Introduction
The Española Lagoon is a shallow freshwater ecosystem situated within the Meta Department, located in the Orinoquía region of Colombia, exhibiting depths that vary between 3 and 8 m. This wetland encompasses an approximate surface area of 0.35 km2 and is the largest lagoon system within the River Manacacías basin. Remarkably, no prior investigations have been conducted concerning the cladoceran fauna within this ecosystem. Taxonomic investigations pertaining to Colombian cladoceran fauna within shallow freshwater habitats have been actively pursued over the past few decades (Fuentes-Reinés et al. 2012, Fuentes-Reinés & Elmoor-Loureiro 2015, Fuentes-Reinés 2014, Fuentes-Reinés et al. 2019, Andrade-Sossa et al. 2020, Garibian et al. 2021a, Fuentes-Reinés et al. 2022, Fuentes-Reinés et al. 2023, Fuentes-Reinés et al. 2024).
However, our understanding of the diversity of this taxonomic group remains incomplete across the entire Orinoquía region. To date, only five species have been documented within this region (Fuentes-Reinés et al. 2018). Cladocerans serve a pivotal role in nutrient recycling and the transfer of energy within aquatic food webs, facilitating the reduction of phytoplankton biomass (Hulot et al. 2014).
The superorder comprises more than 700 species and 100 genera (Jeong et al. 2015, Smirnov 2017). Most biologists regard cladocerans as planktonic; nevertheless, they are more diverse in the littoral area, dwelling amid freshwater macrophytes (Maia-Barbosa et al. 2008).
Based on samples collected from La Española lagoon, we provide a list of species of the cladocerans dwelling in this lagoon system. In addition, descriptive notes, comparative comments on morphology and variability, and complementary figures are provided for the new occurrence (18 species) of Colombian cladoceran fauna. This survey contributes to increasing the knowledge of this group in Colombia and the Neotropical region.
Material and Methods
La Española lagoon is located in the basin of river Manacacías, Meta Department, Colombia (4°20'36.6" N, 72°03'35.2" W) (Fig. 1); in the wavy savannah of the region Orinoquía to the west of the country, which is characterized by bimodal regimen (dry season: December-February; rainy season: march-November) and its varied water richness (Bustamante 2018). Plankton samples were taken in November 2020 with horizontal drags using a plankton net of mesh size of 55 μm and preserved in 96% ethanol.
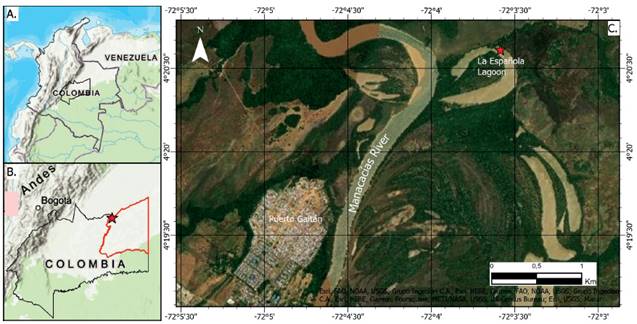
Figure 1 Location of La Española Lagoon. A. Colombia; B. Meta Department, red star indicating the sampling point; C. Aerial view of La Española Lagoon (red star).
Specimens were sorted from all the samples, transferred to 70% ethanol, and then processed for taxonomical identification, including examining the whole specimen and dissection of selected appendages. The dissection was made with two sharp tungsten needles. The dissected appendices were mounted in a drop of glycerin on slides and sealed with Canada balm.
The morphology of appendages and other structures was examined using a compound microscope at 1000× magnification. Images were acquired with a Kodak Easy Share C140 digital camera connected to the microscope. The individuals were positioned in a lateral position and measured from the anterior end of the rostral area to the posterior margin of the valve using an eye micrometer.
The description style follows Van Damme (2016), splitting the anatomic structures into general habitus, carapace, cephalic, thoracic limbs, and postabdomen. The enumeration of limb setae follows the criteria of Kotov (2000a, 2000b) and Kotov et al. (2010).
Identifications of these species were made according to Smirnov (1996), Sinev (1998), Hudec (2000), Sinev (2001), Fuentes-Reinés et al. (2012), Fuentes-Reinés and Zoppi de Roa (2013), Sousa et al. (2018), Sousa and Elmoor-Loureiro (2019, 2021), and Sinev (2020).
The dissected animals (slides) and vouchers were deposited at the Museo de Historia Natural Unillanos (MHNU), Colombia, where they are available for consultation and/or further examination.
Results
The taxonomic analysis of the cladoceran specimens collected yielded the identification of thirty-five species. These are all new records for La Española lagoon. Of these, ten species and one genus are new records for Colombia. They belong to seven families and twenty-six genera (Table 1). Chydoridae showed the highest species richness (23), followed by Macrothricidae (4), Sididae (3), and Bosminidae with 2. The remaining families were represented by one species each. Brief remarks and descriptions, with illustrations (Figs. 2 ‒ 8), for the relevant species are given below.
Table 1 General distribution of different species of Cladocera in the Española lagoon, Meta. * New record for Colombia.
| Taxon | Distribution in Colombia | World distribution | References to Colombia |
|---|---|---|---|
| CTENOPODA | |||
| SIDIDAE | |||
| 1.Diaphanosoma fluviatile* Hansen 1899 | Orinoquía region | Neotropical and Neartic regions (López et al. 2008) | Present data |
| 2.Latonopsis australis Sars, 1888 -group | Caribbean and Orinoquía region | Cosmopolitan (Fuentes-Reinés et al. 2012, Kotov et al. 2013) | Álvarez 2010; Villabona-González et al. 2011; Fuentes-Reinés et al. 2012; Fuentes-Reinés & Elmoor-Loureiro 2015; present data |
| 3.Pseudosida ramosa (Daday, 1904) | Caribbean an Orinoquía region | Neotropical region (Korovchinsky 1992) | Fuentes-Reinés et al. 2012; Fuentes-Reinés et al. 2022; present data |
| ANOMOPODA | |||
| MOINIDAE | |||
| 4.Moina minuta Hansen, 1899 | Andean, Amazon, Caribbean and Orinoquía regions | Neotropical region (Kotov et al., 2013) | Barón-Rodríguez et al. 2006; Gallo-Sánchez et al. 2009; Aranguren-Riaño et al. 2011 and Fuentes-Reinés et al. 2012, Fuentes-Reinés et al. 2022; present data |
| BOSMINIDAE | |||
| 5.Bosmina (Liederobosmina) hagmannii Stingelin, 1904 | Amazon, Orinoquía and Caribbean regions | Nearctic and Neotropical regions (Paggi 1979; Kotov et al. 2013 | Aranguren-Riaño et al. 2011, present data |
| 6.Bosminopsis deitersiRichard, 1895 | Amazon, Orinoquía and Caribbean regions | Tropicopolitan species (Van Damme and Dumont 2010). | Barón-Rodríguez et al. 2006; Gallo-Sánchez et al. 2009; Aranguren-Riaño et al. 2011; present data |
| DAPHNIIDAE | |||
