- | -- | -- | |||
| 12 | 9.5 | 17.5 | 2.92 | 7.90 | 15.17 | 2.36 | 0.17 | 10.4 | 0 | 40.82 | 59.18 | 11.07 | |||
| 13 | 12 | 17.0 | 1.23 | 7.77 | 16.52 | 2.27 | -- | -- | -- | -- | -- | -- | |||
| 14 | 22 | 16.7 | 0.97 | 7.82 | 20.45 | 2.22 | 0.17 | 10.95 | 0 | 56.83 | 43.17 | 12.99 | |||
| 15 | 16 | 16.9 | 2.18 | 7.85 | 15.98 | 2.03 | 0.18 | 9.47 | 0 | 42.48 | 57.52 | 19.87 | |||
| 16 | 11 | 16.9 | 2.12 | 7.86 | 14.02 | 2.32 | 0.19 | 8.81 | 0 | 8.98 | 91.02 | 11.07 | |||
| 17 | 8.5 | 17.2 | 3.1 | 7.93 | 14.03 | 2.19 | 0.22 | 6.58 | 0 | 2.28 | 97.72 | 7.61 | |||
| 18 | 4.5 | 20.3 | 5.49 | 8.14 | 11.92 | 1.52 | 0.20 | 2.16 | 0 | 79.84 | 20.16 | 2.76 | |||
| 19 | 6 | 18.1 | 5.26 | 8.14 | 8.84 | 1.7 | -- | -- | -- | -- | -- | -- | |||
| 20 | 8 | 17.4 | 2.36 | 7.80 | 13 | 2.26 | -- | -- | -- | -- | -- | -- | |||
| 21 | 10 | 17.1 | 1.92 | 7.87 | 12.67 | 2.66 | 0.21 | 6.23 | 0 | 23.01 | 76.99 | 23.34 | |||
| 22 | 11 | 17.1 | 2.53 | 7.90 | 13.86 | 1.9 | 0.17 | -- | -- | -- | -- | -- | |||
| 23 | 5 | 17.9 | 4.22 | 7.95 | 14.09 | 1.92 | -- | -- | -- | -- | -- | -- | |||
| 24 | 5 | 17.8 | 3.4 | 7.97 | 10.38 | 2.16 | 0.19 | 9.29 | 0 | 88.66 | 11.34 | 5.43 | |||
Sedimentary characteristics. The content of sand and mud were complementary (Fig. 2a,b) since gravel was only recorded in site 1 (2.2%). High concentrations of sand, mud and carbonates were found in sites 24, 17 and 21, respectively (Table 2). Chl-a/phaeo ratio showed peaks of concentration in sites 5 and 8 (Fig. 2c). In turn, TOM showed high values near to El Ferrol north island (Fig. 2d).
Species composition. A total of 12 taxa was recorded; 8 of them were identified at species level, Bolivina costata d’Orbigny, 1839, Bolivina striatula Cushman, 1922, Buliminella tenuata Cushman, 1927, Buliminella elegantissima d’Orbigny, 1839, Nonionella auris d’Orbigny, 1839, Virgulinella fragilis Grindell & Collen, 1976, Discorbis peruvianus d’Orbigny, 1839, Buccella peruviana d’Orbigny, 1839; and the others at genus level, Nonionella sp., Globocassidulina spp., Quinqueloculina sp. y Allogromia sp. 1. Bolivina costata was the most dominant species; present for more than 50% in all sites, accompanied by other species such as B. elegantissima, N. auris, V. fragilis, B. peruviana; and Allogromia sp. 1 with relative abundances >5% in each site (Fig. 3). Representative living (stained) and dead (unstained) benthic foraminiferal species are shown in Fig. 4.
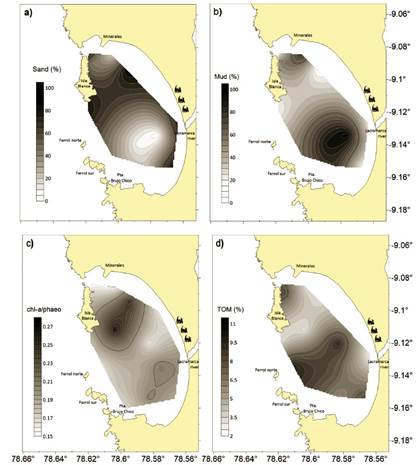
Figure 2 Isoline map of sedimentary characteristics in El Ferrol Bay, September 2015: a) sand (%), b) mud (%), c) chl-a/phaeo ratio and d) total organic matter (TOM).