| 7.Ceriodaphnia cornuta Sars, 1885 s.lat | Amazon, Orinoquía, Andean and Caribbean regions | Tropicopolitan taxon (Smirnov et al. 1995) | Camargo-Fajardo 1994; Barón-Rodríguez et al. 2006; Guevara et al. 2009; Gallo-Sánchez et al. 2009; Álvarez 2010; Villabona-González et al. 2011; Aranguren et al. 2011; Fuentes-Reinés et al. 2012; Fuentes-Reinés 2014; Fuentes-Reinés & Elmoor-Loureiro 2015; Fuentes-Reinés et al. 2019; Fuentes-Reinés et al. 2022; present data |
| ILYOCRYPTIDAE | |||
| 8.Ilyocryptus spinifer Herrick, 1882 | Andean, Orinoquía and Caribbean regions | Cosmopolitan (Sousa & Elmoor-Loureiro 2019) | Stingelin 1913; Gaviria 2001; Barón-Rodríguez et al. 2006; Fuentes-Reinés et al. 2012; Fuentes-Reinés et al. 2022; present data |
| MACROTHRICIDAE | |||
| 9.Grimaldina freyiNeretina & Kotov, 2017 | Caribbean, Andina and Orinoquia regions | Neotropical (Neretina & Kotov 2017) | Fuentes-Reinés et al. 2012; Fuentes-Reinés et al. 2019; present data |
| 10.Macrothrix paulensis (Sars, 1900) | Caribbean and Orinoquía regions | Neotrópical (Kotov & Hollwedel 2004, Neretina and Kotov 2017) | Álvarez, 2010; Fuente-Reinés et al. 2022; present data. |
| 11.Macrothrix elegansSars, 1901 | Caribbean and Orinoquía regions | Neotropical region (Kotov et al. 2004) | Álvarez 2010, Fuentes-Reinés et al. 2012; Fuentes-Reinés & Elmoor-Loureiro 2015; Fuentes-Reinés et al. 2019; present data |
| 12.Streblocerus sp.* | Orinoquía region | Neotropical region | Present data |
| CHYDORIDAE | |||
| 13.Alonella cf. dadayi Birge, 1910 | Andean, Orinoquía and Caribbean regions | Neotropical and Nearctic species (Hollwedel et al. 2003; Kotov et al. 2013; Van Damme & Dumont 2010) | Barón-Rodríguez et al. 2006; Álvarez 2010; Fuentes-Reinés et al. 2012; present data |
| 14.Alonella clathratula*Sars, 1896 | Orinoquia region | Australasian, Afrotropical, Neotropical and oriental region (Sharma & Sharma 2010; Kotov et al. 2013) | Present data |
| 15.Alonella pulchelaHerrick, 1884 | Orinoquia region | Nearctic region (Kotov et al. 2010) | Present data |
| 16.Anthalona neotropica* Sousa, Elmoor-Loureiro & Debastiani-Júnior 2015 | Orinoquía region | Neotropical region (Sousa et al. 2015) | Present data |
| 17.Anthalona verrucosa verrucosa (Sars, 1901) | Caribbean and Orinoquía regions | Neotropical region (Van Damme et al. 2011; Sousa et al. 2022) | Álvarez 2010; Fuentes-Reinés 2014; present data |
| 18.Biapertura ossiani ossiani* (Sinev, 1998) | Orinoquía region | Nearctic and Neotropic regions (Sinev 2020) | Present data |
| 19.Coronatella monacantha (Sars, 1901) | Caribbean and Orinoquia regions | Neotropical (Fuentes-Reinés et al. 2024) | Fuentes-Reinés & Zoppi de Roa 2013; Fuentes-Reinés et al. 2022; Fuentes-Reinés et al. 2024; present data |
| 20.Camptocercus dadayi (Stingelin, 1913) | Andean, Orinoquía and Caribbean regions | Neotropical region (Sinev 2015) | Stingelin 1913; Villabona-González et al. 2011; present data |
| 21.Chydorus eurynotusSars, 1901 | Andina, Caribbean and Orinoquía region | Tropicopolitan taxon (Van Damme & Dumont 2010) | Stingelin 1913; Álvarez 2010; Fuentes-Reinés et al. 2012; Fuentes-Reinés 2014; Fuentes-Reinés et al. 2022; present data |
| 22.Chydorus sp. | Orinoquía region | Neotropical region | Present data |
| 23.Dadaya macrops (Daday, 1898) | Andean, Orinoquía and Caribbean regions. | Tropicopolitan region (Smirnov 1996) | Barón-Rodríguez et al. 2006; Álvarez 2010; Fuentes-Reinés & Zoppi de Roa 2013; Fuentes-Reinés et al. 2022; present data |
| 24.Disparalona (Mixopleuroxus) lucianae*Sousa, Elmoor-Loureiro, Mugnai, Panarelli and Paggi 2018 | Orinoquía region | Neotropical region (Sousa et al. 2018) | Present data |
| 25.Disparalona (Mixopleuroxus) leptorhyncha* (Daday, 1905) | Orinoquía region | Neotropical region (Sousa et al. 2018) | Present data |
| 26.Euryalona orientalis (Daday, 1898) | Caribbean and Orinoquía region | Neotropical, Afrotropical, Australasian and oriental regions (Kotov et al. 2013) | Pearse 1916; Fuentes-Reinés et al. 2012; Fuentes-Reinés et al. 2022; present data |
| 27.Ephemeroporus barroisi-group (Richard, 1894) s.lat | Caribbean and Orinoquía regions | Cosmopolitan (Yalım & Çıplak 2010) | Álvarez 2010; present data |
| 28.Ephemeroporus hybridus (Daday, 1905) | Andean, Caribbean and Orinoquía regions | Nearctic and Neotropical regions (Kotov et al. 2013) | Stingelin 1913; Fuentes-Reinés et al. 2012; present data |
| 29.Ephemeroporus tridentatus (Bergamin, 1939) | Andean, Orinoquía and Caribbean regions | Neotropical region (Fuentes-Reinés et al. 2012) | Álvarez 2010; Barón-Rodríguez & Gavilán-Díaz 2007; Fuentes-Reinés et al. 2012; present data |
| 30.Flavalona iheringula* (Sinev & Kotov, 2004) | Orinoquía region | Neotropical species (Kotov & Sinev 2004) | Present data |
| 31.Graptoleberis occidentalis*Sars, 1901 | Orinoquía region | Neotropical region (Van Damme & Dumont 2010) | Present data |
| 32.Kurzia cf polyspinaHudec, 2000 | Caribbean and Orinoquía regions | Neotropical species (Hudec 2000) | Fuentes-Reinés et al. 2012; Fuentes-Reinés et al. 2019; Fuentes-Reinés et al. 2022, present data |
| 33.Matralona sp. * | Orinoquía region | Neotropical region | Present data |
| 34.Nicsmirnovius incredibilis* (Smirnov, 1984) | Orinoquía region | Neotropical taxon (Sousa & Elmoor-Loureiro 2017) | Present data |
| 35.Notoalona sculpta (Sars, 1901) | Caribbean and Orinoquía Regions. | Neotropical species (Kotov et al. 2013; Van Damme et al. 2013). | Brehm 1956; Fuentes-Reinés et al. 2012; Fuentes-Reinés 2014; present data |
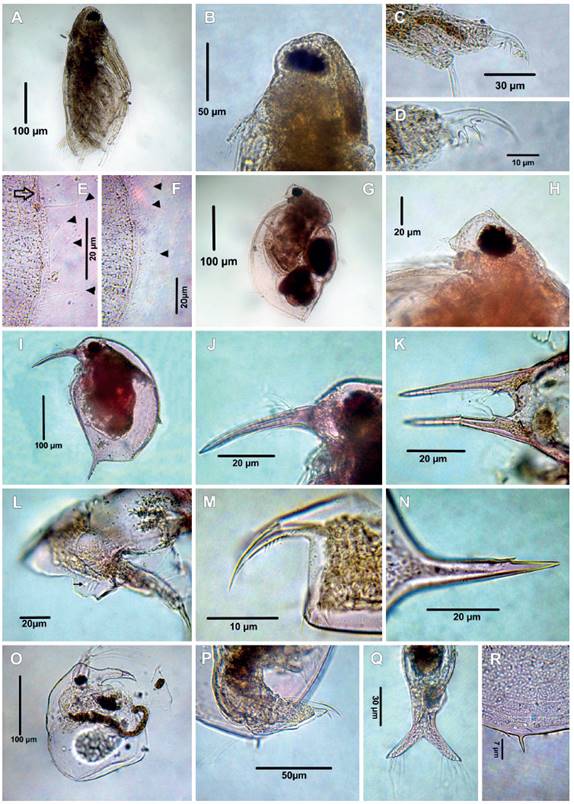
Figure 2 Cladocerans from the Española Lagoon, Meta Department. A-F. Diaphanosoma fluviatile (A. Habitus, B. Head, C. Postabdomen, D. Postabdominal claw, E-F. Valve, arrowheads point at setules); G-H. Ceriodaphnia cornuta (G. Habitus, H. Head); I-N. Bosmina (L.) hagmanni (I. Habitus, J. Rostrum, K. Antennule, dorsal view, L. lateral head pore, M. Postabdominal claw, N. Mucro); O-R. Bosminopsis deitersi (O. Habitus. P. Postabdomen, Q. Antennule, dorsal view, R. Mucro.
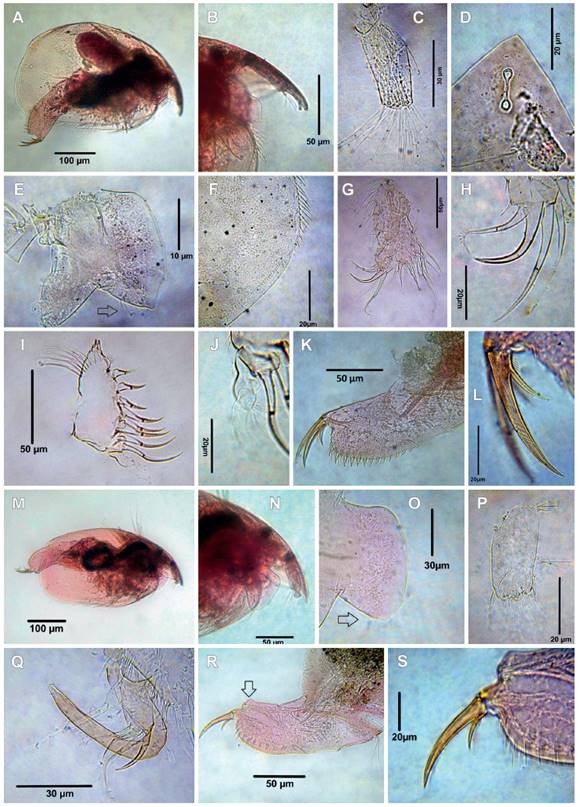
Figure 3 Cladocerans from the Española Lagoon, Meta Department. A-J. Biapertura ossiani ossiani, female (A. Habitus, B. Rostrum, C. Antennule, D. Head pores, E. Labrum, F. Posterodorsal margin of valve, G. Limb I, H. idem, IDL and ODL, I. Limb II, J. idem, exopodite, K. Postabdomen, L. Postabdominal claw,); M-S. Biapertura ossiani ossiani, male (M. Habitus, N. Rostrum, O. Labrum, the arrow points at setulae, P. Antennule, Q. Copulatory hook on IDL of limb I, R. Postabdomen, the arrow points at a small bulge upper to genital pore, S. Postabdominal claw).
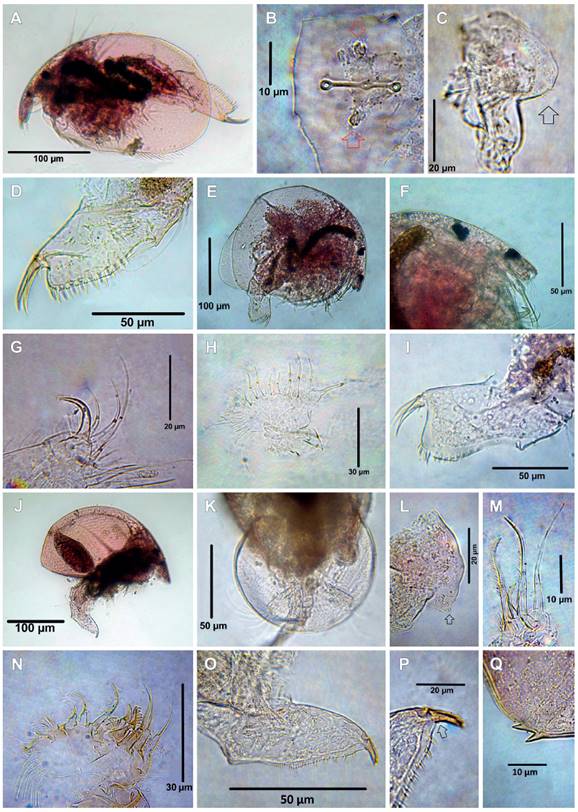
Figure 4 Cladocerans from the Española Lagoon, Meta Department. A-D. Flavalona iheringula (A. Habitus, B. Head pores, C. Labrum, arrow points at cluster of setules, D. Postabdomen); E-I. Nicsmirnovius incredibilis (E. Habitus, F. Rostrum, G. ODL and IDL of limb I, H. Limb II, I. Postabdomen,); J-Q. Graptoleberis occidentalis (J. Habitus, K. Rostrum, dorsal view, L. Labrum, M. ODL and IDL of limb I, N. Limb II, O. Postabdomen, P. Postabdominal claw, Q. posteroventral margin of valve).
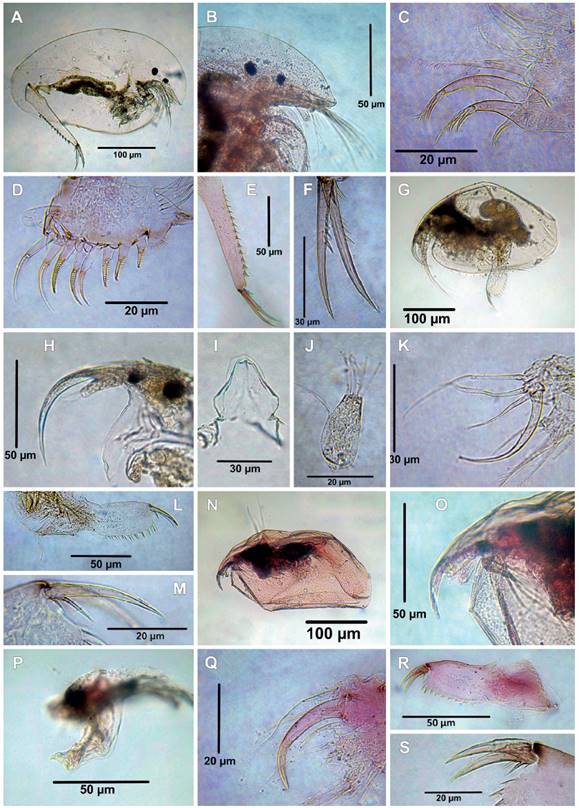
Figure 5 Cladocerans from the Española Lagoon, Meta Department. A-F. Camptocercus dadayi (A. Habitus, B. Rostrum, C. Limb I, D. Limb II, E. Postabdomen, postanal part, F. Postabdominal claw); G-M. Disparalona leptorhyncha (G. Habitus, H. Rostrum, I. Labrum, ventral view, J. Antennule, K. ODL and IDL of limb I, L. Postabdomen, M. Postabdominal claw); N-S. Disparalona lucianae (N. Habitus, O. Rostrum, dorsal view, P. Labrum, Q. ODL and IDL of limb I, R. Postabdomen, S. Postabdominal claw).
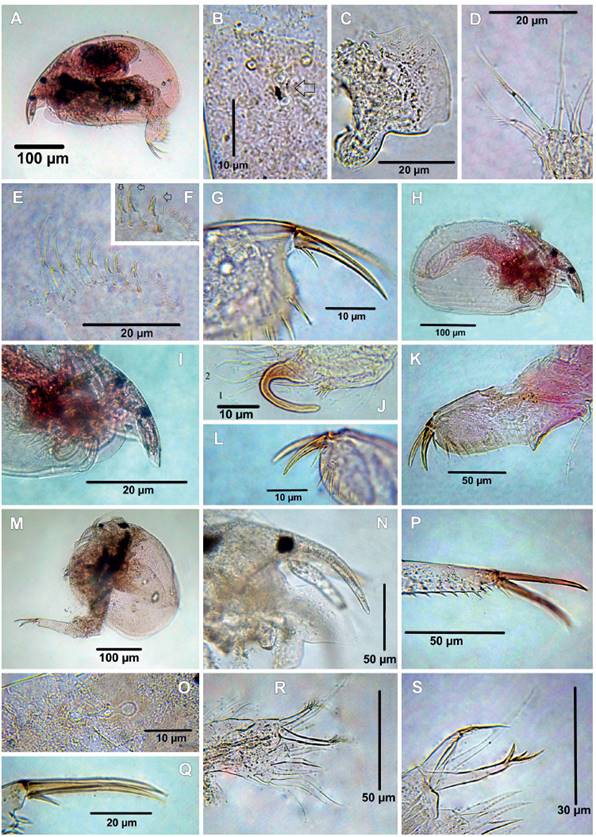
Figure 6 Cladocerans from the Española Lagoon, Meta Department. A-G. Anthalona neotropica, female (A. Habitus, B. Head pores, C. Labrum, D. ODL and IDL of limb I, E. Limb II, F. Idem, scrapers 6-8, G. Postabdominal claw); H-L. Anthalona neotropica, male (H. Habitus, I. Rostrum, J. Copulatory hook on IDL of limb I, K. Postabdomen, L. Postabdominal claw); M-S. Kurzia cf polyspina (M. Habitus, N. Rostrum, O. Head pores, P. Postabdomen, distal part, Q. Postabdominal claw, R. Limb I, S, idem, ODL and IDL).
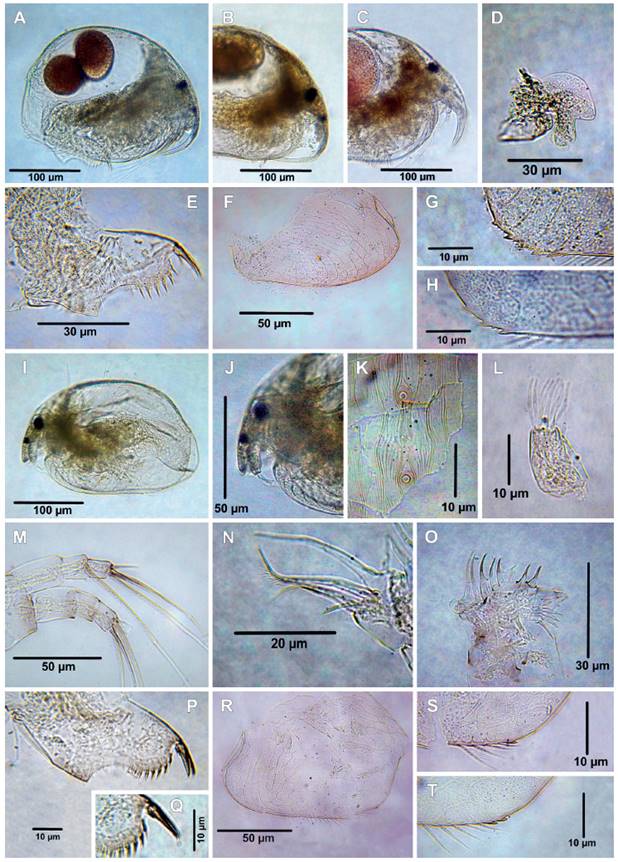
Figure 7 Cladocerans from the Española Lagoon, Meta Department. A-H. Alonella dadayi (A. Habitus, B-C. Rostrum, D. Labrum, E. Postabdomen, F. Valve, G-H. posteroventral margin of valve); I-T. Alonella pulchella (I. Habitus, J. Rostrum, K. Head pores, L. Antennule, M. Antenna, N. ODL and IDL of limb I, O. Limb II, P. Postabdomen, Q. Postabdominal claw, R. Valve, S-T. posteroventral margin of valve).
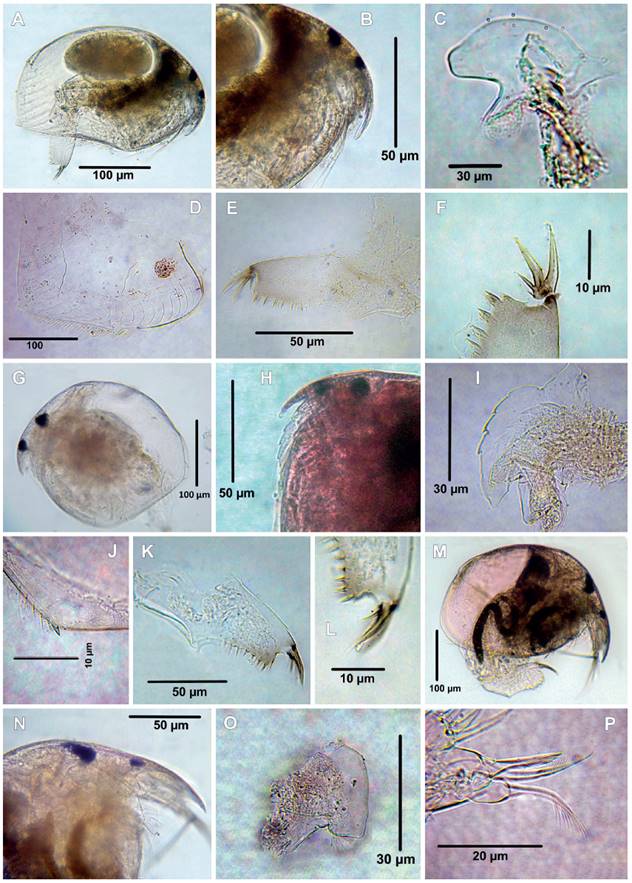
Figure 8 Cladocerans from the Española Lagoon, Meta Department. A-F. Alonella cf clathratula (A. Habitus, B. Rostrum, C. Labrum, D. Valve, E. Postabdomen, F. Postabdominal claw); G-L. Ephemeroporus barroisi group (G. Habitus, H. Rostrum, I. Labrum, J. posteroventral margin of valve, K. Postabdomen, L. Postabdominal claw); M-P. Matralona sp (M. Habitus, N. Rostrum, O. Labrum, P. ODL and IDL of limb I).