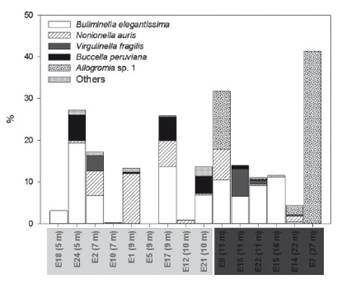
Figure 3 Relative density (%) of the six dominant foraminiferal species in El Ferrol Bay, 2015, except for B. costata, which was not included for a better visualization, composed more than 50% in each sampling site. The inner- and outer-bay sites are indicated in gray and dark gray boxes.
Density and diversity. Living foraminiferal density ranged between 87 and 14576 ind/50cm3 with higher values in the south of the bay (Table 3). Richness ranged between 1 (site 5) and 9 (site 22) taxa. The highest values of Shannon-Wiever, Fisher and Pielou evenness (J’) diversity indices were located in the deepest sites (Table 3). Rather, high dominance was restricted to the shallower sites off Chimbote. Stained shells retained in the small fraction (63 - 150 μm) contributed more than 80% of the abundances in all sites whereas only the sites 7, 8 and 17 showed that the coarser fraction (150 - 500 μm) contributed more than 10%. At the same time, these latter sites showed similar values in dominance (Table 3).
Table 3 Ecological parameters in El Ferrol Bay, September 2015: Foraminiferal density (ind/50cm3), contribution of each size fractions (>150 µm and 63-150 µm), richness (S), Shannon-Wiener (H’, log2), Fisher (α), Pielou evenness (J’) and dominance (λ).
| Site | Water depth (m) | Density (ind/50cm3) | >150 μm (%) | 63-150 μm (%) | S | H' | α | J' | λ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 3916 | 2.66 | 97.34 | 5 | 0.64 | 0.57 | 0.28 | 0.77 |
| 2 | 7 | 893 | 0.75 | 99.25 | 5 | 0.96 | 0.70 | 0.41 | 0.70 |
| 5 | 8.5 | 740 | 0.00 | 100.00 | 1 | 0.00 | 0.11 | -- | 1.00 |
| 7 | 27 | 87 | 16.30 | 83.70 | 2 | 0.98 | 0.37 | 0.98 | 0.52 |
| 8 | 11 | 1150 | 14.78 | 85.22 | 4 | 1.39 | 0.52 | 0.70 | 0.50 |
| 10 | 7 | 1257 | 2.39 | 97.61 | 2 | 0.03 | 0.23 | 0.03 | 0.99 |
| 12 | 9.5 | 226 | 0.88 | 99.12 | 2 | 0.07 | 0.30 | 0.07 | 0.98 |
| 14 | 22 | 1135 | 3.96 | 96.04 | 6 | 0.38 | 1.27 | 0.15 | 0.91 |
| 15 | 16 | 480 | 0.00 | 100.00 | 2 | 0.50 | 0.27 | 0.50 | 0.80 |
| 16 | 11 | 244 | 1.64 | 98.36 | 4 | 0.76 | 0.68 | 0.38 | 0.75 |
| 17 | 8.5 | 440 | 11.11 | 88.89 | 5 | 1.22 | 0.79 | 0.52 | 0.58 |
| 18 | 4.5 | 648 | 0.31 | 99.69 | 2 | 0.20 | 0.26 | 0.20 | 0.94 |
| 21 | 10 | 880 | 6.06 | 93.94 | 5 | 0.79 | 0.70 | 0.34 | 0.75 |
| 22 | 11 | 14576 | 0.99 | 99.01 | 9 | 0.62 | 0.93 | 0.19 | 0.80 |
| 24 | 5 | 4563 | 3.29 | 96.71 | 5 | 1.16 | 0.55 | 0.50 | 0.57 |

Figure 4 Hard-shelled foraminiferal species found in El Ferrol Bay, September 2015. Living (stained) shells of: a) Bolivina costata (dorsal view), b) Buliminella elegantissima, c) Nonionella auris (umbilical view), d) Bolivina striatula, e) Buccella peruviana (umbilical view). Dead (empty) shells of: f) Bolivina costata (dorsal view), g) Nonionella auris (umbilical view), h) ) Bolivina striatula,, i) Discorbis peruvianus (umbilical view), j) Discorbis peruvianus (dorsal view), k) Discorbis corus (dorsal view), l) Buliminella elegantissima, m) Buccella peruviana (umbilical view), n) Textularia spp., o) Quinqueloculina seminulum, p) Robulus sp., q) Virgulinella fragilis, r) Haeuslerella hoeglundi , s) Quinqueloculina spp., t) Remaneica kelletae. Bar scale=100 µm.
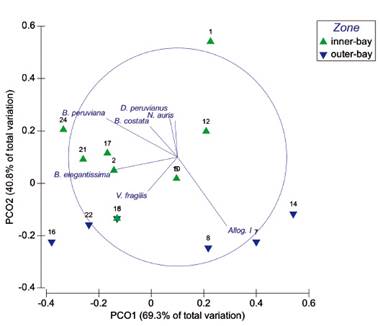
Figure 5 PCoA ordination diagram based on Spearman-rank measure from fourth root transformed abundance data of the dominant foraminiferal species from all the sampling sites in El Ferrol Bay. Longitude of superimposed vectors corresponds to the magnitude of the contribution (r>0.25) of species abundance at each site related to PCoA axes.
Table 4 Results of DistLM routine for (a) marginal and (b) sequential tests. Akaike’s information criterion (AIC). Prop. and Cumul (%): percentage of variance in species data explained by that variable or the model, respectively.
| Variable | pseudo-F | p | Prop. (%) | Cumul. (%) | AIC | df |
|---|---|---|---|---|---|---|
| Marginal tests | ||||||
| Temp | 0.260 | 0.86 | 2.31 | |||
| Depth | 4.455 | 0.01 | 28.83 | |||
| DO | 1.604 | 0.22 | 12.73 | |||
| NO3 | 5.112 | 0.01 | 31.73 | |||
| pH | 1.265 | 0.32 | 10.31 | |||
| chl-a/phaeo | 0.780 | 0.51 | 6.62 | |||
| TOM | 1.555 | 0.27 | 12.39 | |||
| Sand | 2.119 | 0.15 | 16.15 | |||
| Sequential tests | ||||||
| +NO3 | 5.112 | 0.01 | 28.83 | 31.73 | -30.24 | 11 |
| +Depth | 2.811 | 0.08 | 14.69 | 46.71 | -31.46 | 10 |
| +Sand | 2.713 | 0.1 | 16.29 | 59.05 | -32.88 | 9 |
| +TOM | 2.919 | 0.09 | 18.53 | 70.00 | -34.93 | 8 |
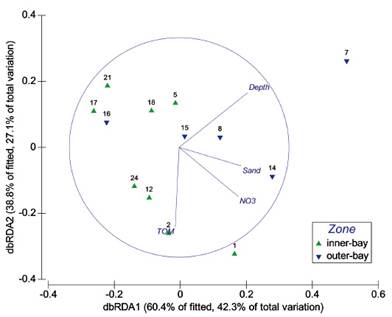
Figure 6 RDA projection biplot based on Spearman-rank measure of fourth root taxa abundances. Numbers indicate sampling sites. Only environmental predictors selected by the forward selection model are shown.
Multivariate analysis. PCoA ordination showed differences in the assemblage composition between the inner- and the outer-bay sites (Fig. 5). Resembling to Fig. 3, superimposed vectors of B. costata, B. elegantissima y B. peruviana; N. auris, D. peruvianus and V. fragilis were oriented to the sites in which these species were important. For instance, a greater contribution of Allogromia sp. 1 was observed in deeper sites (Fig. 5).