Family SIDIDAE Baird, 1850
Genus Diaphanosoma Fischer, 1850
1. Diaphanosoma fluviatile Hansen, 1899
Material examined: Five adult females, catalog number: MHNU-P 002.
Remarks: Diaphanosoma fluviatile has a widespread distribution, and it has been reported in Brazil, Argentina (Fernándes et al. 2012), Venezuela (Zoppi de Roa & López 2008), Nicaragua (Cisneros et al. 1991), Haiti (Korovchinsky 1992), Mexico (Elías-Gutierrez et al. 2001) and United State (Korovchinsky 2002, Lopez et al. 2008 and Whitmore et al. 2019). This is the first record for Colombia. Diaphanosoma fluviatile has an elongated body (Fig. 2A), head rectangular and compound eye large (Fig. 2B), postabdomen small (Fig. 2C) with three spines on the base of claw, the distal one is the longest (Fig. 2C-D). It can be easily distinguished, having posteroventral and posterior valve margins with very fine long denticles (4-12) alternated by long thin setulae (arrowhead, figs. 2E-F). The ventral margin of the carapace is not folded, a feature shared with D. birgei, but this species possesses one setula between pair of massive denticles on posteroventral margin (Sousa and Elmoor-Loureiro 2021, Fig. 10A), so easily distinguished from D. fluviatile.
Family Daphniidae Straus, 1820
Genus Ceriodaphnia Dana, 1853
2. Ceriodaphnia cornuta Sars, 1885
Material examined: Five adult females, catalog number: MHNU-P 007.
Remarks: Ceriodaphnia cornuta was described from Australia and has a worldwide distribution (Fuentes-Reinés et al. 2015). This species has and oval body, body length is between 364 ‒ 462 μm (mean = 414 µm) (Fig. 2G) and can be easily identified by the presence of horn on the rostrum, (Fig. 2H) and fornices. The specimens from La Española lagoon were much bigger than the ones reported for the Caribbean region (Fuentes-Reinés et al. 2012, 290 µm), Fuentes-Reinés and Elmoor-Loureiro (2015, 350 ‒ 352 µm), evidencing variability in size among Colombia populations. Ceriodaphnia cornuta is considered a species complex (Sharma & Kotov 2013).
Family Bosminidae Baird, 1845
Genus Bosmina Baird, 1845
3. Bosmina (Liederobosmina) hagmanni Stingelin, 1904
Material examined: Six adult females, catalog number: MHNU-P 005.
Remarks: The genus Bosmina is divided into 5 subgenera: Bosmina s. str., B. (Sinobosmina) Lieder, B. (Eubosmina) Seligo, B. (Lunobosmina) Taylor, Ishikane et Haney and B. (Liederobosmina) Brték (Lieder 1983, Kotov et al. 2009). Bosmina hagmanni Stingelin, 1904 is included in the last subgenera. In Colombia, the genus Bosmina is represented by five species, one belonging to subgenus Bosmina s. str: B. (Bosmina) longirostris (O. F. Müller, 1776), another to subgenus Eubosmina: B. (Eubosmina) coregoni (Baird, 1857) and the others to the subgenus Liederobosmina:B (Liederobosmina) tubicen Brehm, 1953, B. (Liederobosmina) hagmanni Stingelin, 1904 and B. (Liederobosmina) korinekiGaribian, Juračka and Kotov, 2021.
The specimen examined bears the diagnostic features B. (Liederobosmina) hagmanni as described by Paggi (1979) and Elmoor-Loureiro (1988). Habitus ovoid (Fig. 2I), body length is between 280 ‒ 322 μm (n = 6), rostrum rounded (Fig. 2J), antenna I fused with rostrum and arranged parallel to each other forming two different arcs (Fig. 2K), antennal formula: setae 0-1-3/1-1-3, lateral head pore small and circular (Fig. 2L) located far away from the fornix, postabdomen short and quadrangular-like, postabdominal claw slightly bent with proximal pecten of 6 ‒ 7 relatively strong, sparsely located teeth (Fig. 2M), mucro with small denticle on the dorsal margin (Fig. 2N).
In Colombia, B. (Liederobosmina) hagmanni can be confused with B. (Liederobosmina) korineki and B. (Liederobosmina) tubicen. Nevertheless, they can be separated by the following characters: 1) heavily striated valves in B. (Liederobosmina) korineki (Garibian et al. 2021a, fig. 2A-D, 3A) while in B. (Liederobosmina) hagmanni (Paggi 1979, figs 85 ‒ 87; Elmoor-Loureiro 1997, fig. 21a, present data, fig. 2I) and B. (Liederobosmina) tubicen (Elmoor-Loureiro 1997, Fig. 24a) the valve is smooth in adults or fine striae in juveniles; 2) antennule arranged parallel to each other in B. (Liederobosmina) hagmanni (Paggi 1979, fig. 88, Elmoor-Loureiro 1997, fig. 21b, present data, Fig. 2K) and B. (Liederobosmina) korineki (Garibian et al. 2021a, fig. 3D) whereas in B. (Liederobosmina) tubicen (Lieder 1961, figs 4 ‒ 5) they are divergent, 3) rostrum rounded in B. (Liederobosmina) hagmanni (Paggi 1979, figs. 85-85, Elmoor-Loureiro 1997, fig. 21a, present data, Fig. 2J) and B. (Liederobosmina) korineki (Garibian et al. 2021a, figs. 3A ‒ C) vs. flatten in B. (Liederobosmina) tubicen (Elmoor-Loureiro 1997, fig. 24a).
Genus BosminopsisRichard, 1895
4. Bosminopsis deitersiRichard, 1895
Material examined: 12 adult females, catalog number: MHNU-P 006.
Three valid species of genus Bosminopsis have been described worldwide: B. deitersi (Richard, 1895), B. brandorffi (Rey and Vasquez, 1989) and B. negrensis (Brandorff, 1976). Of these, the two latter are endemic from Amazon River basin (Garbian et al. 2021b, Kotov & Garibian 2021).
Bosminopsis deitersi was originally described by Richard (1895) from Río de la Plata, Argentina. Subsequently, this species has been reported from tropical and subtropical regions of all continents (Elmoor-Loureiro 1988, Smirnov & Timms 1983). This taxon is represented by a series of cryptic species forming the Bosminopsis deitersi group, and only two valid species-group are recognized within it: Bosminopsis zernowi Linko, 1901, B. deitersi birgei Burckhardt, 1924 (Garibian et al. 2021b), the former is distributed in Palearctic region while the latter in the Nearctic zone (Beaver et al. 2018). The deitersi-group still needs further revision (Kotov & Garibian 2021).
Bosminopsis deitersi has a subovoid body (Fig. 2O), body length is between 280 ‒ 294 μm (mean = 280 μm, n = 12), antennal formula: setae 0-0-3/1-1-3 , antennule fused to rostrum (Fig. 2Q), rounded labrum, postabdomen compressed laterally, narrowing distally (Fig. 2P), posteroventral valve with a single short mucro (Fig. 2R) which differentiate from B. zernowi (with several mucro-like spines). In the Neotropical region, this species can be differentiated from B. negrensis and B. brandorffi by: 1) posterodorsal angle of valve with a long caudal spine in B. negrensis (Brandorff 1976, fig. 1, 3) vs. smooth in B. brandorffi (Rey & Vásquez 1989, fig. 1) and short in B. deitersi (Garibian et al. 2021b, figs. 5A, H, present data, Fig. 2R), 2) ocular dome slightly projected in B. deitersi female (Garibian et al. 2021b, fig. 6B, present data, Fig. 2O) and B. negrensis female (Brandorff 1976, Fig. 3, 5; Kotov & Garibian 2021, Figs. 3A, B) vs. strongly projected in B. brandorffi (Rey and Vásquez, 1989, figs 1,2), 3), posterior part of the postabdomen with a long projection for the natatory setae in B. brandorffi (Rey and Vásquez, 1989, figs. 10,11, 12; Kotov & Garibian 2021, figs. 5A, 6A, G ) while the posterior part of postabdomen of B. negrensis (Kotov & Garibian 2021, figs. 3A, I) and B. deitersi (Garibian et al. 2021b, figs. 7A, B, present data, Fig. 2P) lack this projection.
Familia Chydoridae Dybowsky & Grochowski, 1894 emend. Frey, 1967
Subfamily Aloninae Dybowsky & Grochowski, 1894 emend. Frey, 1967
Genus BiaperturaSmirnov, 1971 emend. Sinev, 2020
5. Biapertura ossiani ossiani (Sinev, 1998)
Material examined: Nine adult females, 2 adults male, catalog number: MHNU-P 013.
Remarks: The genus Biapertura was created by Smirnov 1971 to include all species of Alona s. lato with two main head pores, (Sinev 2020) but this proposal was rejected because some taxon with two main head pores in that time ‒i.e. Alona karua King, 1853 = Karualona karua (King, 1853) and A. verrucosa verrucosaSars, 1901 = Anthalona verrucosa verrucosa (Sars, 1901)‒ bear six setae on exopodite III vs. seven in the genus Biapertura. In fact, Frey (1987) considered that the presence of two main head pores was a convergent character in some Chydoridae (Geoffreyia, Flavalona, Alpinalona, Matralona).
A new recent research made by Sinev (2020) revalidated the genus Biapertura, which encompassed eight taxa: B. lepida (Birge, 1892), B. sibirica (Sinev, Karabanov & Kotov, 2020), B. elliptica (Sinev, 1997), B. martensi (Sinev, 2007), B. kendallensis (Henry, 1919), B. affinis (Leydig, 1860), B. ossiani ossiani (Sinev, 1998), and B. ossiani herricki Sinev, 2013 (USA, Canada, South Korea). This genus has a worldwide distribution (Sinev 2020) with records in Africa, North and Central Asia, North Europe, Australia, North and South America (Henry 1919, Sinev 1997, 1998, 2009, 2013). In South America, hitherto, only B. ossiani ossiani has been reported from Brazil (Sinev 1998, Sousa et al. 2014, 2017). The studied specimens (nine adult females) fully agreed with the descriptions of Sinev (1998) and Sousa et al. (2017).
Habitus irregularly oval as in Figure 3A, body length is between 644 ‒ 756 µm (mean = 678 µm, n = 9), antennule of moderate size, about 2.28 as long as wide, with nine aesthetascs, the two lateral strong (Figs. 3B ‒ C), reaching the tip of the rostrum (Fig. 3B). Antenular seta thin more than ½ length of antennule, antennal formula: setae 0-0-3/1-1-3, seta arising from first segment of endopodite thin, spine on first segment of exopodite longer than middle segment, two main head pore connected with PP/IP = 1.27 (Fig. 3D), labrum triangular with cluster of setulae on the posterior margin (arrowed in Fig. 3E), posterior margin of valves followed by a row of spinules (Fig. 3F). Limb I of moderate size (Fig. 3G), inner distal lobe (IDL) and outer distal lobe of trunk limb I with three and one setae respectively (Fig. 3H). Limb II subtriangular, exopodite of irregular shape, with apical long setulae and one distal long angulate seta as long as exopodite itself, endopodite with eight scrappers (Fig. 3I - J). Postabdomen subrectangular large (Fig. 3K), about 2.6 as long as wide, with 12 - 14 massive denticles and 11 fascicles in the postanal portion, postabdominal claw equal in length to preanal part of postabdomen, with a basal spine shorter than anal margin, and 5 long proximal setulae arising from the base of spines (Fig. 3L). Male smaller than female, body length 518 µm (n = 2), habitus elongated, height/length ratio about 0.54 (Fig. 3M), rostrum short (Fig. 3N), labrum as in females (Fig. 3O), antennule stouter than female, not reaching the tip of the rostrum, aesthetascs projecting beyond tip of rostrum (Fig. 3N), male seta short and subterminal (Fig. 3P). IDL and ODL of P1 with four and one setae, respectively (Fig. 3Q), copulatory hook U-shaped. Postabdomen about 3 times as long as high, with a small bulge in the ventral portion of the distal margin (arrowed in Fig. 3R), postabdominal claws shorter than that of female. Basal spine about 0.3 times as short as claw proper (Fig. 3S).
The specimen from Colombia agrees with descriptions by Sinev (1998), however, Colombian specimens possess in ventral portion of distal margin of male postabdomen a small bulge (Fig. 3R), but we do not regard this difference as sign of a separate status of the Colombian populations.
In the Neotropical region, B. ossiani ossiani resembles B. ossiani herricki (Sinev 2013), but they can be distinguished by: 1) Postabdomen of adult male of B. ossiani herricki with 1 - 3 marginal denticles at postanal angle (Sinev 2020, fig. 16L) whereas in B. ossiani ossiani they are absent (Sinev 2020, fig.16O, present data, Fig. 3R); 2) seta 1 on the IDL P1 smaller than seta 2 in B. ossiani herricki (Sinev 2020, fig. 16M) whereas in B. ossiani ossiani both setae almost equal in length (Sinev 1998, figs. 4D, E, present data, Fig. 3O).
Females of B. ossiani ossiani and B. affinis (Leydig, 1860) can be confused since both species have subtle differences; however, the male of both species shows marked differences, for example: i) the seta 1 of P1 IDL of B. affinis is located laterally (Sinev 2020, fig. 5F) vs., distally in B. ossiani ossiani (Sinev 1998, figs. 4D, E, present data, Fig. 3O); ii) male seta of antennule is straight in B. affinis (Sinev 2020, fig. 5D) vs. curved in B. ossiani ossiani (Sinev 1998, fig. 4C, present data, Fig. 3P); iii) claw base of the male with projection in B. ossiani ossiani (Sinev 2020, fig. 16O, present data, Figs. 3R - S) whereas in B. affinis without projection (Sinev 2020, figs. 1N, 5B, C). Although Stingelin (1913) recorded Alona affinis for Colombia, its taxonomic status remains questionable since the author did not deposit material in a museum, and no adequate illustrations were depicted in the paper.
Genus FlavalonaSinev and Dumont, 2016
6. Flavalona iheringula (Kotov & Sinev, 2004)
Material examined: Two adult females, catalog number: MHNU-P 032-1: MHNU-P 032-6.
Remarks: The genus Flavalona is distributed worldwide (Sinev & Dumont 2016) and comprises 14 taxa: Flavalona costata (Sars, 1862), F. cheni (Sinev, 1999), F. margipluma (Sousa, Santos, Güntzel, Diniz, de Melo Junior & Elmoor-Loureiro, 2015), F. natalensis (Sinev, 2008), F. setigera (Brehm, 1931), F. weltneri (Keilhack, 1905), F. rustica rustica (Scott, 1895), F. rustica americana (Flössner & Frey, 1970), F. bicolor (Frey, 1965), F. hudeci (Sinev, 1999), F. iheringula (Kotov & Sinev, 2004), F. sphagnophila (Van Damme & Eggermont, 2011), F. asymmetrica Sousa & Elmoor-Loureiro, 2017 and F. darkovi (Nerentina & Sinev, 2021). Of these, F. margipluma, F. iheringula, F. hudeci and F. asymmetrica occur in the Neotropical region (Sousa and Elmoor-Loureiro 2018). Sinev and Dumont (2016) divided the genus into two lineages: the costata and the rustica-clades. Flavalona iheringula is included in the latter.
Flavalona inheringula was originally described by Sars (1901) and redescribed by Sinev (2001) as Alona iheringi, later the name of this taxon was amended by Kotov and Sinev (2004), and created a nomem novum A. iheringula because A. iheringi was a junior homonym of A. dadivi var. iheringi
The Colombian specimens fully agree with the description by Kotov and Sinev (2004), Van Damme and Dumont (2010), and Sinev and Dumont (2016). Habitus oval (Fig. 4A), body length 322 µm (n=2), height/length ratio ca 0.61, rostrum short, ocellus longer than eye, with three connected main head pores (Fig. 4B), lateral head pores with a shallow and semicircular pocket-shape structure underneath them (arrowed in Fig. 4B), labrum of moderate size with two clusters of setulae (Fig. 4C), antennule not reaching the tip of rostrum, with nine aesthetascs, antennular sensory seta slender, located sub-distally, antennal formula, setae 0-0-3/1-1-3, IDL of limb I with three setae, limb II subtriangular, inner portion with eight scrappers increasing distally, third and seven scrappers with robust denticles, exopodite globular, with one slender, naked seta. Postabdomen tapering distally (Fig. 4D), postanal margin with 11 merged denticles. Postabdominal claw 1.2 times longer than preanal portion of postabdomen (Fig. 4D). Basal spine short, about 0.16 ‒ 0.2 of claw length (Fig. 4D).
The specimen from Colombia agree with descriptions by Sinev (1998); however, the spine basal/claw ratio and the distance between the postabdominal claw and preanal portion of Colombian specimens were shorter than Brazilian population (Sinev 2001). Nevertheless, we do not regard this difference as a sign of a separate status the Colombian populations.
Genus Nicsmirnovius Chiambeng & Dumont, 1999
7. Nicsmirnovius incredibilis (Smirnov, 1984)
Material examined: Two adult females, catalog number: MHNU-P 034-1: MHNU-P 034-4.
Remarks: This species was originally described as Alona incredibilis by Smirnov (1984) and transferred to the genus Nicsmirnovius by Kotov (2003). The observed specimens fit with the description of Kotov (2003) and Sousa and Elmoor-Loureiro (2017). Habitus ovoid (Fig. 4E), body length is between 350 ‒ 364 µm (n= 2), height/length ratio about 0.8, rostrum short (Fig. 4F), labrum triangular, apex short and round, antennule almost reaching the tip of the rostrum (Fig. 4F), length about 2-times the width, with nine aesthetascs, two of them longer than the others, antennal formula, setae 0-1-3/0-0-3. IDL of limb I with two slender unequal setae and one hook seta, ODL with one slender, single, long seta (Figs. 4G ‒ H). Inner portion of limb II with eight scrappers; scrappers 1 ‒ 4 decreasing distally, scrappers 5, 6 the shortest and scrappers 7 and 8 similar in length (Fig. 4H). Postabdomen about 2 times as long as wide, postanal margin rounded with seven modified denticles in the distal portion (Fig. 4I), postabdominal claw shorter than anal margin (Fig. 4I), basal spine short about 0.2 of length of the postadominal claw (Fig. 4I).
In Colombia, it was also reported Nicsmirnovius fitzpatricki (Chien, 1970) (Fuentes-Reinés & Zoppy 2013, Fuentes-Reinés 2014), but probably these records belong to Nicsmirnovius paggii. Indeed, Sousa and Elmoor-Loureiro (2017) reported N. paggii from the Colombian Amazon Departament, reinforcing this idea. Certainly, this hypothesis needs to be investigated.