The DistLM marginal analysis indicated that depth and nitrates were the main significant predictor explaining the greatest amount of variability in the assemblage (Table 4). These two variables were significant among four explanatory variables selected by the sequential test (Table 4) and are shown in the dbRDA plot (Fig. 6). The first two axes on the biplot projection explained more than 98% of fitted model which corresponds to 39% of the variation in the species (Fig. 6). The first axis was correlated with depth and sand in a lesser extent while the second axis was inversely correlated to TOM. Conspicuously, nitrates tended to be positively correlated with sand (Fig. 6).
Discussion
Environmental conditions: The sampling survey was conducted in September during the closed fishing season, when fish meal factories were not in operation. Dissolved oxygen (DO) levels observed in all surveyed sites were comparable to concentrations (>1 mL/L) reported by Guzmán et al. (1997), Jacinto et al. (1997a), and Tresierra et al. (2007) during periods of low fishing activity. However, deeper sites exhibited severe hypoxia (<1 mL/L) (Table 2). pH values above 7.6 were higher compared to measurements taken during the intense fishing season reported by Jacinto et al. (1996). While salinity was not considered in this study, its influence should be assessed.
Bottom phosphate concentrations were similar to the average values (2.2 µM) recorded by García et al. (2019) during the season (November 2018). However, the average nitrate values (8.9 µM) reported by these authors were lower compared to our data. Previous studies have emphasized the role of coastal upwelling in shaping the physical and chemical distribution patterns in the bay (Orozco et al. 1997, Sánchez et al. 2008). Shallower depths become enriched with nitrate from upwelled waters originating from greater depths in the open sea. Thus, the high nitrate content observed in our study may be linked to a more relaxed OMZ during the 2015 - 2016 El Niño event, as its influence extended to central Peru (Graco et al. 2016). This water column condition could enhance primary production, leading to oxygen depletion including the seafloor. Furthermore, the Lacramarca River serves as an important nitrogen source for the bay, exhibiting high values during certain seasons (García et al. 2015). On the other hand, the spatial distribution of phosphates typically shows higher concentrations near the shoreline (Jacinto et al. 1996, 1997a, b, Guzmán et al. 1997), which is attributed to industrial, agricultural, and anthropogenic inputs (Orozco et al. 1997).
Significant concentrations of TOM were observed in the central area of El Ferrol Bay, particularly near the bay's mouth (Fig. 2, Fig. 5). El Ferrol Bay stands out for its high TOM content (>9%) compared to other bays in the region (García et al. 2019), with predominantly muddy sand sediments (Ganoza et al. 2020). The elevated TOM levels can be attributed to the discharges from industrial and domestic sources, as well as the primary productivity in the water column, as emphasized by Tresierra et al. (2007). According to Ganoza et al. (2020), the central zone near the bay's mouth is expected to have lower mud accumulation due to increased exposure to resuspension and exportation towards the open sea. The hydrodynamic regime, including near-bottom currents, plays a role in the deposition of recent material, as indicated by the distribution of the chl-a/phaeo ratio (Fig. 2c).
Spatial distribution and environmental predictors. The majority of benthic foraminiferal species found in this study have previously been recorded in the inner-shelf region (Cardich et al. 2012, 2015, Romero 2021), as well as in other embayments such as Paracas Bay (Merma-Mora 2019), and even in the intertidal zone (Verano 1974). Bolivina costata and B. elegantissima were the most abundant species in the studied area. Bolivina costata was the dominant species, while B. elegantissima was the co-dominant species in most of the sites (Fig. 3). Only these two species were found in sites adjacent to fish processing plants (Table 3). Both species have been described as opportunistic detritivores associated with relatively low concentrations of dissolved oxygen (e.g., dysoxia) (Paéz et al. 2001). This observation aligns with the conceptual model proposed by Merma-Mora (2019), in which these two species characterize sheltered environments with high organic loads and periodic redox oscillations.
No spatial distribution pattern was found in the living benthic foraminiferal assemblage, despite a significant relationship between bottom dissolved nitrates and the model fit (Table 4). It has been demonstrated that the reduction of nitrate is the preferred respiration mechanism in certain benthic foraminiferal species within the Peruvian OMZ (Glock et al. 2019). Among the species analysed by these authors, shallow-water species represented by B. costata exhibited low denitrification capacities. Therefore, the absence of a spatial distribution pattern is reasonable considering that this species dominated the entire assemblage.