Despite shallow similarities with N. fitzpatrick and N. paggii, N. incredibilis can be easily differentiated by: 1) basal spine short in N. incredibilis (Kotov 2003, figs. 6, 14 ‒ 16, Sousa and Elmoor-Loureiro 2017, figs 3a,b, present data, Fig. 4I) vs. long in N. fitzpatricki (Van Damme et al. 2003, figs. 63 ‒ 65, Fuentes-Reinés & Zoppy 2013, figs. 11B ‒ C, Fuentes-Reinés 2014, figs. 4A, B, E) and N. paggii (Sousa & Elmoor-Loureiro 2017 fig. 6a); 2) postanal margin with modified denticles in N. incredibilis (Kotov 2003, figs. 6, 14 ‒ 16, Sousa & Elmoor-Loureiro 2017, figs.1a, 3a ‒ b, present data, Fig. 4I) vs. not modified denticles in N. fitzpatricki and N. paggii (Van Damme et al. 2003, figs. 63 ‒ 65; Sousa & Elmoor-Loureiro 2017, fig. 6a; Fuentes-Reinés 2014, fig. 4D); 3) ODL seta of limb I longer than the longer IDL seta in N. incredibilis (Kotov 2003, fig. 8, Sousa et al. 2017, fig. 2C, present data, Fig. 4G) whereas in N. fitzpatricki and N. paggii the ODL seta is almost as long as the longer IDL seta (Van Damme et al. 2003, fig. 67, Fuentes-Reinés & Zoppy 2013, fig. 11A, Fuentes-Reinés 2014, fig. 3F, Sousa & Elmoor-Loureiro 2017, fig. 5c); 4) scrappers 5 and 6 equal in size in N. incredibilis (Kotov 2003, figs. 6, 14 ‒ 16, Sousa & Elmoor-Loureiro 2017, figs 3a, b, present data, Fig. 4H) vs. different in size in N. fitzpatricki (Van Damme et al. 2003, fig. 68a) and N. paggii (Sousa & Elmoor-Loureiro 2017, fig. 5d).
Genus Graptoleberis G.O. Sars, 1862
8. Graptoleberis occidentalis Sars, 1901
Material examined: Five adult females, catalog number: MHNU-P 018.
Remarks: The genus Graptopleberis is widespread and can be found among macrophytes (Sinev & Gavrilko 2020), this genus comprises four valid species: G. testudinaria (Fischer, 1851), G. occidentalisSars, 1901, and G. smirnovi Sinev et Gavrilko 2020. Of these, hitherto, only G. occidentalis has been recorded in the Neotropical region; nevertheless, Graptoleberis occidentalis was originally described by Sars (1901) as a subspecies of G. testudinaria, and taking the consideration of non-cosmopolitanism of Chydoridae (Frey, 1982a), some researchers have suggested that it is a valid species (Elmoor-Loureiro 2007, Van Damme & Dumont 2010). Nevertheless, it is necessary to make an exhaustive comparison between both species, G. testudinaria and G. occidentalis, for the formal status definitions (Kotov & Fuentes-Reinés 2015). In Colombia, Barón-Rodríguez et al. (2006) recorded G. testudinaria from Ciénaga de Paredes (Santander-Colombia), but its taxonomic status remains doubtful since this species has a Palearctic distribution and, in addition, no material was deposited by the author and no adequate illustrations were represented in the paper.
The Colombian specimens have the habitus elongated (Fig. 4J), body length between 350 ‒ 540 µm (mean = 420 µm, n = 5), rostrum short (Fig. 4J), dorsal frontal view rounded (Fig. 4K), three main head pores connected, labrum subtriangular with small spinules on the apex (Fig. 4L, arrow points at the spinules), antennule about two times as long as wide, with nine aesthetascs, antennal formula seta: 0-0-3/0-1-3, IDL of limb I with three unequal setae as in figure 4M, ODL with one long seta (Fig. 4M), limb II (Fig. 4N) with eight scrappers, the scrappers 4 with strong denticles, exopodite long with some setulae on the apical portion, postabdomen short, tapering distally with fewer marginal denticles per group and decreasing in number distally (Fig. 4O), postabdominal claw very short, shorter than claw base (Fig. 4P), posteroventral margin of valve with two or three small denticles (Fig. 4Q).
This species can be confused with G. testudinaria, but they can be separated by: 1) denticles on the posteroventral valve corner smaller in G. occidentalis (Van Damme & Dumont 2010, fig. 8b, present data, Fig. 4Q) than in G. testudinaria (Smirnov 1971, figs. 545, b ‒ f, 546, Alonso 1996, figs. 170 H ‒ I, Van Damme & Dumont 2010, fig. 8g); 2) scrappers 4, 5 of limb II with fine denticles in G. testudinaria (Alonso 1996, fig. 171B) whereas in G. occidentalis only scrappers 4 with strong denticles and scrapper 5 fine bristles (Present data, Fig. 4N); 3) distal portion of postabdomen wider in G. testudinaria (Van Damme & Dumont 2010, fig. 8h) than G. occidentalis (Van Dammen & Dumont 2010, fig. 8e, present data, Figs. 4O ‒ P); 4) distal margin of postabdomen with fewer marginal denticles per group and decreasing in number distally in G. occidentalis (Van Damme & Dumont 2010, fig. 8e-f, present data, Figs 4O ‒ P) in comparison with G. testudinaria (Van Damme & Dumont 2010, fig. 8h ‒ i).
Genus Camptocercus Baird, 1943
9. Camptocercus dadayi (Stingelin, 1913)
Material examined: Six adult females, catalog number: MHNU-P 01.
Remarks: Camptocercus dadayi was originally described by Stingeling (1913) as a variety of C. australisSars, 1896. Latter, Smirnov (1971) treated it as variety of C. lillejborgi Schoedler, 1862, and finally established as valid species by Rey and Vasquez (1986) and confirmed by Sinev (2015). This species has a wide distribution in the Neotropical region. It has been reported in Mexico, Central, and South America (Sinev 2015). All specimens studied agree with the description by Sinev (2015). Habitus elongated (Fig. 5A), body length is between 574 ‒ 798 µm (mean = 420 µm, n = 6), height/length ratio about 0.59 ‒ 0.62 in adult, rostrum truncated (Fig. 5B), labrum triangular with rounded apex and posterior margin with tiny spine setulae, antennule about 3.7 times as long as wide, sensory seta slender, antennal formula seta: 0-0-3/0-1-3. IDL of limb I with three unequal setae, seta 1 thin and short, setae 2 and 3 strong and curved armed with strong spines distally (Fig. 5C). ODL of limb I with one thick seta armed by thin setulae (Fig. 5C). Limb II with eight scrappers in the inner portion, exopodite oval, seta was not studied (Fig. 5D). Postabdomen very long and narrow, postanal margin with 16 denticles (Fig. 5E), postabdominal claw about two times longer than anal margin, with spines increasing distally (Fig. 5F), basal spine about 1.2 length of the claw, margin posterior ventral of the valve with uniform spinulae.
Camptocercus dadayi has been confused with C. australis; however, they can be separated by: 1) truncated rostrum in C. dadayi (Sinev 2015, fig. 6F, present data, Fig. 5B) vs. not truncated in C. australis (Sinev 2015, fig. 3H); 2) IDL setae 2 - 3 armed with strong spines in C. dadayi (Sinev 2015, fig. 8D, present data, Fig. 5C), vs. fine spine in C. australis (Sinev 2015, fig. 5D); 3) postabdominal claw with several spines increasing distally in C. dadayi (Sinev 2015, figs. 6K, 7H, present data, Fig. 5F) vs. one spine in C. australis (Sinev 2015, figs. 3M, 4J).
Genus Disparalona Fryer, 1968
10. Disparalona (Mixopleuroxus) leptorhyncha (Daday, 1905)
Material examined: three adult females, catalog number: MHNU-P 029.
Remarks: Disparalona is a widespread genus recorded in Palearctic, Nearctic, Neotropical, Afrotropical, and Australasian region (Smirnov 1996, Neretina et al. 2018, Sousa et al. 2018). This genus was divided in to two subgenera: Disparalona (Disparalona) and Disparalona (Mixopleuroxus) (Neretina et al. 2018) and comprise 12 valid species: D. (D.) rostrata (Koch, 1841); D. acutirostris (Birge, 1879); D. (M) leptorhyncha (Daday, 1905); D. (M) hamata (Birge, 1879); D. (M) chappuisi Brehm, 1934; D. (M) striatoides (Šrámek-Hušek, 1946); D. (D.) leei (Chien Shing-Ming 1970); D. (M) caudata Smirnov, 1996; D. (D.) ikarus Kotov and Sinev, 2011; D. (D.) smirnovi Sinev, 2015; D. (M) lucianae Sousa, Elmoor-Loureiro, Mugnai, Panarelli & Paggi, 2018; and D. (M) tenuispina Sousa, Elmoor-Loureiro, Mugnai, Panarelli & Paggi, 2018. Of these, hitherto D. (M) leptorhyncha, D. (M) hamata and D. (M) lucianae have been recorded in Colombia (Fuentes-Reinés et al. 2018, present data)
Disparalona (M) leptorhyncha was originally described as Leptorhynchus rostratus by Daday (1905) from Paraguay and transferred to Disparalona by Smirnov (1996). The Colombian specimens fully agree with the description by Van Damme and Dumont (2010) and Sousa et al. (2018). Habitus elongated (Fig. 5G), body length 490 µm (n=2), height/length ratio 0.57, rostrum very long and strongly curved (Fig. 5H), ocellus smaller than eye, labrum without keel with short lateral expansion (Fig. 5I), antennule not reaching the tip of rostrum (Fig. 5H), antennular sensory seta slender, located 1/3 from the tip of antennule (Fig. 5J), antennal formula, setae 0-0-3/1-1-3, IDL of limb I with three setae (Fig. 5K), seta 3 hook-like and heavily chithinized. The inner portion of limb II with eight scrappers, the first one longest, exopodite armed with a shot seta. Postabdomen (Fig. 5L) about 3.8 times as long as wide, preanal margin about 1.4 times as long as the anal margin, post anal margin very long about 2.38 times as long as anal margin, with 14 strong denticles. Two basal spines, the proximal one shortest, distal about 0.3 of length of the postabdominal claw, proximal about 0.44 times shorter than distal one (Fig. 5M). Marginal setae on valves densely setulated.
The specimen from Colombia bears the diagnostic feature of D. (M) leptorhyncha as described by Sousa et al. (2018), but some fine scale differences were observed in our specimens: 1) the antennular sensory seta arises from 1/3 of antennule in Colombian specimens (Present data, Fig. 5J) vs. ¼ in population from Brazil (Sousa et al. 2018, fig. 1O); 2) lateral expansions of the labrum are short in Colombian specimens (Present data, Fig. 5I) vs. large in Brazil population (Sousa et al. 2018, fig. 1N), but we do not regard such differences as signs of a separate status the Colombian populations.
11. Disparalona (Mixopleuroxus) lucianae Sousa, Elmoor-Loureiro, Mugnai, Panarelli & Paggi, 2018
Material examined: Two adult females; catalog number: MHNU-P 028.
Remarks: The Colombian specimens fully agree with the description by Sousa et al. (2018). Habitus elongated (Fig. 5N), body length 364 ‒ 434 µm (mean = 399 µm, n = 2), rostrum short, antennule not reaching the tip of the rostrum (Fig. 5O), labral keel not prominent, distal portion rounded (Fig. 5P), IDL of limb I with three setae, the third seta thick, hook-like, and heavily chithinized (Fig. 5Q). ODL with a long slender seta. Postabdomen large, about 3.7 times as long as wide, post anal margin with 10 denticles the distalmost thicker (Fig. 5R), postabdomen claw about 1 times as long as anal margin, with two basal spines (Fig. 5S).
Among the Neotropical species of this genus, D. (M.) lucianae most resemble D. (M.) tenuispina, and both species present the labrum short and rounded; nevertheless, they can be distinguished by the number of denticles on the postanal margin with 3 in D. (M.) tenuispina vs 10 in D. (M.) lucianae.
Genus AnthalonaVan Damme, Sinev & Dumont, 2011
12. Anthalona neotropica Sousa, Elmoor-Loureiro & Debastiani-Júnior, 2015
Material examined: Four adult females and two adult males. Catalog number: MHNU-P 014.
Remarks: Morphology of the species fully agrees with the description by Sousa et al. (2015). Habitus moderately elongated (Fig. 6A), body length is between 296 ‒ 336 µm (mean = 312 µm, n = 4) rostrum short, antennule does not reach the tip of the rostrum, ocellus smaller than eyes (Fig. 6A). Two connected main pores, lateral pores located above the anterior main pore (Fig. 6B, main pores arrowed). Labrum quadrangular-like with a notch in the apex (Fig. 6C), antennal formula setae: 0-1-3/0-0-3, seta on the second segment very slender almost unnoticeable, antennal formula: spines 0-0-1/1-0-1, the spine on the first segment of endopodite going beyond of second segment. IDL of limb I with two thin setulae setae of similar length and ODL with one long thin seta (Fig. 6D). Exopodite limb II elongated with fine setulae and without seta, inner portion with eight scrappers on the inner portion (Fig. 6E), scrappers 6 - 8 bend in the tip (arrowed in Fig. 6F). Postabdomen short, about 2.5 times longer than wide, postanal margin with 6 lateral fascicules, first setulae of each fascicle longer and thicker than the others (Figs. 6A, G), postabdominal claw long, about 1.36 times as long as the anus, with one basal spine armed with spinulae along its dorsal margin, its length about 0.2 of the claw (Fig. 6G), posteroventral corner of valve armed with spinulae not arranged in groups.
Male shorter than female, body length 245 ‒ 262 µm (mean = 254 µm, n = 2), habitus elongated, height/length ratio about 0.54 (Fig. 6H), rostrum short (Fig. 6I). Limb I proportionally smaller than on female, copulatory hook U-shaped, copulatory brush present, IDL with two setae (2 ‒ 3) of different size, male seta similar to the longest IDL seta (Fig. 6J). Postabdomen tapering distally (Fig. 6K), about 2.65 times as long as height; preanal angle well defined with strongly developed triangular projection, distalmost spine of the three first lateral fascicle on postabdomen long and reaching beyond dorsal margin of postabdomen, gonopores situated ventrally to the postabdominal claw base, postabdominal claw long, about ½ of postabdominal claw length (Fig. 6L).
The morphology of A. neotropica resembles its Neotropical congeners, but this species is easily distinguished from them by bear: 1) IDL setae armed with fine setulae; 2) scrappers 6 - 8 bend in the tip; 3) male postabdominal claw with long basal spine, about ½ of claw length.
Genus Kurzia Dybowski & Grochowski, 1894
13. Kurzia cf polyspina Hudec, 2000
Material examined: Two adult females, catalog number: MHNU-P 019.
Remarks: The specimens possess the general characteristics of K. polyspina, such as the habitus (Fig. 6M), body length 476 µm (n = 2), rostrum short (Fig. 6N), three connected head pores (Fig. 6O), triangular labrum with a notch on the apex (Fig. 6N), postabdomen long and tapering distally (Figs. 6M, P), one long basal spine about two times longer than claw width (Fig. 6Q) and marginal setulae along posteroventral corner of the valves. However, some subtle differences can be observed in our specimen: 1) the two longest IDL seta bear different spiculae (5+2) (Figs. 6R ‒ S); 2) distal portion of postabdomen with three spiculae (Fig. 6P). These traits observed in our specimens do not entirely fit with K. polyspina, suggesting a possible new species. Nevertheless, the affordable material was insufficient for a complete comparison and description of a new species. Therefore, more specimens should be analyzed.
Genus Alonella Sars, 1862
14. Alonella dadayi Birge, 1910
Material examined: Six adult females. Catalog number: MHNU-P 023.
Remarks: The specimens share the diagnostic features from A. dadayi previously recorded from the Magdalena department in Colombia (Fuentes-Reinés et al. 2012). Habitus as in Figure 7A, body length range between 234 ‒ 238 µm (mean = 276 µm, n = 6), rostrum long and curved (Figs. 7A ‒ C), labral keel elongated more or less triangular (Fig. 7D), postabdomen short and robust, tapering in the distal portion (Fig. 7E), postanal margin with seven denticles, preanal margin with angle clearly prominent, postabdominal claw shorter than anal margin, basal spine about ¼ of postabdominal claw length (Fig. 7E), valve ornamented with longitudinal lines or hexagons and striae (Fig. 7F) posteroventral margin of the valve with 3 ‒ 5 small denticles (Figs. 7G ‒ H). The specimens of the Orinoquía region present variabilities in the rostrum (Figs. 7A ‒ C) and in the number of denticles in the posteroventral margin of the valve (Figs. 7G ‒ H). Alonella dadayi is a species with a high morphological variability (Sousa et al. 2020).
15. Alonella pulchella Herrick, 1884
Material examined: Two adult females, catalog number: MHNU-P 035-1: MHNU-P 035-4.
Remarks: Alonella pulchella was originally described from Minnesota by Herrick (1984) and has been recorded in Canada (Hann & Chengalath 1981). This is the first record for Colombia. The female specimens examined were assigned to Alonella pulchella following the diagnostic characters presented by Hann and Chengalath (1981) and Smirnov (1996). Habitus as in Figure 7I, rostrum short (Fig. 7J), body length 234 µm (n = 2), antennule almost reaching the tip of the rostrum, two main head pores, and two very small, closely-set pores between them (Fig. 7K), antennules with nine aesthetascs extend beyond the length of the antennular body, sensory seta slender and inserted 1/7 from the apex of the antennular body (Fig. 7L), antennal formula, setae 0-0-3/0-1-3 (Fig. 7M). IDL of limb I with three unequal setae, seta 1 thin and short, setae 2 and 3 armed with fine setualae distally (Fig. 7N). ODL armed with a short seta and a thin seta longer than IDL longer seta (Fig. 7N). Exopodite of limb II oval and armed with a long seta (Fig. 7O), inner portion with eight scrappers, scraper 1 and 2 almost equal in length, scrappers 3 ‒ 6 decreasing distally, scrapper 7 and 8 similar in length, proximal portion of the gnathobase flattened; distal portion armed with three elongated elements, lateral portion with a small spicule, filter comb with seven setulated setae, postabdomen short tapering distally (Fig. 7P), preanal margin with an angle very prominent, postanal margin with seven strong denticles (Fig. 7P) postabdominal claw shorter than anus, basal spine short (Fig. 7Q), patterns of reticulation of valve with longitudinal lines or hexagons and striae (Fig. 7R), posteroventral of the valve with 2 or 1 denticles (Figs. 7S ‒ T).