In shallower sites (<10 m), the presence of B. peruviana was limited to the southern part of the bay, contributing to a relatively low proportion (~6%) of the assemblage (Fig. 3). A recent study indicates that this species primarily inhabits shallower depths characterized by more oxygenated and warmer waters (Merma-Mora 2019). This finding confirms its successful representation in the continental shelf (Bernasconi 2020) and even in polluted areas of the South Atlantic Ocean (Eichler et al. 2012).
The soft-walled foraminifera Allogromia sp. exhibited high abundance (>40% at 27 m depth) in sites with a substantial proportion of sand near Isla Blanca (Fig. 3, 5). Previous studies have identified this species as a spherical-type taxon in silty-clay mud sediments of the inner-shelf off Callao (Cardich et al. 2012, 2015, Aramayo 2018). Thus, our observations differ from those of Merma-Mora (2019) and Gooday (2002), who suggested that fine-grained substrates are more suitable for the settlement of this group.
The species composition of both living and dead assemblages indicates the alternation between anoxic and oxygenated conditions in the bay. Environmental data from the area have documented periods of oxygenation and hypoxia (Sánchez et al. 2008). The relatively high proportion of certain calcareous species, such as Nonionella auris, Virgulinella fragilis, and Allogromia sp., all known to be tolerant to sulphidic conditions in the inner-shelf off Callao (Cardich et al. 2012), suggests that the bottom environment of the bay is exposed to anoxic events. Furthermore, empty calcareous shells are well-preserved in the sediment under these conditions. In contrast, the presence of other hard-shelled species (both hyaline and agglutinated) in surface sediments indicates periods of oxygenated conditions in El Ferrol Bay.
The potential utility of shallow-water benthic foraminifera in monitoring studies. Due to the historical discharge of industrial wastes and sewage into El Ferrol Bay (Tresierra et al. 2007), there have been numerous reports of anoxic episodes (<0.2 mL/L) occurring during industrial fishing and fish meal production activities (Jacinto et al. 1996, 1997b, Sánchez et al. 2008, Ganoza et al. 2020) as well as during the fishing closed season (Jacinto et al. 1997a). The bay's geomorphological configuration and the prolonged residence time of water masses exacerbate contamination in the area (Jacinto et al., 1996). This chronic situation is particularly evident in the northern part of the bay, where macrofauna is scarce (Orozco et al. 1997, Tresierra et al. 2007).
In our study, we demonstrate that certain benthic foraminifera are capable of thriving under these hostile conditions, exhibiting high population densities. This adaptation has also been observed in other polluted environments, where the diversity of major taxonomic groups is reduced (Rabalais et al. 2010). Further investigations are warranted to evaluate the potential use of chemical signatures, such as trace metals, within foraminiferal shells as indicators of pollution. Additionally, a better understanding of their vertical distribution within the sediment column is essential for calibration in paleo ecological records.
Conclusions
El Ferrol Bay exhibited hypoxic conditions at the bottom with dissolved nitrate concentrations during September 2015. The distribution of grain sizes (sand/mud) indicated the presence of high-energy and low-energy environments adjacent to Isla Blanca and in the central area of the bay, respectively. The latter environment may promote the accumulation of high organic loadings (TOM) in surface sediments compared to other sedimentary settings in the region.
The living benthic foraminiferal fauna in this bay is characterized by a poor species composition but moderate to high population densities. The dominant species in the assemblage was predominantly B. costata, followed by B. elegantissima, along with eight other calcareous hyaline species, one porcelanous species, and one soft-shelled taxon. Conversely, the presence of numerous empty shells in the dead assemblage suggests periods of more oxygenated conditions, although hydrodynamic transport might also have influenced their distribution. Therefore, the preservation of hard-shelled foraminifera serves as a valuable tool for assessing the environmental quality of the bay.
No clear spatial distribution pattern was observed in the living benthic foraminiferal assemblage in relation to any specific environmental parameter, despite the significant correlation between bottom dissolved nitrates and the assemblage in our multivariate analysis.












 uBio
uBio 