Alonella pulchela resembles A. dadayi but they can be separated mainly by: 1) rostrum short in A. pulchella (Hann & Chengalath 1981, fig. 1, present data Figs. 7I ‒ J) vs. long in A. dadayi (Fuentes-Reinés et al. 2012, figs. 33A ‒ B, Sousa et al. 2020, figs. 1A ‒ B, 2A ‒ J, present data, Figs. 7A ‒ C); 2) seta on exopodite of limb II as long as exopodite itself in A. dadayi (Sousa et al. 2020, fig. 3E) while in A. pulchella the seta is longer than exopodite (present data, Fig. 7O).The specimens from Colombia bear the diagnostic feature of A. pulchella as described by Hann and Chengalath, 1981, but some fine scale differences were observed in our specimens: 1) scrapper 1 of limb II a little longer than scrapper 2 in Colombian specimens (Present data, Fig. 7O) while the opposite condition occurs in Canadian population (Hann & Chengalath, 1981, fig. 27, 2), scrapper 4 of limb II shorter than 5 in Canadian population while in Colombia specimens both scrapers, 4 and 5 almost equal in size; 3) lateral spicule on gnathobase of limb II present in Colombia population (present data, Fig. 7O) vs. absent in Canadian population (Hann & Chengalath 1981, fig 27). Alonella constitutes a genus that displays a high level of both intra-and interpopulation morphological variability (Hollwedel et al. 2003, Van Damme & Dumont 2010, Smirnov 1996, Sousa et al. 2020), therefore, we do not consider such differences as signs of a separate status of the Colombia populations.
16. Alonella cf. clathratula Sars, 1896
Material examined: Four adult females. Catalog number: MHNU-P 024.
Remarks: Alonella cf. clathratula was originally described from Australia by Sars (1896), and has been recorded in Brazil (Sars 1901, Van Damme & Dumont 2010), India (Sharma & Sharma 2001, 2010), Perú (as A. excisa var clathratula, Harding 1955, Uéno 1967), Cambodia (Tanaka & Ohtaka 2009), Thailand (Maiphae et al. 2005), Java, and Ethiopians regions (Smirnov 1996). This is the first record for Colombia. Habitus elongated (Fig. 8A), body length between 259 ‒ 291 µm (mean = 283 µm, n = 4) length/height ratio of the body about ca 1.42 ‒ 1.6, ocellus smaller than compound eye (Fig. 8B), labrum curved in the anterior margin (Fig. 8C), valve covered with lines and polygons, posteroventral corner of the valve with a small prominence as a blunt indentation (Fig. 8D), postabdomen elongated (Fig. 8E), about 3.7 times as long as wide, preanal margin with angle well defined, postanal margin with 8 denticles, postanal margin/anal margin ratio about 1, postabdominal claw shorter than anal margin, with two basal spines (Fig. 8F), the distal one about 0.36 of length of the postabdominal claw, the proximal spine the shortest, about 0.28 times shorter than the distal one.
Some morphological characteristics of the Colombian specimens do not agree with the populations reported by Smirnov (1996), Sharma and Sharma (2001, 2010) and Van Damme and Dumont (2010). For example, postanal margin/anus ratio is about 1 in Colombian specimens vs. 0.8 in specimens from Brazil (Van Damme & Dumont 2010), 1.5 in specimens from Australia (Smirnov 1996) suggesting that non-Australian populations may be forming a cryptic species complex.
Alonella clathratula was considered a variety of A. excisa (Uéno 1967, Harding 1955) or a subspecies of A. excisa (Smirnov 1971) but was finally reinstated as valid species by Smirnov (1996). This species is widespread in the tropics (Van Damme & Dumont 2010) and, owing to its poor original description and illustrations, needs to be redescribed. In addition, populations outside the Australasian region should constitute new taxa; therefore, the confirmation of Neotropical region population status is still needed.
17. Ephemeroporus barroisi (Richard, 1894) - group
Material examined: Tree adult females, catalog number: MHNU-P 025.
Remarks: This species was originally described from Syria as Pleuroxus barroisi by Richard, 1894, then transferred to the genus Chydorus by Sars (1895), and finally relocated to Ephemeroporus by Frey (1982b). Habitus ovoid (Fig. 8G), body length between 235 ‒ 280 µm (mean = 250 µm, n = 3), rostrum short (Fig. 8H), labrum with four denticles (Figs. 8H ‒ I). Valves with a spine on posteroventral corner (Fig. 8J). Postabdomen narrowing distally with prominent and sharp preanal angle (Fig. 8K), postabdominal claw with two basal spines, the first one smallest (Fig. 8L).
Ephemeroporus barroisi can be differentiated by its congeners by bearing: 1) labrum with 4 o 5 denticles, 2) one denticle on the posteroventral corner of the valve. These distinctive traits are also characteristics of Colombian populations. Ephemeroporus barroisi is considered a complex species group (Smirnov 1996) with recorded from different geographies worldwide (Zoppi de Roa & López 2008, Yalım & Çıplak 2010, Kotov et al. 2013, Korovchinsky 2013, Debastiani-Júnior et al. 2015, Sinev & Yusoff 2016, Etilé et al. 2020). In Colombia has been recorded in Córdoba Department (Alvarez 2010) without description and illustration. This is the first illustrated record of E. barroisi group confirming their presence in Colombia and Meta department.
Genus MatralonaVan Damme & Dumont, 2009
18. Matralona sp.
Material examined: One adult females. Catalog number: MHNU-P 033-1: MHNU-P 033-4.
Remarks: Matralona was proposed by Van Damme and Dumont (2009) after revising the species Alona simoneae Dumont, 1981 and including it in this genus. A similar case occurred with Alona freyi Idris & Fernando, 1981, which was also allocated in this genus (Sinev & Kotov 2012). Since then, the genus has two members: Matralona simoneae (Dumont, 1981) and M. freyi (Idris & Fernando, 1981). According to available data, this genus has a tropical distribution, with records from West Africa and West Asia. Here, the genus is formally reported for the first time in South America, although there is an unpublished record from Brazilian Amazon (Ghidini 2011). According to Van Damme and Dumont (2009) the genus can be recognized by the following characteristics: 1) body oval (Fig. 8M); 2) ocellus smaller than compound eye (Fig. 8N); 3) labrum quadrangular-like, with ventral setulae (Fig. 8O); 4) IDL and ODL of P1 with two a one setae respectively, both ornamented with long setulae (Fig. 8P). The specimen's morphology from La Española lagoon did not match any of the species already described, suggesting that the Colombian population constitutes a new species waiting for description.
Discussion
With this research, the total number of cladocerans for Colombia is increased to 124 species, constituting approximately 17.71% of the worldwide fauna of Cladocera. Furthermore, with these new records and the reported previously by Fuentes et al. (2018) from Tomo River, Vichada, 38 species have been hitherto registered for Orinoquía region, which represent 30.64 % of the whole Colombia territory, constituting the third region of Colombia with more records after Caribbean and Andean regions (Kotov & Fuentes-Reinés 2015).
Nevertheless, this new score is yet low in comparison with Brazil (approximately 169 species) (Elmoor-Loureiro et al. 2023) and Argentina (about 170 species) (José de Paggi et al. 2023). On the other hand, the richness observed in Colombia is superior to values in Paraguay (Villalba Duré et al. 2023), Uruguay (Caraballo et al. 2023), and Venezuela (González-Rivas et al. 2023).
The most of cladoceran species found in La Española lagoon, Meta-Department should be considered tropical forms, and most of them have been found in other Colombian body water (Fuente-Reinés et al. 2012, Reinés & Zoppi de Roa 2013, Fuentes-Reinés et al. 2022). Nevertheless, some species have also been recorded in the Nearctic region - for example Diaphanosoma fluviatile, Bosmina (Liederobosmina) hagmanni, Alonella dadayi, Alonella pulchella, Euryalona orientalis - and other are considered cosmopolitan (Ceriodaphnia cornuta, Ilyocryptus spinifer, Alonella clathratula) (Kotov et al. 2013).
Cladoceran diversity analysis for La Española lagoon displayed that Anomopoda was the most dominant order (90.3%), followed by Ctenopoda (9.67%). This structure of cladoceran diversity is like the observed in Afrotropical and Neotropical regions (Forró et al. 2008, Etilé et al. 2020), being a typical pattern related to the number of species of each order.
Despite the advance in investigations of the Colombian fauna of Cladocera in recent years, few studies have sampled in the Orinoquía region (Fuentes-Reinés et al. 2018) and no reports of this superorder have been recorded from Meta department, which makes it challenging to carry out comparisons. Here, a total of 35 species were found, a richness superior to that presented by Fuentes-Reinés et al. (2018), with five species from Tomo River (Orinoquia region), Fuentes-Reinés and Elmoor-Loureiro (2015) with 13 species from the Ciénaga El Convento (Caribbean region), Fuentes-Reinés (2014) with 17 species from the Laguna Navío Quebrado (Caribbean region) and Fuentes-Reinés et al. (2019) with 18 species from a small temporary pond (Caribbean region). Nevertheless, Fuentes-Reinés et al. (2013) reported 45 species for Ciénaga Grande de Santa Marta (Caribbean region). We believe that the richness of cladoceran fauna from La Española lagoon is probably underestimated due to sampling effort, considering that sampling campaigns were carried out only in a month and probably the reproduction and hatching of eggs of these organisms in the body water can occur in different periods (Pasos 2017).
The family Chydoridae was the most dominant, with 64.67% of the total species. Chydoridae family is represented by species related to some kind of substrate in aquatic ecosystems, which allows them to have a greater richness in the tropical regions (Debastiani-Junior et al. 2015; Gogoi et al. 2018). Finally, some species found here ‒ i.e. Streblocerus sp., Matralona sp., Chydorus sp., Kurzia cf. polyspina and Alonella cf. clathratula ‒ deserve attention concerning their taxonomic status because they can represent new species for the science.
In summary, this study contributes to the knowledge of the diversity and distribution of cladocerans of the central region of Colombia, especially in the Orinoquia region, a zone with little information about this superorder. Furthermore, here we reported for the first time in South America Matralona genus and provided the male description of Anthalona neotropica. Such a result reinforces the necessity to increase effort sampling in other regions with little or no information to improve the comprehension of Colombian cladoceran fauna's diversity.












 uBio
uBio 


